Effects of Total Solid Content on Anaerobic Fermentation Performance and Biogas Productivity of Tail Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Tail Vegetables and Inoculum
2.2. Anaerobic Fermentation Experimental Design and Apparatus
2.3. Determination and Methodology of Liquid Phase
2.4. Determination and Methodology of Gas Phase
2.5. Kinetic Characteristics of Methane Production
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Raw Materials and Inoculants
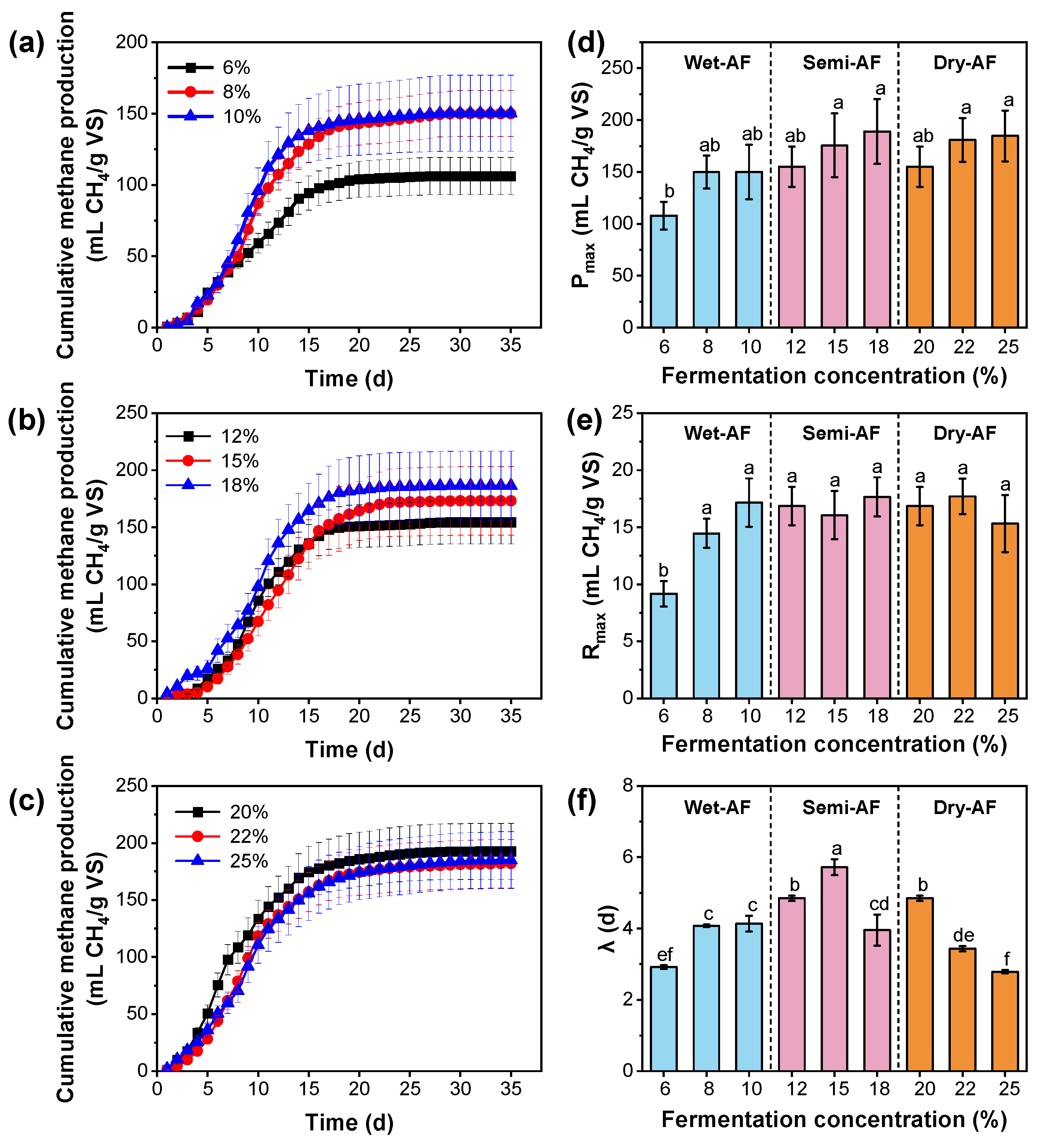
3.2. The Effect of TS Content on Biogas Production Performance
3.3. The Performances of Cumulative Methane Production under Different TS Content Treatments
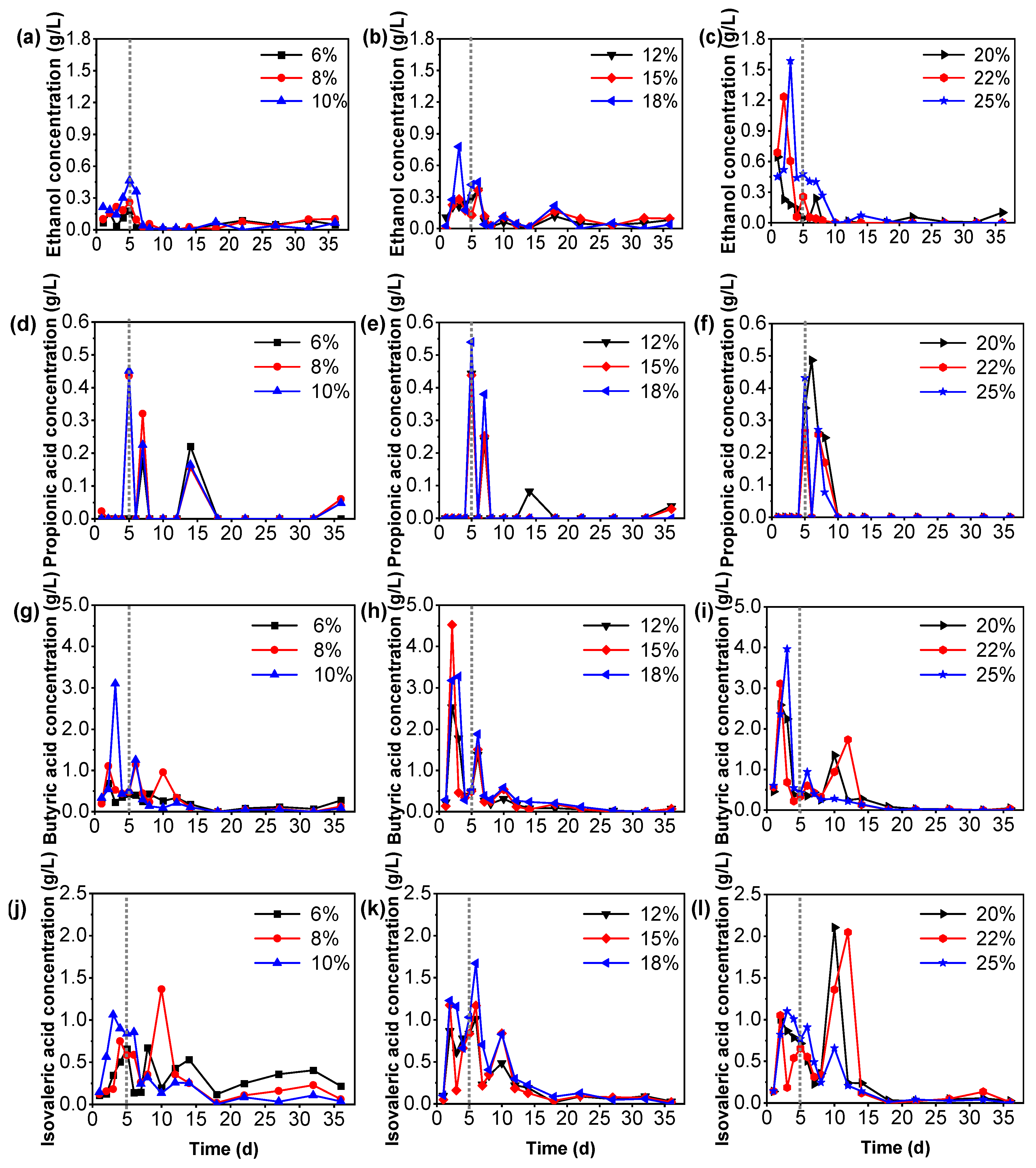
3.4. The Variation in Acetic Acid and VFAs in Tail Vegetables Anaerobic Fermentation
3.5. The Differences in Acidogenic Types in Tail Vegetables’ Anaerobic Fermentation
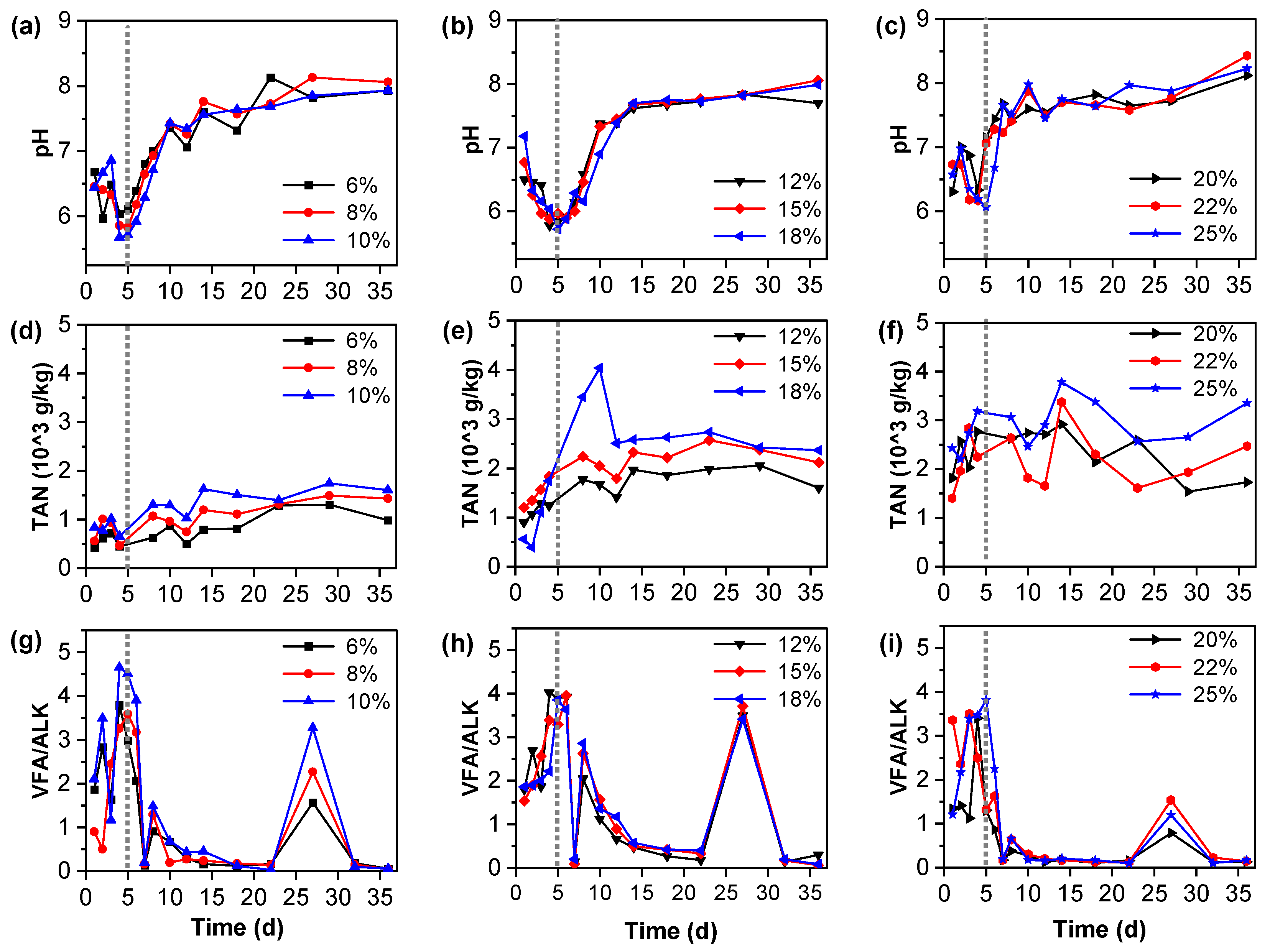
3.6. The Characteristics of pH, VFA/ALK, and TAN in the Fermentation Broth
3.7. Economic and Environmental Analysis of Rotten Vegetable Methane Source
