Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria bassiana Conidia Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Inoculum Preparation
2.2. Raw Materials
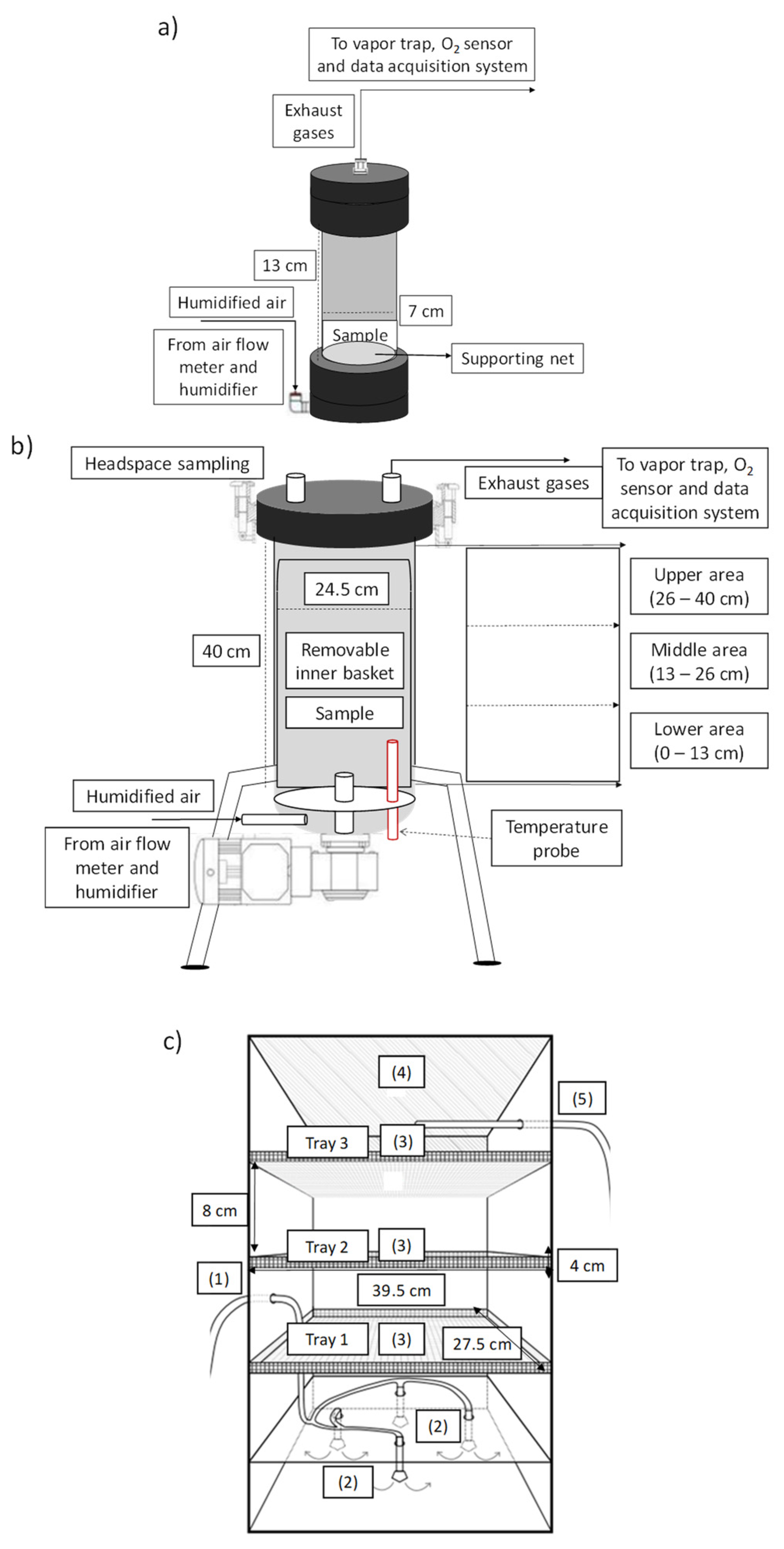
2.3. Solid-State Fermentation Experimental Setups
2.3.1. Packed-Bed Reactors: 0.5 L
2.3.2. Packed-Bed Reactors: 22 L Bioreactor
2.3.3. Tray Bioreactor
