Role of Wilting Time on the Chemical Composition, Biological Profile, and Fermentative Quality of Cereal and Legume Intercropping Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Productivity Determination
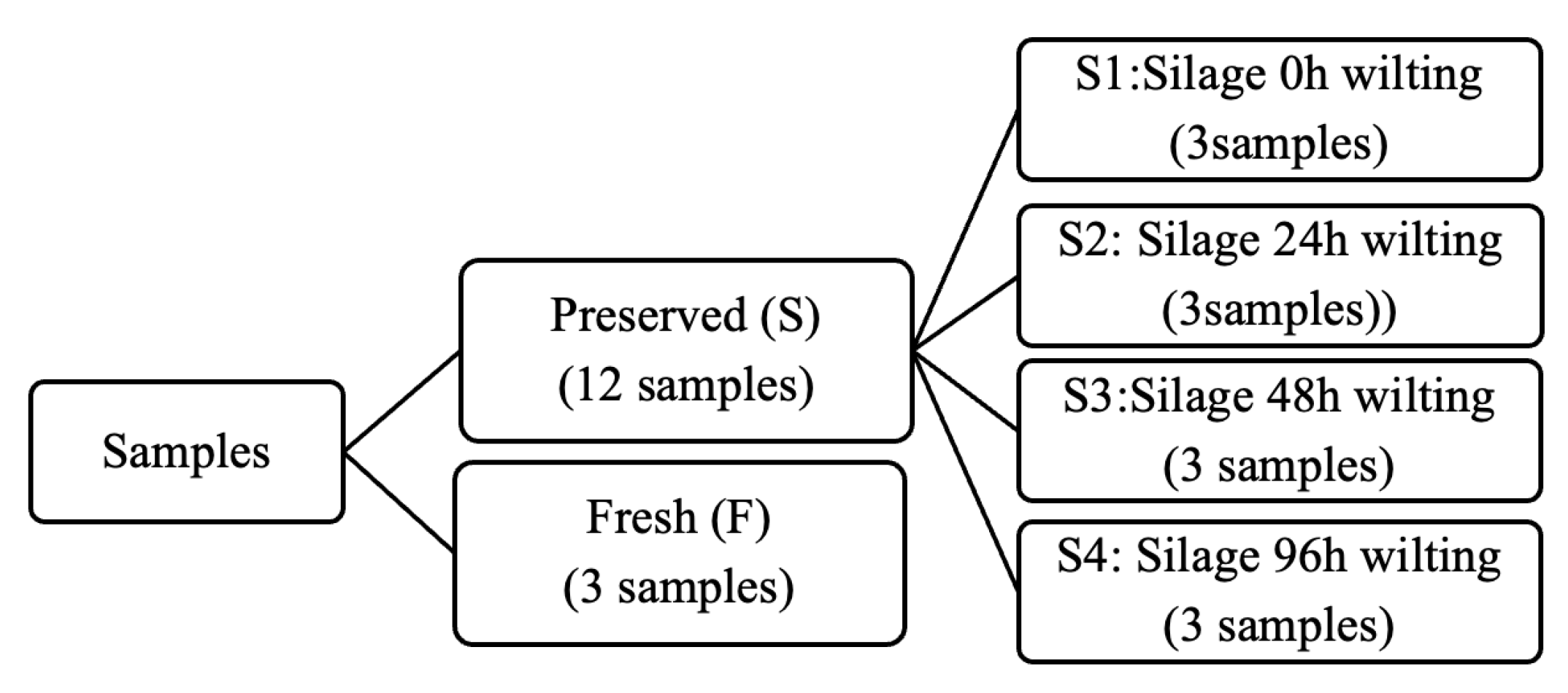
2.2. Sample Preparation
Ensiling Procedure
2.3. Chemical Analyses
2.4. Energy Estimates
2.5. Determination of Biological Parameters
2.6. Statistical Analyses
3. Results
3.1. Productivity
3.2. Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Givens, D.I.; Deaville, E.R. The current and future role of near infrared reflectance spectroscopy in animal nutrition: A review. Aust. J. Agric. Res. 1999, 50, 1131–1145. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review: Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, P.; Starck, T.; Voisin, A.S.; Nesme, T. Biological nitrogen fixation of legumes crops under organic farming as driven by cropping management: A review. Agric. Syst. 2023, 205, 103579. [Google Scholar] [CrossRef]
- Weinert, C.; Sousa, R.O.; de Bortowski, E.M.; Campelo, M.L.; da Silva Pacheco, D.; Santos, L.V.d.; Deuner, S.; Valente, G.B.; Matos, A.B.; Vargas, V.L.; et al. Legume winter cover crop (Persian clover) reduces nitrogen requirement and increases grain yield in specialized irrigated hybrid rice system. Eur. J. Agron. 2023, 142, 126645. [Google Scholar] [CrossRef]
- Ciaccia, C.; Montemurro, F.; Campanelli, G.; Diacono, M.; Fiore, A.; Canali, S. Legume cover crop management and organic amendments application: Effects on organic zucchini performance and weed competition. Sci. Hortic. 2015, 185, 48–58. [Google Scholar] [CrossRef]
- Mori Alvez, C.; Perdomo Varela, C.; González Barrios, P.; Bentos Guimaraes, A.; del Pino Machado, A. Lupine Cultivation Affects Soil’s P Availability and Nutrient Uptake in Four Contrasting Soils. Agronomy 2024, 14, 389. [Google Scholar] [CrossRef]
- Lopes, D.J.H. Estratégia para o Desenvolvimento da Agricultura Biológica e Plano de Ação Para a Produção e Promoção de Produtos Biológicos; Governo dos Açores: Ponta Delgada, Portugal, 2022.
- Ţiţei, V. Some biological features and biomass quality of Lupinus albus and Lupinus luteus in Moldova. Lucr. Ştiinţifice. Ser. Agron. 2020, 63, 19–26. [Google Scholar]
- Fontaine, R.; Zimbron Silva, D.; Gonçalves, D. Vertebrate damage to Azorean vineyards: The role of the endemic Azores Woodpigeon Columba palumbus azorica. Environ. Monit. Assess. 2024, 196, 258. [Google Scholar] [CrossRef]
- Corredor, F.A.; Passuni, J.F.; Noli, E.C.; Bernaola-Paucar, R.M. Nutritional and energy value of Vicia sativa pods. Agroindustrial Sci. 2020, 10, 307–309. [Google Scholar] [CrossRef]