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Bremer, P.; Harder, M.K.; Lee, M.S.W.; Parker, K.; Gaugler, E.C.; Mirosa, M. A systematic review of food loss and waste in China: Quantity, impacts and mediators. J. Environ. Manag. 2022, 3031, 14092. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities-A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liao, W.; Yu, Y.; Li, G.; An, T. How Does Vegetable Waste Decomposition Influence the Antibiotic Resistome and the Human Bacterial Pathogen Structure in Leachates? ACS ES&T Water 2021, 2, 226–236. [Google Scholar]
- Lin, D.; Huang, D.; Zhang, J.; Yao, Y.; Zhang, G.; Ju, F.; Xu, B.; Wang, M. Reduction of antibiotic resistance genes (ARGs) in swine manure-fertilized soil via fermentation broth from fruit and vegetable waste. Environ. Res. 2022, 214, 113835. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Cao, J.; Wang, P.; Li, R.; Qi, Z.; Fu, J.; Chen, Y.; Chen, M. Volatile organic compounds and dominant bacterial community during aerobic composting of vegetable waste and cow manure co-complexing. BioResources 2022, 17, 1338–1353. [Google Scholar] [CrossRef]
- Liang, Z.; Feng, Q.; Zhang, Y.; Yu, Y.; Liao, W.; Li, G.; An, T. Odorous VOCs released from bio-decomposition and its interaction mechanism with bacteria: Compared inter-type with intra-type household garbage. J. Clean. Prod. 2024, 447, 141523. [Google Scholar] [CrossRef]
