Modifications of Constitutive Promoter to Large-Scale Synthesize Porcine Myoglobin in Komagataella phaffii
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Strains, Plasmids, and Culture Conditions
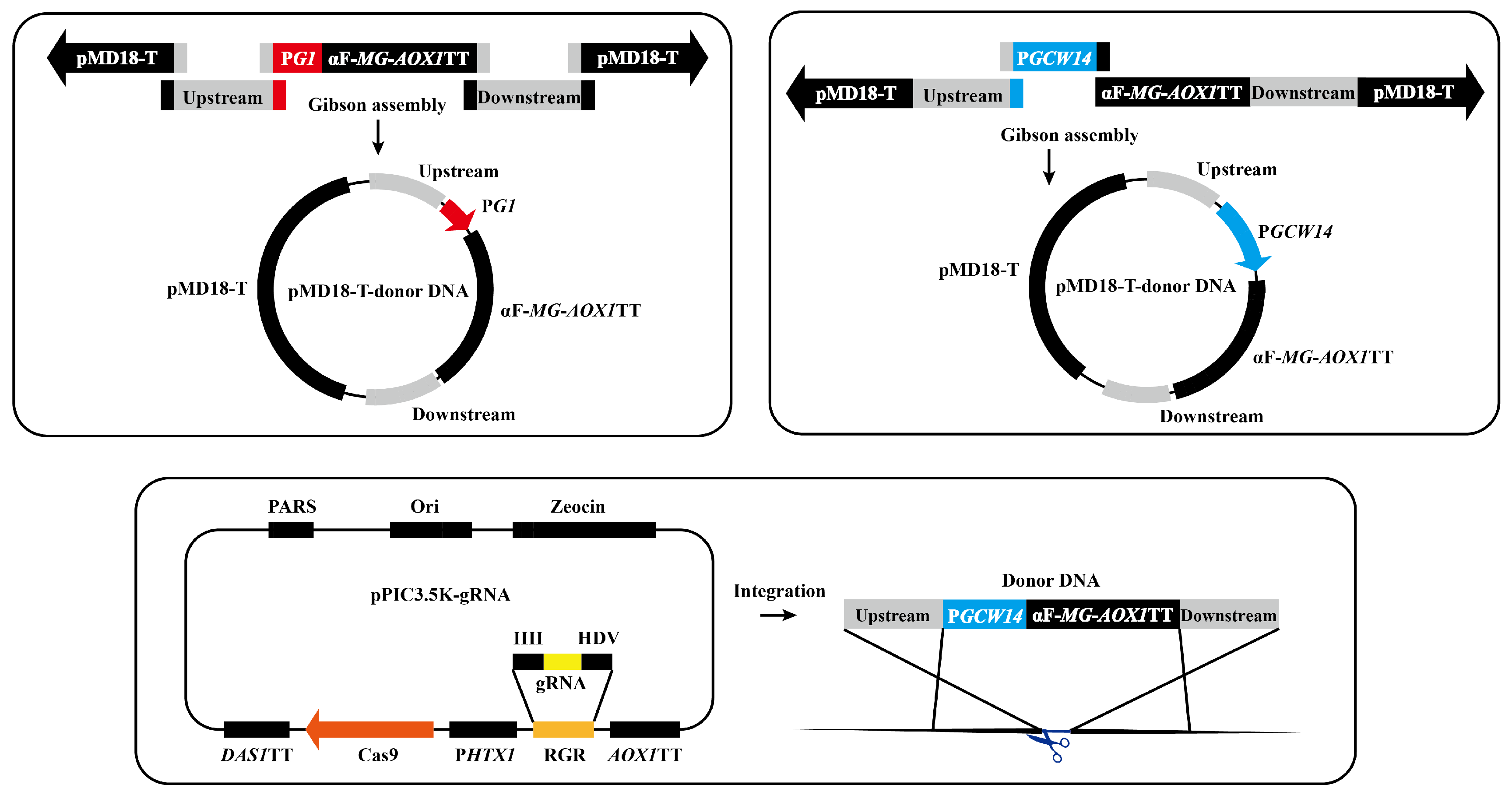
2.2. Optimization of Promoter and Copy Number for pMG Expression
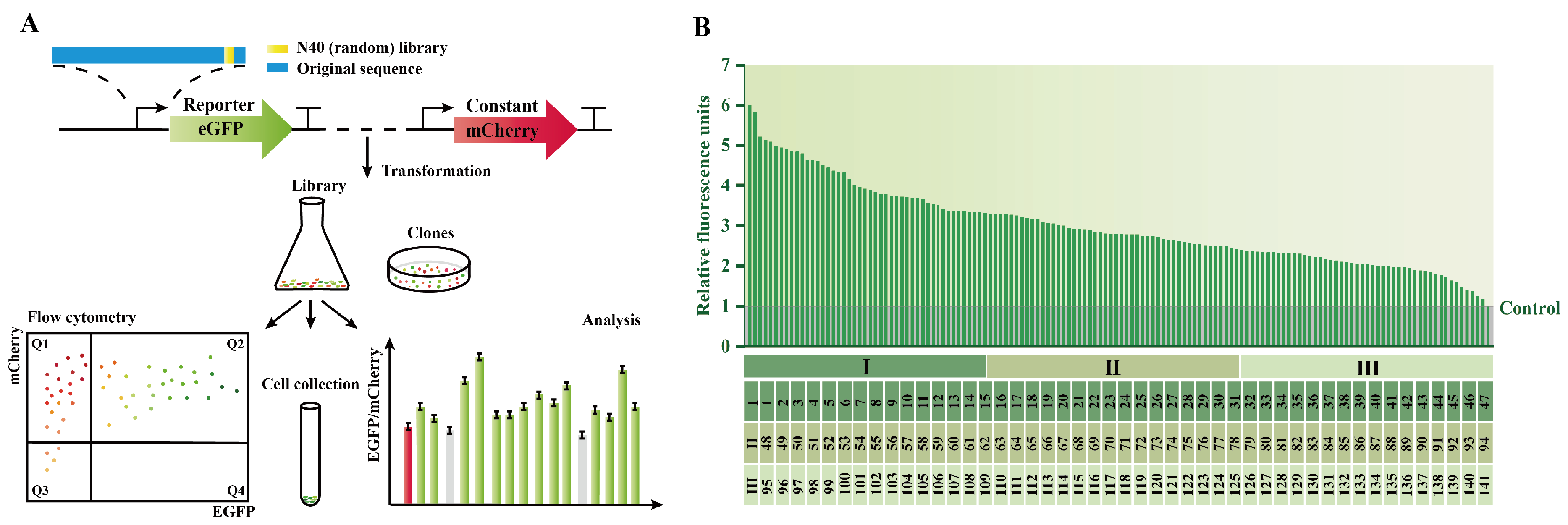
2.3. Construction of PGCW14 Mutant Library
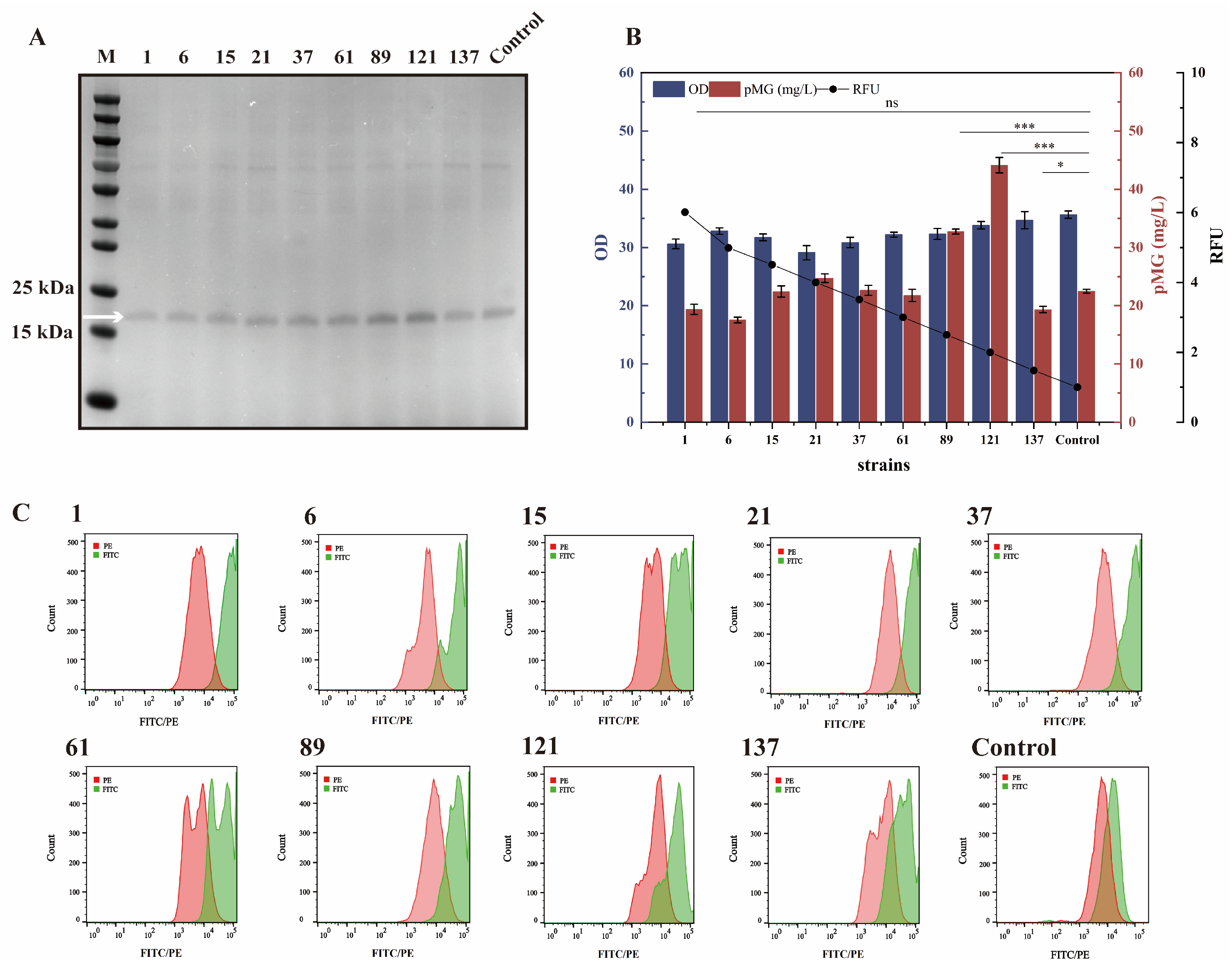
2.4. Screening of the PGCW14 Mutant with Enhanced Strength
2.5. The Enhancement of pMG Expression by the Inhibition of Protein Degradation
2.6. The Enhancement of pMG Expression by the Co-Expression of Proteins of Anti-Stress Response Systems
2.7. Cultural Conditions for pMG Expression at Shaking-Flask Level
2.8. Conditions for pMG Expression at Fermenter Level
2.9. Purification of Food-Grade pMG
2.10. The Peroxidase-Specific Activity of Synthesized pMG
3. Results and Discussion
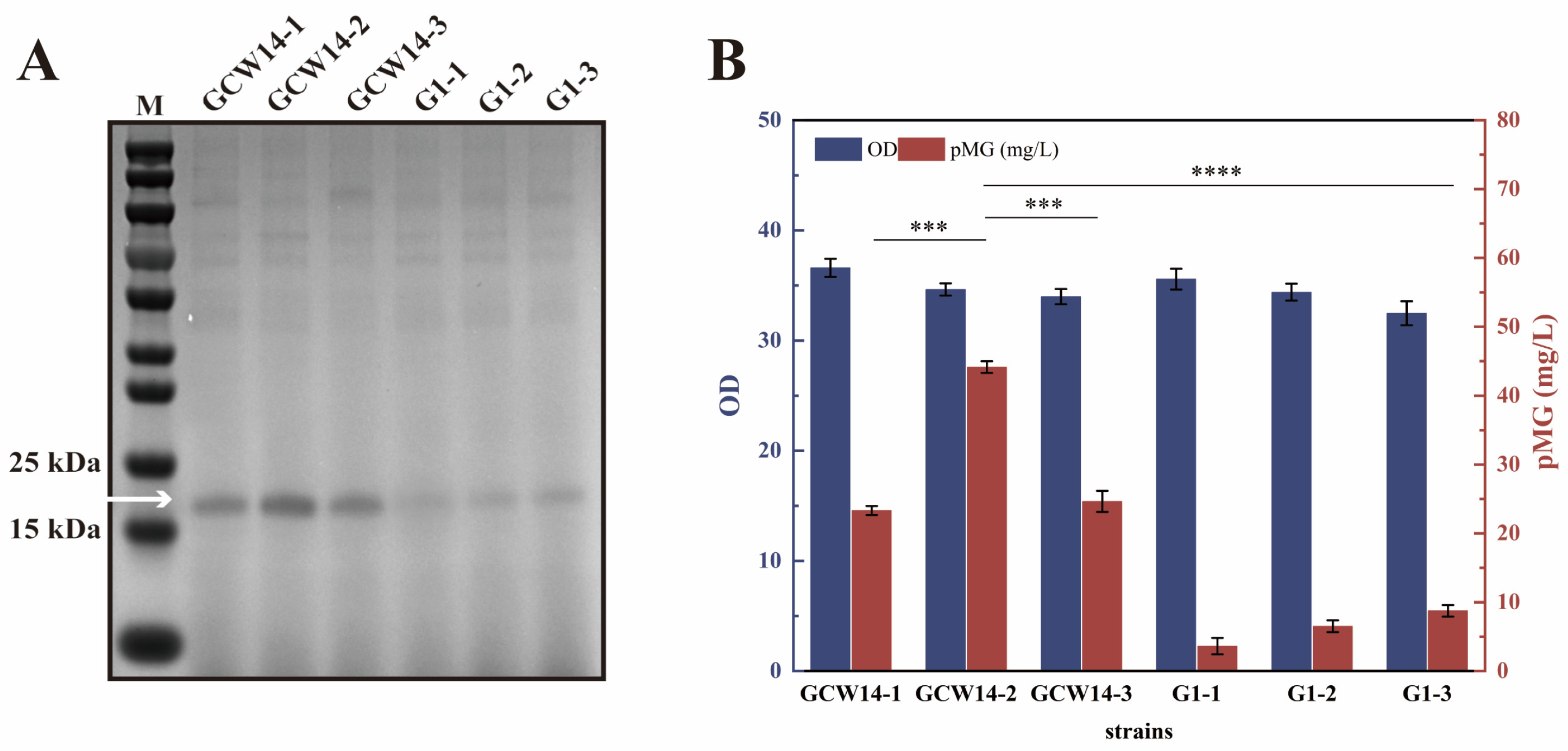
3.1. Selection of Proper Promoter and Integrated Copy Number for pMG Expression
3.2. The Construction of PGCW14 Mutant Libraries to Enhance Its Strength
3.3. The Application of PGCW14 Mutants to Increase pMG Expression
3.4. The Construction of a Proper K. phaffii Host for the Efficient pMG Expression
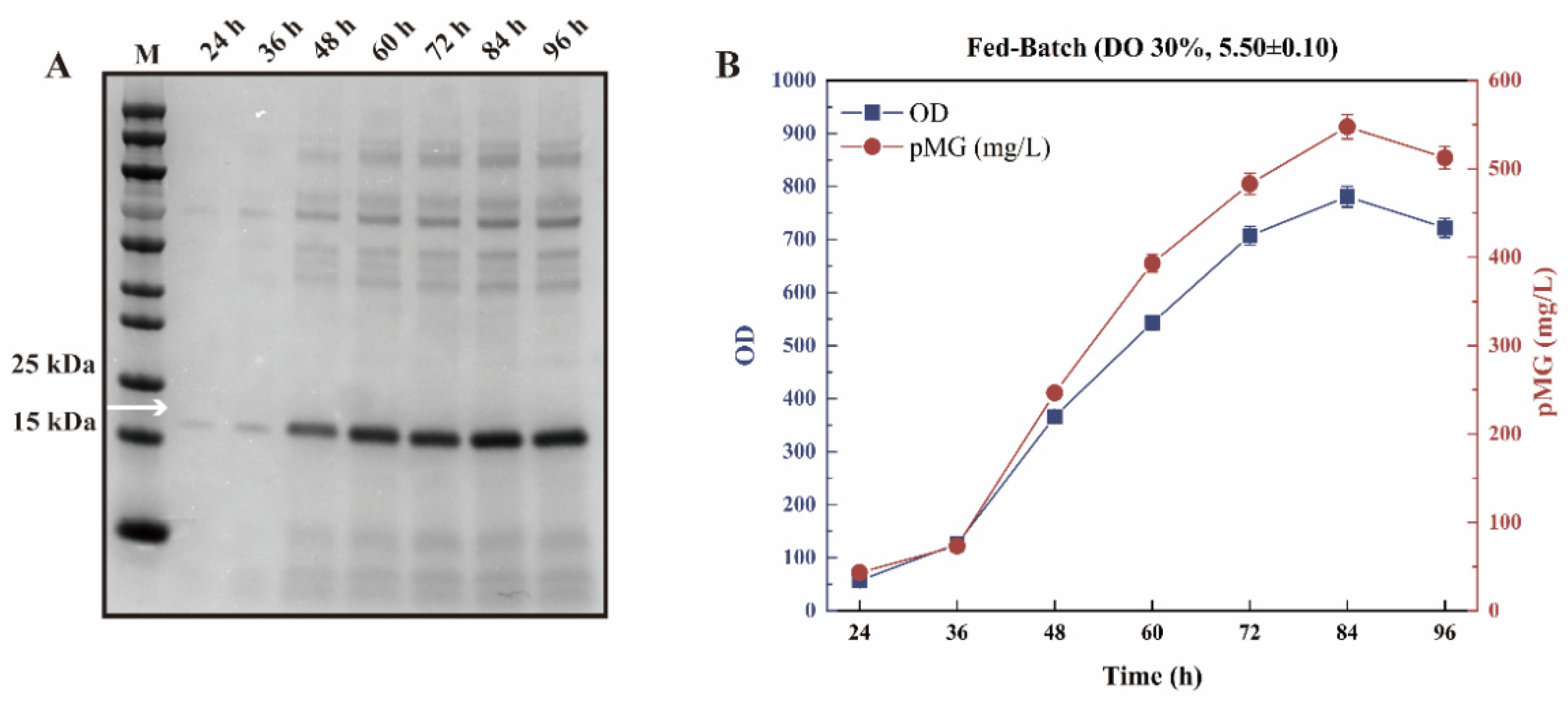