- A.O.A.C.—Association of Official Analytical Chemists. Official Methods of Analysis, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures, and some Applications); ARS, USDA: Washington, DC, USA, 1970.
- NRC. Nutrient Requirements of Beef Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Yan, T.; Agnew, R.E. Prediction of nutritive values in grass silages: I. Nutrient digestibility and energy concentrations using nutrient compositions and fermentation characteristics. J. Anim. Sci. 2004, 82, 1367–1379. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Alexander, R.H.; McGowan, M. The routine determination of in vitro digestibility of organic matter in forages. An investigation of the problems associated with continuous large-scale operation. J. Br. Grassl. Soc. 1966, 21, 140–147. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- McDonald, P. The Biochemistry of Silage; John Wiley and Sons Ltd.: Chichester, UK, 1981. [Google Scholar]
- Ørskov, E.R.; McDonald, P. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Dias, C.S.A.M.M.; Nunes, H.P.B.; Vouzela, C.F.M.; Madruga, J.S.; Borba, A.E.S. Influence of the Season on the Nutritive Value and Gas Production of Opuntia ficus-indica and Agave americana L. in Ruminant Feed. Animals 2023, 13, 1008. [Google Scholar] [CrossRef]
- Marques, D.E.O.; Bonfá, C.S.; Magalhães, M.A.; Guimarães, C.G.; Rodrigues, R.C.; Nobre, D.A.C.; Barroso, E.G. Implicações no uso da técnica de realocação de silagens: Uma revisão. Res. Soc. Dev. 2022, 11, e57811831338. [Google Scholar] [CrossRef]
- Nunes, H.; Maduro Dias, C.; Borba, A. Chemical composition and gas production kinetic parameters of sweet potato vine waste silage after preserved for short and prolonged periods. Multidiscip. Sci. J. 2023, 6, 2024041. [Google Scholar] [CrossRef]
- Mariotti, M.; Masoni, A.; Ercoli, L.; Arduini, I. Above- and below-ground competition between barley, wheat, lupin, and vetch in a cereal and legume intercropping system. Grass Forage Sci. 2009, 64, 401–412. [Google Scholar] [CrossRef]
- Van der Colf, J.R.; Botha, P.; Meeske, R.; Truther, W. Seasonal drymatter production, botanical composition and forage quality of kikuyu over-sown with annual or perennial ryegrass. Afr. J. Range Forage Sci. 2015, 33, 133–142. [Google Scholar] [CrossRef][Green Version]
- Prates, L.L.; Yu, P. Interconnection between protein-related chemical functional group spectral features of prairie oat (Avena sativa L.) varieties and ruminant relevant nutrition—Degradation, intestinal digestion, and true nutrient supply to dairy cows. J. Anim. Physiol. Anim. Nutr. 2023, 107, 783–793. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2012, 68, 1–19. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Bolsen, K.K.; Lin, C.J. History of silage. Silage Sci. Technol. 2003, 42, 1–30. [Google Scholar] [CrossRef]