- Agrawal, A.; Chaudhari, P.K.; Ghosh, P. Anaerobic digestion of fruit and vegetable waste: A critical review of associated challenges. Environ. Sci. Pollut. Res. 2022, 30, 24987–25012. [Google Scholar] [CrossRef]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The influence of co-fermentation of agri-food waste with primary sludge on biogas production and composition of the liquid fraction of digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Li, L.; Zhou, H.; Wang, K.; Ding, J.; Jiang, J.; Wei, L.; Zhao, Q. The stress of bioactive compounds on microbes in anaerobic digestion of food waste and mitigation strategies: A critical review. Chem. Eng. J. 2024, 493, 152764. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Xing, T.; Xiao, F.; Liu, Y.; Zhen, F.; Sun, Y. An integrated evaluation strategy for anaerobic digestion monitoring based on acid-base balance and thermodynamics of volatile fatty acid degradation. Chem. Eng. J. 2024, 486, 150340. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, L.; Li, Y. Comparison of premixing methods for solid-state anaerobic digestion of corn stover. Bioresour. Technol. 2015, 175, 430–435. [Google Scholar] [CrossRef]
- Liotta, F.; d’Antonio, G.; Esposito, G.; Fabbricino, M.; Frunzo, L.; van Hullebusch, E.; Lens, P.; Pirozzi, F. Effect of moisture on disintegration kinetics during anaerobic digestion of complex organic substrates. Waste Manag. Res. J. A Sustain. Circ. Econ. 2014, 32, 40–48. [Google Scholar] [CrossRef]
- Xu, F.; Wu, Z.; Li, Y. Predicting the methane yield of lignocellulosic biomass in mesophilic solid-state anaerobic digestion based on feedstock characteristics and process parameters. Bioresour. Technol. 2014, 173, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Mohan, S.V. Effect of food to vegetable waste ratio on acidogenesis and methanogenesis during two-stage integration. Bioresour. Technol. 2018, 254, 256–263. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Liu, X.; Mei, Z.; Ren, H.; Cao, Q.; Yan, Z. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste. Bioresour. Technol. 2017, 245, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y. Anaerobic digestion of giant reed for methane production. Bioresour. Technol. 2014, 171, 233–239. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste: Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- HJ 643-2013; Solid Waste—Determination of Volatile Organic Compounds—Headspace-Gas Chromatography/Mass Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2013.