2.3.4. Oxygen Uptake Rate
2.4. Conidia Counting
2.5. Total Sugar Content Analysis
2.6. Analytical Methods
2.7. Statistical Analyses
3. Results
3.1. Substrate Selection
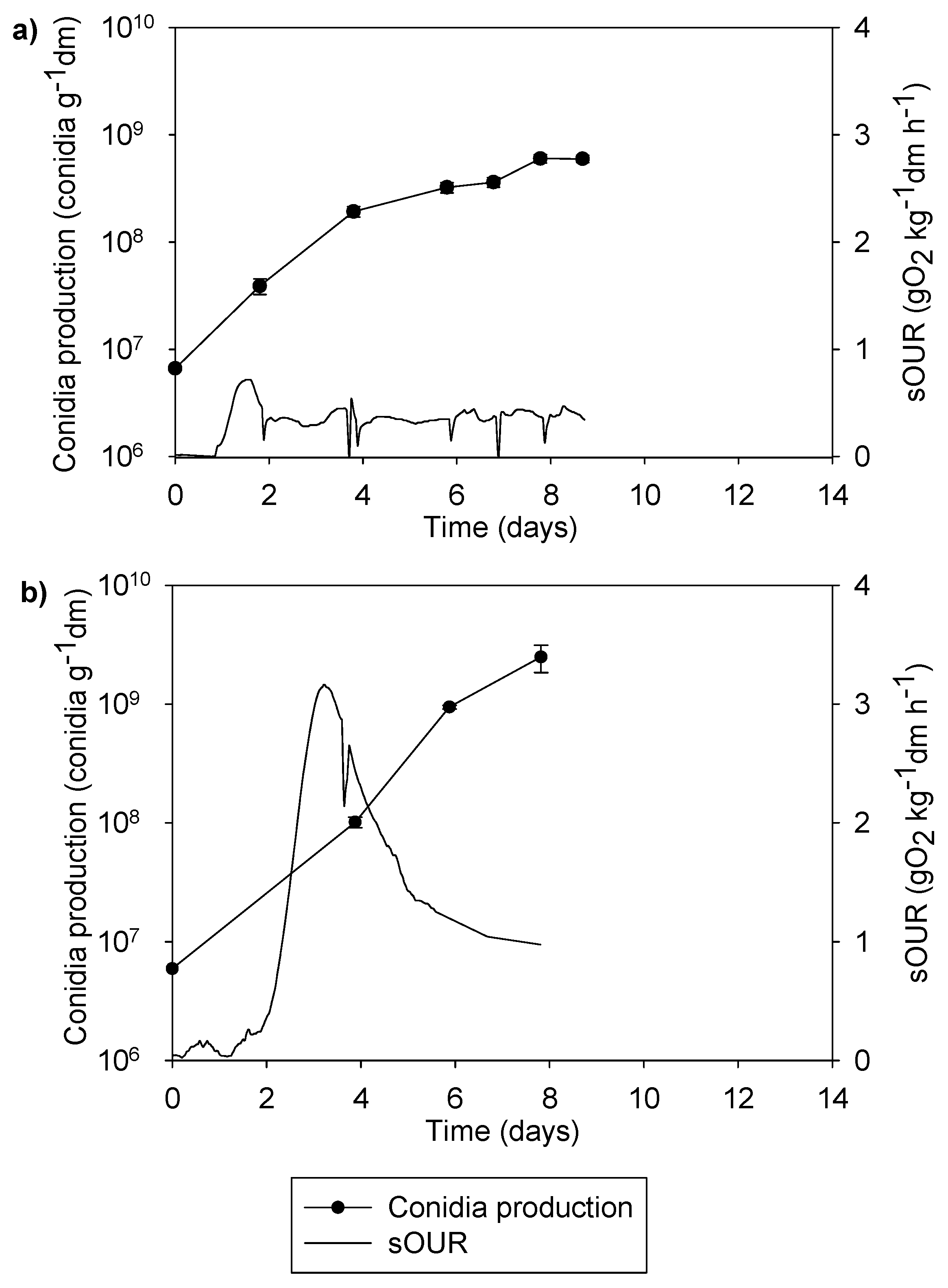
3.2. Optimization in 0.5 L Reactors
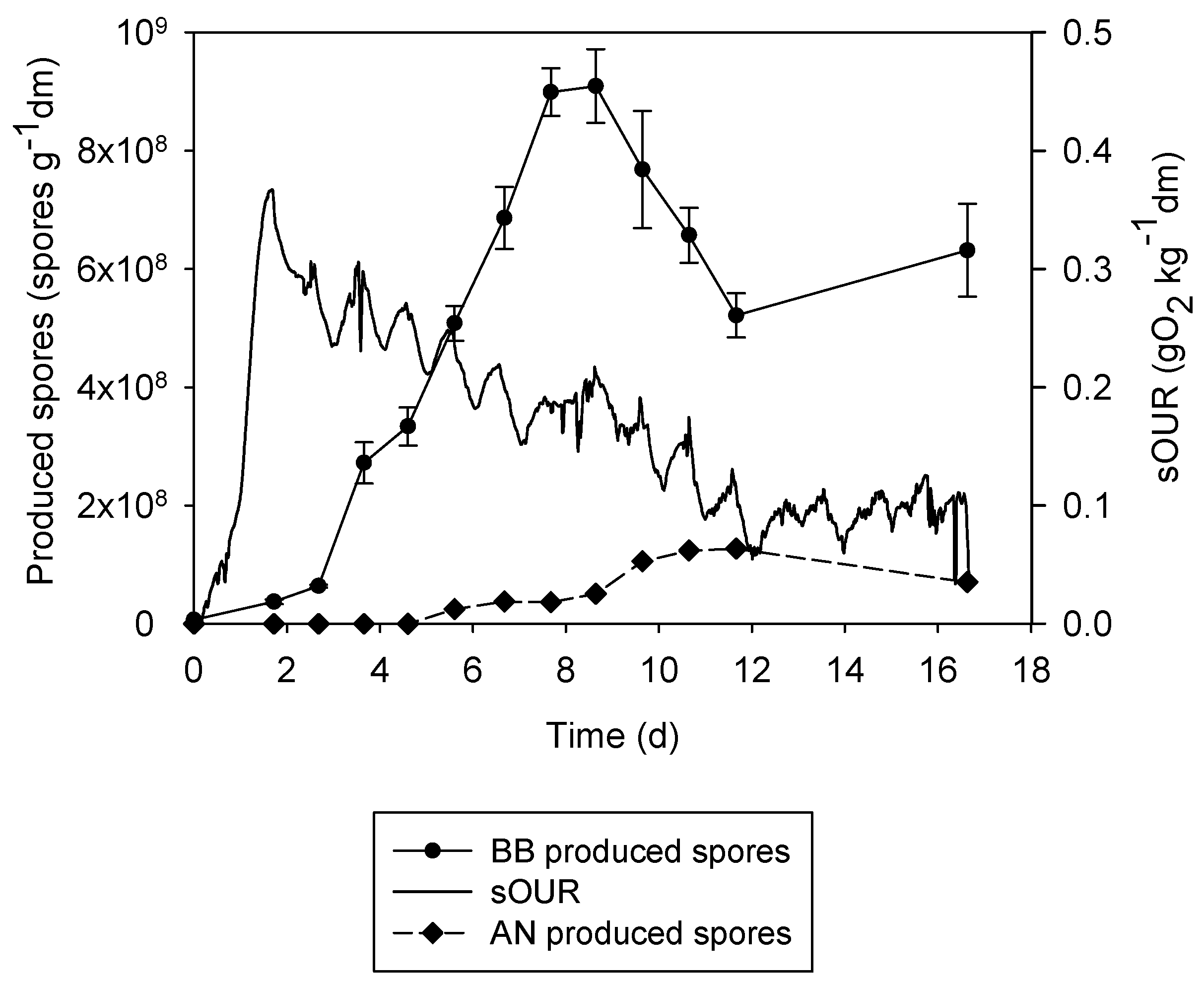
3.3. The 22 L Experiments
3.4. Tray Bioreactor Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Olde, E.M.; Ripoll-Bosch, R.; Van Zanten, H.H.E.; Metze, T.A.P.; Termeer, C.J.A.M.; van Ittersum, M.K.; de Boer, I.J.M. Principles, drivers and opportunities of a circular bioeconomy. Nat. Food 2021, 2, 561–566. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals (SDGs). Available online: https://sdgs.un.org/goals (accessed on 9 June 2024).