- Chamberlain, A.; Wilkinson, J.M. Feeding the Dairy Cow; Chalcombe Publications: Wickham, UK, 1996. [Google Scholar]
- Costa, C.A.D.; Gaião, D.; Teixeira, D.; Esteves Correia, H.; Guerra, L.T.; Guiné, R.P. Pontes entre agricultura familiar e biológica através da formação em contexto de trabalho. Inovação Tecnol. Nas Ciências Agrárias 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Kung, L., Jr. A Review on Silage Additives and Enzymes; Department of Animal and Food Sciences, University of Delaware: Newark, DE, USA, 2010. [Google Scholar]
- Wang, J.; Wang, J.Q.; Zhou, H.; Feng, T. Effects of addition of previously fermented juice prepared from alfalfa on fermentation quality and protein degradation of alfalfa silage. Anim. Feed. Sci. Technol. 2009, 151, 280–290. [Google Scholar] [CrossRef]
- Silva, T.C.d.; Leandro, D.d.S.; Santos, E.M.d.; Oliveira, J.S.d.; Perazzo, A.F. Importance of the Fermentation to Produce High-Quality Silage. Ferment. Process. InTech 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Fonseca, A.J.M.; Cabrita, A.R.J.; Nogueira, C.S.S.; Melo, D.S.P.; Lopes, Z.M.C.; Abreu, J.M.F. Lactation responses of dairy cows to whole-crop wheat or ryegrass silages. Anim. Feed. Sci. Technol. 2005, 118, 153–160. [Google Scholar] [CrossRef]
- Bernard, J.K.; West, J.W.; Trammel, D.S. Effect of Replacing Corn Silage with Annual Ryegrass Silage on Nutrient Digestibility, Intake, and Milk Yield for Lactating Dairy Cows. J. Dairy Sci. 2002, 85, 2277–2282. [Google Scholar] [CrossRef]
- Abreu, J.M.; Bruno-Soares, A.M.; Calouro, F. Intake and Nutritive Value of Mediterranean Forages and Diets; Instituto Superior de Agronomia: Lisboa, Portugal, 2000. [Google Scholar]
- Huhtanen, P.; Rinne, M.; Nousiainen, J. Effects of Silage Soluble Nitrogen Components on Metabolizable Protein Concentration: A Meta-Analysis of Dairy Cow Production Experiments. J. Dairy Sci. 2007, 91, 1150–1158. [Google Scholar] [CrossRef]
- Martinez, V.C.; Saldaña, J.L.S. Ensilado de Forrajes y su Empleo en la Alimentación de Ruminantes; Ediciones Mundi-Prensa: Madrid, Spain, 1998. [Google Scholar]
- Frame, J. Improved Grassland Management; Farming Press Books: Ipswich, UK, 1992. [Google Scholar]
- Jarrige, R. Alimentação de Bovinos Ovinos e Caprinos; Publicações Europa-America: Sintra, Portugal, 1998. [Google Scholar]