3.5. The Biochemical Properties of pMG Efficiently Expressed by Fed-Batch Fermentation Using an Economical Medium
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ordway, G.A.; Garry, D.J. Myoglobin: An essential hemoprotein in striated muscle. J. Exp. Biol. 2004, 207, 3441–3446. [Google Scholar] [CrossRef]
- Tan, B.; Sun, B.; Sun, N.; Li, C.; Zhang, J.; Yang, W. Structure, functional properties and iron bioavailability of Pneumatophorus japonicus myoglobin and its glycosylation products. Int. J. Biol. Macromol. 2021, 173, 524–531. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, P.; Zhang, J.; Huang, T.; Li, C.; Yang, W. Preparation, characterization and bioavailability studies of Tegillarca granosa hemoglobin and its glycosylated products. Int. J. Biol. Macromol. 2022, 219, 11–20. [Google Scholar] [CrossRef]
- Chen, J.; Ran, F.; Chen, Q.; Luo, D.; Ma, W.; Han, T.; Wang, C.; Wang, C. A fluorescent biosensor for cardiac biomarker myoglobin detection based on carbon dots and deoxyribonuclease I-aided target recycling signal amplification. RSC Adv. 2019, 9, 4463–4468. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Wang, Z.; Wang, H.; Zhou, J.; Du, G.; Chen, J.; Li, J. Efficient secretory expression and purification of food-grade porcine myoglobin in Komagataella phaffii. J. Agric. Food Chem. 2021, 69, 10235–10245. [Google Scholar] [CrossRef]