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Mingzhen, L.; Zhou, C.; Wang, C.; Jackson, R.B.; Kempes, C.P. Worldwide scaling of waste generation in urban systems. Nat. Cities 2024, 1, 126–135. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.; Gnana Soundari, P.; Chen, H.; Kumar Awasthi, S.; Varjani, S.; Pandey, A.; Zhang, Z.; Kumar Awasthi, M. Chapter 7—Waste Biorefinery Development Toward Circular Bioeconomy with a Focus on Life-Cycle Assessment. In Biomass, Biofuels, Biochemicals, 1st ed.; Pandey, A., Dayal Tyagi, R., Varjani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 199–230. [Google Scholar]
- Molina-Peñate, E.; Artola, A.; Sánchez, A. Organic municipal waste as feedstock for biorefineries: Bioconversion technologies integration and challenges. Rev. Environ. Sci. Biotechnol. 2022, 21, 247–267. [Google Scholar] [CrossRef]
- Thapa, P.; Hasnine, M.T.; Zoungrana, A.; Thakur, S.; Yuan, Q. Food waste treatments and the impact of composting on carbon footprint in Canada. Fermentation 2022, 8, 566. [Google Scholar] [CrossRef]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-State Fermentation from Organic Wastes: A New Generation of Bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Porras, R.C.S.; Artola, A.; Barrena, R.; Ghoreishi, G.; Matos, C.B.; Sánchez, A. Breaking new ground: Exploring the promising role of Solid-State Fermentation in harnessing natural biostimulants for sustainable agriculture. Processes 2023, 11, 2300. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Artola, A.; Sánchez, A. Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Crit. Rev. Environ. Sci. Technol. 2019, 49, 655–694. [Google Scholar] [CrossRef]
- Kurpnieks, O.; Senkovs, M.; Rimkus, A. Challenges of SSF Process for Pea and Wheat Bran Valorization Using Trichoderma spp. for Biocontrol Agent Production. In Proceedings of the Environment, Technologies, Resources, Proceedings of the International Scientific and Practical Conference, Rezekne, Latvia, 15–16 June 2023; pp. 93–100. [Google Scholar] [CrossRef]
- Pallín, M.Á.; Gonzalez-Rodriguez, S.; Eibes, G.; Lopez-Abelairas, M.; Moreira, M.T.; Lema, J.M.; Lu-Chau, T.A. Towards industrial application of fungal pretreatment in 2G biorefinery: Scale-up of solid-state fermentation of wheat straw. Biomass Conv. Bioref. 2024, 14, 593–605. [Google Scholar] [CrossRef]
- Stenmarck, A.; Jensen, C.; Quested, T.; Moates, G. Fusions: Estimates of European Food Waste Levels; European Comission (EU): Brussels, Belgium, 2016.
- Russ, W.; Schnappinger, M. Waste related to the food industry: A challenge in material loops. In Utilization of by-Products and Treatment of Waste in the Food Industry; Springer: Boston, MA, USA, 2007; pp. 1–13. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2011; Available online: https://www.madr.ro/docs/ind-alimentara/risipa_alimentara/presentation_food_waste.pdf (accessed on 6 September 2024).