- Dias, C.S.A.M.M.; Nunes, H.P.B.; Borba, A.E.S. Influence of the Physical Properties of Samples in the Use of NIRS to Predict the Chemical Composition and Gas Production Kinetic Parameters of Corn and Grass Silages. Fermentation 2023, 9, 418. [Google Scholar] [CrossRef]
- Hartinger, T.; Gresner, N.; Südekum, K.-H. Effect of Wilting Intensity, Dry Matter Content, and Sugar Addition on Nitrogen Fractions in Lucerne Silages. Agriculture 2019, 9, 11. [Google Scholar] [CrossRef]
- Seglar, B. Fermentation Analysis and Silage Quality Testing; College of Veterinary Medicine, University of Minnesota: Minnesota, MN, USA, 2003. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice Hall: Hoboken, NJ, USA, 2011. [Google Scholar]
- Getachew, G.; Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed. Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Lopez, S.; Dijkstra, J.; Davies, D.R.; Sanderson, R.; Williams, B.A.; France, J. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Comparison of models. Br. J. Nutr. 2000, 83, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed. Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Chesson, A.; Forsberg, C.W. Polysaccharide degradation by rumen microorganisms. In The Rumen Microbial Ecosystem; Hobson, P.N., Ed.; Elsevier: New York, NY, USA, 1988; pp. 329–381. [Google Scholar] [CrossRef]
| Parameter | Fresh (F) | Preserved (Silage) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| DM (%) | 10.00 a | 11.78 b | 13.13 c | 13.98 c | 26.47 d | 1.56 | <0.001 |
| CP (%DM) | 22.51 a | 21.97 ab | 21.66 bc | 20.77 c | 19.67 d | 0.28 | <0.001 |
| EE (%DM) | 1.96 | 2.05 | 2.05 | 2.07 | 2.08 | 0.02 | 0.443 |
| Ash (%DM) | 8.72 | 8.07 | 8.19 | 8.27 | 8.32 | 0.08 | 0.107 |
| IVDMD (%) | 77.31 a | 69.36 b | 65.29 c | 64.08 c | 62.36 c | 0.94 | <0.001 |
| IVOMD (%) | 68.3 a | 58.03 b | 56.95 bc | 55.63 bd | 48.12 c | 0.67 | <0.001 |
| Parameter | Fresh (F) | Preserved (Silage) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| NDF (%DM) | 51.26 ab | 50.80 a | 53.06 bc | 54.79 c | 57.04 d | 0.67 | <0.001 |
| ADF (%DM) | 35.50 a | 39.75 b | 43.26 c | 44.54 c | 45.99 c | 1.03 | <0.001 |
| ADL (%DM) | 5.39 a | 7.37 b | 7.99 bc | 8.10 c | 9.99 d | 0.39 | <0.001 |
| HEM (%DM) | 16.12 a | 11.05 b | 9.80 c | 10.25 b | 11.00 b | 0.90 | <0.001 |
| CEL (%DM) | 30.11 a | 32.38 b | 35.27 c | 36.44 c | 36.00 c | 0.85 | <0.001 |
| Parameter | Preserved (Silage) | SEM | p Value | |||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |||
| pH | 4.61 a | 4.56 ab | 4.52 b | 4.42 c | 0.02 | <0.001 |
| %N-NH3/N | 13.25 a | 12.94 a | 12.46 bc | 11.78 c | 0.18 | 0.003 |