- Liu, Q.; Long, Y.; Zhang, Y.F.; Zhang, Z.Y.; Yang, B.; Chen, C.Y.; Huang, L.S.; Su, Y. Phenotypic and genetic correlations of pork myoglobin content with meat colour and other traits in an eight breed-crossed heterogeneous population. Animal 2021, 15, 100364. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yong, H.; Kim, M.; Choi, Y.; Jo, C. Status of meat alternatives and their potential role in the future meat market—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ushio, H.; Ochiai, Y. Expression and characterization of rainbow trout Oncorhynchus mykiss recombinant myoglobin. Fish Physiol. Biochem. 2021, 47, 1477–1488. [Google Scholar] [CrossRef]
- Meng, Y.; Xie, L.; You, K.; Chen, W. High-level secretory production of leghemoglobin and myoglobin in Escherichia coli through inserting signal peptides. Food Biosci. 2023, 56, 103356. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, J.; Xu, J.; Li, P.; Sheng, L.; Qian, Y.; Ji, J.; Han, X.; Zhao, X.; Zhou, J.; et al. Allergenic risk assessment of porcine myoglobin expressed by engineered Komagataella phaffii. Fundam. Res. 2024, 4, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, X.; Zhou, J.; Lu, W.; Li, J.; Chen, J.; Du, G. Biosynthesis of high-active hemoproteins by the efficient heme-supply Pichia Pastoris chassis. Adv. Sci. 2023, 10, 2302826. [Google Scholar] [CrossRef] [PubMed]