- van Soest, P. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 10, 3583–3597. [Google Scholar] [CrossRef]
- ISO 5664-1984; Water Quality—Determination of Ammonium—Distillation and Titration Method. ISO: Geneva, Switzerland, 1984.
- Xiao, L.; Zheng, S.; Lichtfouse, E.; Luo, M.; Tan, Y.; Liu, F. Carbon nanotubes accelerate acetoclastic methanogenesis: From pure cultures to anaerobic soils. Soil. Biol. Biochem. 2020, 150, 107938. [Google Scholar] [CrossRef]
- Farghali, M.; Mayumi, M.; Syo, K.; Satoshi, A.; Seiichi, Y.; Takashima, S.; Ono, H.; AP, Y.; Yamashiro, T.; Ahmed, M.; et al. Potential of biogas production from manure of dairy cattle fed on natural soil supplement rich in iron under batch and semi-continuous anaerobic digestion. Bioresour. Technol. 2020, 309, 123298. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Li, X.; Wang, K.; Yuan, H. Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pre- treatment. Energy Fuel 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.; Tang, L.; Li, Y. A mass diffusion-based interpretation of the effect of total solids content on solid-state anaerobic digestion of cellulosic biomass. Bioresour. Technol. 2014, 167, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, M.; Torii, S. Comparison effect of feedstock to inoculum ratios on biogas yields of cafeteria, vegetable, fruit wastes with cattle manure using co-digestion process. Int. J. Green. Energy 2017, 14, 665–673. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, Z.; Lu, J.; Li, D.; Wang, G.; Li, Y.; Wu, Y.; Li, S.; Xu, F.; et al. Effect of inoculum and substrate/inoculum ratio on the performance and methanogenic archaeal community structure in solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover. Waste Manag. 2018, 81, 117–127. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, J. The effect of total solids concentration and temperature on biogas production by anaerobic digestion. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3534–3541. [Google Scholar] [CrossRef]
- Liu, W.; Liao, B. Anaerobic co-digestion of vegetable and fruit market waste in LBR + CSTR two-stage process for waste reduction and biogas production. Appl. Biochem. Biotechnol. 2018, 188, 185–193. [Google Scholar] [CrossRef]