| Parameter | Fresh (F) | Preserved (Silage) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| GE (MJ/KgDM) | 13.94 a | 13.98 a | 13.78 b | 13.63 bc | 13.43 c | 0.32 | 0.03 |
| DE (MJ/KgDM) | 10.78 a | 9.7 ab | 9 b | 8.73 bc | 8.38 c | 0.13 | <0.001 |
| ME (MJ/KgDM) | 8.84 a | 7.95 b | 7.38 bc | 7.16 c | 6.87 c | 0.18 | <0.001 |
| NEL (MJ/KgDM) | 6.18 a | 6.48 a | 5.67 b | 5.61 b | 5.51 b | 0.29 | 0.02 |
| Incubation Time (h) | Fresh (F) | Preserved (Silage) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| 4 | 4.82 a | 5.76 b | 4.49 c | 2.93 d | 3.40 e | 0.5 | <0.001 |
| 8 | 11.06 a | 13.07 b | 9.67 c | 8.46 d | 8.57 e | 0.49 | <0.001 |
| 12 | 16.55 a | 19.11 b | 14.09 c | 13.16 d | 13.01 d | 0.95 | <0.001 |
| 24 | 29.38 a | 31.65 b | 23.82 c | 23.44 c | 22.90 d | 1.06 | <0.001 |
| 48 | 44.11 a | 42.73 b | 33.63 c | 33.63 c | 33.11 c | 1.18 | <0.001 |
| 72 | 50.95 a | 46.27 b | 37.42 c | 37.47 c | 37.19 c | 1.46 | <0.001 |
| 96 | 54.14 a | 47.40 b | 38.88 c | 38.92 c | 38.82 c | 1.76 | <0.001 |
| Kinetics Parameters | Fresh (F) | Preserved (Silage) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| a (mL/0.2 gDM) | –2.27 a | –1.58 b | –2.63 c | –3.08 d | –3.58 e | 0.18 | <0.001 |
| b (mL/0.2 gDM) | 59.18 a | 51.02 b | 43.37 c | 42.54 d | 41.39 e | 1.8 | <0.001 |
| c (mL/h) | 0.0322 a | 0.047 b | 0.0406 c | 0.0396 d | 0.0382 e | 0.01 | <0.001 |
| tlag (h) | 1.20 ab | 0.97 a | 1.00 ab | 1.70 c | 2.10 d | 0.13 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.M.; Nunes, H.; Aguiar, M.; Pereira, A.; Madruga, J.; Borba, A. Role of Wilting Time on the Chemical Composition, Biological Profile, and Fermentative Quality of Cereal and Legume Intercropping Silage. Fermentation 2024, 10, 448. https://doi.org/10.3390/fermentation10090448
Dias CM, Nunes H, Aguiar M, Pereira A, Madruga J, Borba A. Role of Wilting Time on the Chemical Composition, Biological Profile, and Fermentative Quality of Cereal and Legume Intercropping Silage. Fermentation. 2024; 10(9):448. https://doi.org/10.3390/fermentation10090448
Chicago/Turabian StyleDias, Cristiana Maduro, Hélder Nunes, Mariana Aguiar, Arnaldo Pereira, João Madruga, and Alfredo Borba. 2024. "Role of Wilting Time on the Chemical Composition, Biological Profile, and Fermentative Quality of Cereal and Legume Intercropping Silage" Fermentation 10, no. 9: 448. https://doi.org/10.3390/fermentation10090448
APA StyleDias, C. M., Nunes, H., Aguiar, M., Pereira, A., Madruga, J., & Borba, A. (2024). Role of Wilting Time on the Chemical Composition, Biological Profile, and Fermentative Quality of Cereal and Legume Intercropping Silage. Fermentation, 10(9), 448. https://doi.org/10.3390/fermentation10090448