- Bräutigam, K.R.; Jörissen, J.; Priefer, C. The extent of food waste generation across EU-27: Different calculation methods and the reliability of their results. Waste Manag. Res. 2014, 32, 683–694. [Google Scholar] [CrossRef]
- De la Cruz Quiroz, R.; Roussos, S.; Hernández, D.; Rodríguez, R.; Castillo, F.; Aguilar, C.N. Challenges and opportunities of the bio-pesticides production by solid-state fermentation: Filamentous fungi as a model. Crit. Rev. Biotechnol. 2014, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Barrena, R.; Sánchez, A.; Artola, A. Fungal biopesticide production: Process scale-up and sequential batch mode operation with Trichoderma harzianum using agro-industrial solid wastes of different biodegradability. Chem. Eng. J. 2021, 425, 131620. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.A.; Agosin, E.; Cotoras, M.; Martin, R.S.; Volpe, D. Comparison of aerial and submerged spore properties for Trichoderma harzianum. FEMS Microbiol. Lett. 1995, 125, 63–69. [Google Scholar] [CrossRef]
- Faria, M.R.D.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A compre-hensive list with worldwide coverage and international classification of formulation types. Biol. Contr. 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Muñiz-Paredes, F.; Miranda-Hernández, F.; Loera, O. Production of conidia by entomopathogenic fungi: From inoculants to final quality tests. World J. Microb. Biotechnol. 2017, 33, 1–9. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, J.J.; Kim, K. Optimization of solid-state fermentation for improved conidia production of Beauveria bassiana as a mycoinsecticide. Mycobiology 2010, 38, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Artola, A.; Sánchez, A.; Barrena, R. Rice husk as a source for fungal biopesticide production by solid-state fermentation using B. bassiana and T. harzianum. Bioresour. Technol. 2020, 296, 122322. [Google Scholar] [CrossRef]
- Puyuelo, B.; Gea, T.; Sánchez, A. A new control strategy for the composting process based on the oxygen uptake rate. Chem. Eng. J. 2010, 165, 161–169. [Google Scholar] [CrossRef]
- Santa, H.S.D.; Santa, O.R.D.; Brand, D.; Vandenberghe, L.P.D.S.; Soccol, C.R. Spore production of Beauveria bassiana from agro-industrial residues. Braz. Arch. Biol. Technol. 2005, 48, 51–60. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Malik, A. Suitability of agricultural by-products as production medium for spore production by Beauveria bassiana HQ917687. Int. J. Recycl. Org. Waste Agricult. 2016, 5, 179–184. [Google Scholar] [CrossRef]
- Scott, T.A., Jr.; Melvin, E.H. Determination of dextran with anthrone. Anal. Chem. 1953, 25, 1656–1661. [Google Scholar] [CrossRef]
- US Composting Council. Test Methods for the Examination of Composting and Compost; Edaphos International: Houston, TX, USA, 2001. [Google Scholar]
- Richard, T.L.; Veeken, A.H.; De Wilde, V.; Hamelers, H.V.M. Air-filled porosity and permeability relationships during solid-state fermentation. Biotechnol. Progr. 2004, 20, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Gaona, O.; Saucedo-Castañeda, G.; Alatorre-Rosas, R.; Loera, O. Effect of moisture content and inoculum on the growth and conidia production by Beauveria bassiana on wheat bran. Braz. Arch. Biol. Technol. 2010, 53, 771–777. [Google Scholar] [CrossRef]