- Labatut, R.; Angenent, L.; Scott, N. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Motte, J.; Escudié, R.; Bernet, N.; Delgenes, J.; Steyer, J.; Dumas, C. Dynamic effect of total solid content, low substrate/inoculum ratio and particle size on solid-state anaerobic digestion. Bioresour. Technol. 2013, 144, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; Cledera-Castro, M.d.M.; Moratilla Soria, B.Y. Biogas production from vegetable and fruit markets waste-compositional and batch characterizations. Sustainability 2019, 11, 6790. [Google Scholar] [CrossRef]
- Wu, B.; Lin, T.; Lu, Z.; Yu, X.; Huang, M.; Yang, R.; Wang, C.; Tian, C.; Li, J.; Sun, Y.; et al. Fe binuclear sites convert methane to acetic acid with ultrahigh selectivity. Chem 2022, 8, 1658–1672. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Triolo, J.; Sommer, S.; Møller, H.; Weisbjerg, M.; Jiang, X. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Bouallagui, H.; Touhami, Y.; Cheikh, R.B.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejóa, E.; Gonzalez-Fernández, C. Food waste valorization into bioenergy and bioproducts through a cascade combination of bioprocesses using anaerobic open mixed cultures. J. Clean. Prod. 2022, 372, 133680. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Liu, X.; Du, M.; He, D.; Fu, Q.; Pan, M.; Leu, S.; Wang, D. Cetyltrimethylammonium bromide enhances anaerobic fermentative production of short-chain fatty acids from waste activated sludge. ACS ES&T Eng. 2023, 3, 2051–2061. [Google Scholar]
- Feng, L.; Chen, Y.; Zheng, A. Enhancement of waste activated sludge protein conversion and volatile fatty acids accumulation during waste activated sludge anaerobic fermentation by carbohydrate substrate addition: The Effect of pH. Environ. Sci. Technol. 2009, 43, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Buyukkamaci, N.; Filibeli, A. Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem. 2004, 39, 1491–1494. [Google Scholar] [CrossRef]
- Lahav, O.; Morgan, B. Titration methodologies for monitoring of anaerobic digestion in developing countries: A review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Gins, G.; Degrève, J.; Van Impe, J.; Dewil, R. Parameter identification and modeling of the biochemical methane potential of waste activated sludge. Environ. Sci. Technol. 2011, 45, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Jiang, Y.; Zhi, H.; Xue, S.; Chen, Z.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating methane production from anaerobic mono- and co-digestion of kitchen waste, corn stover, and chicken manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Qiao, Y.; Tie, J.; Wang, X.; Wei, B.; Zhang, W.; Liu, Z.; Zhang, G.; Lyu, J.; Liao, W.; Liu, L.; et al. Comprehensive evaluation on effect of planting and breeding waste composts on the yield, nutrient utilization, and soil environment of baby cabbage. J. Environ. Manag. 2023, 341, 117941. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Y.; Du, L.; Yu, Y. Contamination level; sources, and health risk of polycyclic aromatic hydrocarbons in suburban vegetable field soils of Changchun, Northeast China. Sci. Rep. 2022, 12, 11301. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, D.; Proshad, R.; Uwiringiyimana, E.; Wang, Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci. Rep. 2021, 11, 22824. [Google Scholar] [CrossRef]
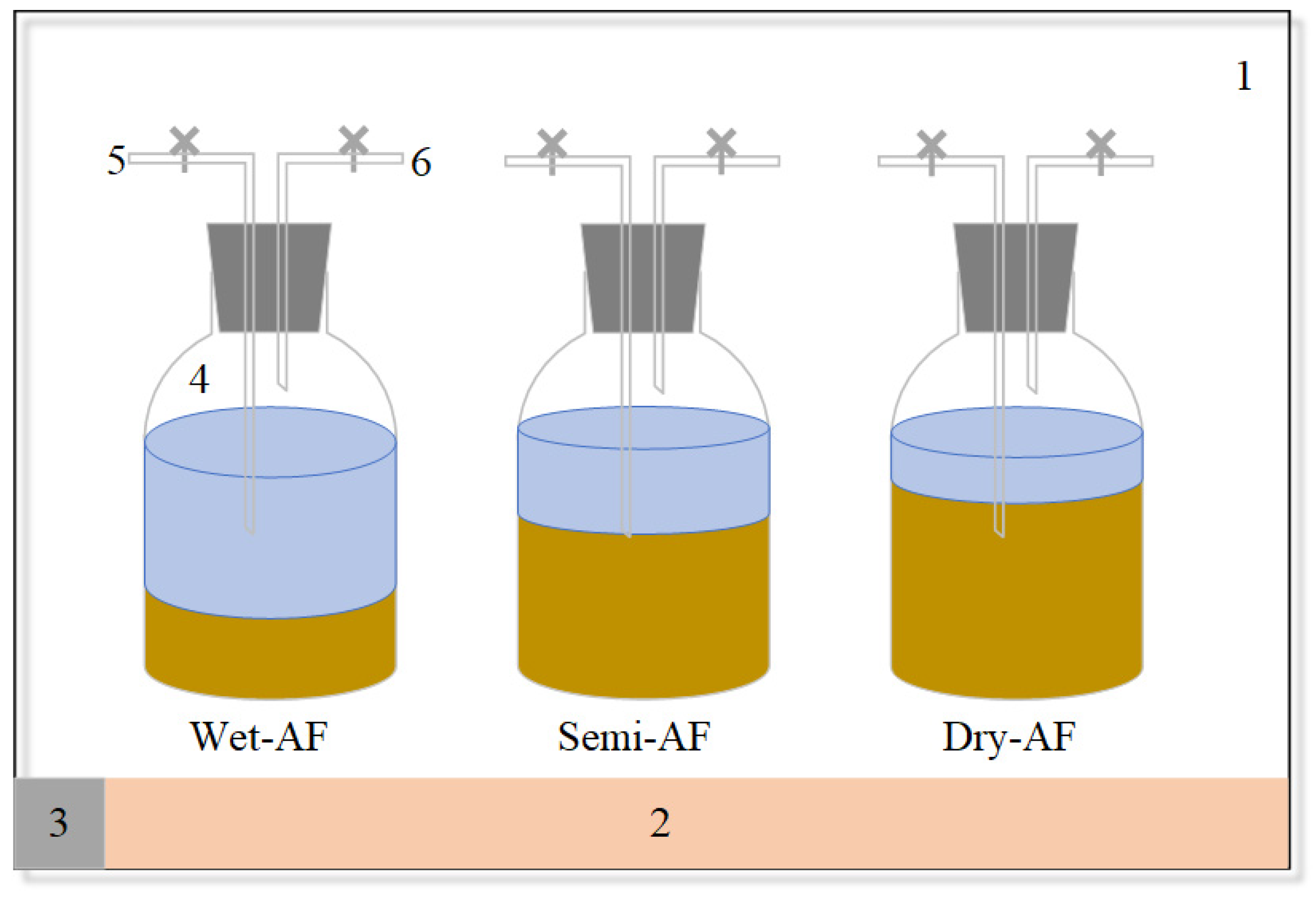

| Parameters | Vegetable Residues | Inoculum |
|---|---|---|
| TS (%) | 16.1 ± 0.3 | 42.7 ± 0.3 |
| VS (%) | 10.9 ± 0.3 | 10.8 ± 0.3 |
| TC (%) | 41.3 ± 1.2 | 24.4 ± 0.3 |
| TN (%) | 2.1 ± 0.1 | 1.9 ± 0.0 |
| pH | 6.5 ± 0.1 | 7.9 ± 0.1 |
| C/N Ratio | 19.7 ± 0.5 | 13.2 ± 0.5 |
| Cellulose (%) | 28.3 ± 1.4 | 12.5 ± 1.6 |
| Hemicellulose (%) | 26.4 ± 0.5 | 2.0 ± 0.3 |
| Lignin (%) | 11.4 ± 1.3 | 7.8 ± 1.0 |
| Treatment | Vegetable Residues (g) | Inoculum (g) | Water (g) | TS Content (%) | |
|---|---|---|---|---|---|
| Wet-AF | 6% | 295 | 175 | 1530 | 6 |
| 8% | 395 | 235 | 1370 | 8 | |
| 10% | 495 | 294 | 1211 | 10 | |
| Semi-AF | 12% | 570 | 339 | 1091 | 12 |
| 15% | 740 | 440 | 820 | 15 | |
| 18% | 890 | 529 | 581 | 18 | |
| Dry-AF | 20% | 985 | 585 | 430 | 20 |
| 22% | 1085 | 645 | 270 | 22 | |
| 25% | 1235 | 734 | 31 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Wang, Y.; Li, R.; Fu, L.; Liu, Z.; Gao, X. Effects of Total Solid Content on Anaerobic Fermentation Performance and Biogas Productivity of Tail Vegetables. Fermentation 2024, 10, 437. https://doi.org/10.3390/fermentation10080437
Yao L, Wang Y, Li R, Fu L, Liu Z, Gao X. Effects of Total Solid Content on Anaerobic Fermentation Performance and Biogas Productivity of Tail Vegetables. Fermentation. 2024; 10(8):437. https://doi.org/10.3390/fermentation10080437
Chicago/Turabian StyleYao, Li, Yanqin Wang, Ran Li, Longyun Fu, Zhaodong Liu, and Xinhao Gao. 2024. "Effects of Total Solid Content on Anaerobic Fermentation Performance and Biogas Productivity of Tail Vegetables" Fermentation 10, no. 8: 437. https://doi.org/10.3390/fermentation10080437