- Fan, S.; Li, S.; Fan, A.; ter Heijne, A.; Buisman, C.J.; Chen, W.S. Heat potential, generation, recovery and utilization from composting: A review. Resour. Conserv. Recy. 2021, 175, 105850. [Google Scholar] [CrossRef]
- Barrena, R.; Gea, T.; Ponsá, S.; Ruggieri, L.; Artola, A.; Font, X.; Sánchez, A. Categorizing raw organic material biodegradability via respiration activity measurement: A review. Compost. Sci. Util. 2011, 19, 105–113. [Google Scholar] [CrossRef]
- Molina-Peñate, E.; del Carmen Vargas-García, M.; Artola, A.; Sánchez, A. Filling in the gaps in biowaste biorefineries: The use of the solid residue after enzymatic hydrolysis for the production of biopesticides through solid-state fermentation. Waste Manag. 2023, 161, 92–103. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W. Development and temperature gradient online monitoring of a vehicular rotary solid-state bioreactor: A novel device for large-scale preparation of Aspergillus niger spore inoculum. J. Chem. Technol. Biotechnol. 2019, 94, 3883–3894. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and engineering aspects on the scale-up of bioreactors for different bioprocesses. Syst. Microbiol. Bioman. 2024, 4, 365–385. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Ruiz, H.A.; Krieger, N. A critical evaluation of recent studies on packed-bed bioreactors for solid-state fermentation. Processes 2023, 11, 872. [Google Scholar] [CrossRef]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Dallastra, E.D.G.; Dias, A.C.P.; de Morais, P.B.; da Silva, J.F.M.; Casciatori, F.P.; Grajales, L.M. Development of a novel pilot-scale tray bioreactor for solid-state fermentation aiming at process intensification. Chem. Eng. Process. 2023, 193, 109526. [Google Scholar] [CrossRef]
- da Cunha, L.P.; Casciatori, F.P.; de Cenço Lopes, I.; Thoméo, J.C. Production of conidia of the entomopathogenic fungus Metarhizium anisopliae ICB 425 in a tray bioreactor. Biopr. Biosyst. Eng. 2019, 42, 1757–1768. [Google Scholar] [CrossRef]
- Fredlund, E.; Thim, A.M.; Gidlund, A.; Brostedt, S.; Nyberg, M.; Olsen, M. Moulds and mycotoxins in rice from the Swedish retail market. Food Addit. Contam. 2009, 26, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Aksu, H.; Gunsen, U. Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess 2011, 178, 271–280. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Nicolau, A.; Aprodu, I.; Puel, O.; Oswald, I.P. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Curiel, J.A.; Poutanen, K.; Katina, K. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innov. Food Sci. Emerg. 2014, 25, 19–27. [Google Scholar] [CrossRef]
- Nandakumar, R.; Bollich, P.A.; Shahjahan, A.K.M.; Groth, D.E.; Rush, M.C. Evidence for the soilborne nature of the rice sheath rot and panicle blight pathogen, Burkholderia gladioli. Can. J. Plant Pathol. 2008, 30, 148–154. [Google Scholar] [CrossRef]
- Stoyanova, M.; Pavlina, I.; Moncheva, P.; Bogatzevska, N. Biodiversity and incidence of Burkholderia species. Biotechnol. Biotech. Eq. 2007, 21, 306–310. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.A. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Barrera, M.C.; Gómez, M.I.; Serrato Bermúdez, J.C. Towards the production of fungal biocontrol candidates using inert supports: A case of study of Trichoderma asperellum in a pilot fixed bed fermenter. Biocontrol Sci. Technol. 2019, 29, 162–184. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; Weber, M.Z.; Fuchs, G.A.; Scholz, L.A.; de Lima Luz, L.F., Jr.; Krieger, N.; Mitchell, D.A.; Matos Jorge, L.M. Estimation of heat and mass transfer coefficients in a pilot packed-bed solid-state fermentation bioreactor. Chem. Eng. J. 2021, 408, 127246. [Google Scholar] [CrossRef]


| Parameter/ Substrate | MC (%) | OM (%) | C/N Ratio | pH | AFP (%) | TSC (mg g−1 dm) | Mixture (w:w) (%:%) (RH: Other) |
|---|---|---|---|---|---|---|---|
| RH | 62.2 ± 0.4 | 83.5 ± 0.5 | 95.3 ± 13 | 5.6 ± 0.2 | 85.7 ± 2.2 | 17.9 ± 0.2 | 100:0 |
| AP | 73.7 ± 0.5 | 89.3 ± 0.7 | 86.6 ± 4.1 | 5.1 ± 0.1 | 60.2 ± 1.8 | 163 ± 7.1 | 20:80 |
| SF | 74.2 ± 2.5 | 92.2 ± 1.5 | 12.2 ± 0.4 | 6.6 ± 0.2 | 45.4 ± 1.2 | 22.1 ± 0.4 | 5:95 |
| BDr | 79.9 ± 3.4 | 95.0 ± 0.5 | 10.6 ± 2.5 | 5.5 ± 0.3 | 65.2 ± 1.3 | 120 ± 4.4 | 5:95 |
| OP | 84.2 ± 0.8 | 94.6 ± 1.9 | 41.1 ± 2.7 | 4.6 ± 0.4 | 59.8 ± 1.2 | 242 ± 9.5 | 0:100 |
| PP | 91.1 ± 0.7 | 81.6 ± 1.2 | 23.8 ± 0.9 | 5.4 ± 0.3 | 59.4 ± 0.7 | 40.8 ± 2.4 | 0:100 |
| DoE1 | DoE2 | |||||
|---|---|---|---|---|---|---|
| Run/ Parameter | MC (%) | IC (Conidia g−1 dm) | AF (mL min−1) | T (°C) | C/N | MC (%) |
| 1 | 45 | 1 × 106 | 40 | 25 | 25 | 60 |
| 2 | 65 | 1 × 106 | 40 | 39 | 25 | 60 |
| 3 | 45 | 1 × 107 | 40 | 25 | 55 | 60 |
| 4 | 65 | 1 × 107 | 40 | 39 | 55 | 60 |
| 5 | 45 | 5.5 × 106 | 20 | 25 | 40 | 50 |
| 6 | 65 | 5.5 × 106 | 20 | 39 | 40 | 50 |
| 7 | 45 | 5.5 × 106 | 60 | 25 | 40 | 70 |
| 8 | 65 | 5.5 × 106 | 60 | 39 | 40 | 70 |
| 9 | 55 | 1 × 106 | 20 | 32 | 25 | 50 |
| 10 | 55 | 1 × 107 | 20 | 32 | 55 | 50 |
| 11 | 55 | 1 × 106 | 60 | 32 | 25 | 70 |
| 12 | 55 | 1 × 107 | 60 | 32 | 55 | 70 |
| 13 | 55 | 5.5 × 106 | 40 | 32 | 40 | 60 |
| 14 | 55 | 5.5 × 106 | 40 | 32 | 40 | 60 |
| 15 | 55 | 5.5 × 106 | 40 | 32 | 40 | 60 |
| Parameter/Bioreactor | Tray Bioreactor | 22 L Bioreactor |
|---|---|---|
| Mean produced conidia (conidia g−1 dm) | 1.2 × 109 | 2.5 × 109 |
| Time (d) | 8.8 | 7.8 |
| Grams dry matter (g dm) | 540 | 1400 |
| Total volume (L) | 43.5 | 22 |
| Productivity (conidia g−1 dm d−1) | 1.4 × 108 | 3.2 × 108 |
| Total produced conidia | 6.5 × 1011 | 3.5 × 1012 |
| Total produced conidia L−1 | 1.5 × 1010 | 1.6 × 1011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, A.; Artola, A.; Barrena, R.; Sánchez, A. Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria bassiana Conidia Production. Fermentation 2024, 10, 481. https://doi.org/10.3390/fermentation10090481
Sala A, Artola A, Barrena R, Sánchez A. Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria bassiana Conidia Production. Fermentation. 2024; 10(9):481. https://doi.org/10.3390/fermentation10090481
Chicago/Turabian StyleSala, Arnau, Adriana Artola, Raquel Barrena, and Antoni Sánchez. 2024. "Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria bassiana Conidia Production" Fermentation 10, no. 9: 481. https://doi.org/10.3390/fermentation10090481
APA StyleSala, A., Artola, A., Barrena, R., & Sánchez, A. (2024). Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria bassiana Conidia Production. Fermentation, 10(9), 481. https://doi.org/10.3390/fermentation10090481