- Unver, Y.; Ari, B.; Acar, M.; Arslan, S.Y. A self-inducible heterologous protein expression system in Komagataella phaffii (Pichia pastoris). 3 Biotech 2024, 14, 193. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liang, S.; Ye, Y.; Lin, Y. Key regulatory elements of a strong constitutive promoter, PGCW14, from Pichia pastoris. Biotechnol. Lett. 2013, 35, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zou, C.; Lin, Y.; Zhang, X.; Ye, Y. Identification and characterization of PGCW14: A novel, strong constitutive promoter of Pichia pastoris. Biotechnol. Lett. 2013, 35, 1865–1871. [Google Scholar] [CrossRef]
- Shen, Q.; Cui, J.; Wang, Y.; Hu, Z.; Xue, Y.; Zheng, Y. Identification of a novel growth-associated promoter for biphasic expression of heterogenous proteins in Pichia pastoris. Appl. Ind. Microbiol. 2024, 90, e0174023. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dong, H.; Zhang, Z.; Wu, X.; Bao, T.; Gao, L.; Chen, X. Enhancing the heterologous expression of a thermophilic endoglucanase and its cost-Effective production in Pichia pastoris using multiple strategies. Int. J. Mol. Sci. 2023, 24, 15017. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, X.; Song, L.; Liu, H.; Zhou, X.; Wang, Q.; Zhang, Y.; Cai, M. CRISPR–Cas9-mediated genomic multiloci integration in Pichia pastoris. Microb. Cell Fact. 2019, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Schusterbauer, V.; Fischer, J.E.; Gangl, S.; Schenzle, L.; Rinnofner, C.; Geier, M.; Sailer, C.; Glieder, A.; Thallinger, G.G. Whole genome sequencing analysis of effects of CRISPR/Cas9 in Komagataella phaffii: A budding yeast in distress. J. Fungi 2022, 8, 992. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, Z.; Zhou, X.; Zhang, S.; Zou, S.; Xiong, N.; Xue, Y.P.; Liu, Z.Q.; Zheng, Y. Identification of a novel promoter for driving antibiotic-resistant genes to reduce the metabolic burden during protein expression and effectively select multiple integrations in Pichia pastoris. Appl. Microbiol. Biotechnol. 2021, 105, 3211–3223. [Google Scholar] [CrossRef]
- de Boer, C.G.; Vaishnav, E.D.; Sadeh, R.; Abeyta, E.L.; Friedman, N.; Regev, A. Deciphering eukaryotic gene-regulatory logic with 100 million random promoters. Nat. Biotechnol. 2019, 38, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lubliner, S.; Regev, I.; Lotan-Pompan, M.; Edelheit, S.; Weinberger, A.; Segal, E. Core promoter sequence in yeast is a major determinant of expression level. Genome Res. 2015, 25, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Chen, M.; Yang, M.; Li, L.; Mou, H. Enhancing the expression of recombinant κ-carrageenase in Pichia pastoris using dual promoters, co-expressing chaperones and transcription factors. Biocatal. Biotransform. 2019, 38, 104–113. [Google Scholar] [CrossRef]
- Huang, M.; Gao, Y.; Zhou, X.; Zhang, Y.; Cai, M. Regulating unfolded protein response activator HAC1p for production of thermostable raw-starch hydrolyzing α-amylase in Pichia pastoris. Bioprocess Biosyst. Eng. 2016, 40, 341–350. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour. Technol. 2022, 363, 127884. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Schmelzer, B.; Wilkinson, S.; Mattanovich, D. From natural to synthetic: Promoter engineering in yeast expression systems. Biotechnol. Adv. 2024, 77, 108446. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qian, J.; Yao, G.; Zhuang, Y.; Zhang, S.; Chu, J. GAP promoter library for fine-tuning of gene expression in Pichia pastoris. Appl. Environ. Microbiol. 2011, 77, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yu, W.; Zhai, X.; Yao, L.; Guo, X.; Gao, J.; Zhou, Y.J. Characterizing and engineering promoters for metabolic engineering of Ogataea polymorpha. Synth. Syst. Biotechnol. 2022, 7, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, Y.; Yu, X. Efficient CRISPR-mediated C-to-T base editing in Komagataella phaffii. Biotechnol. J. 2024, 19, 2400115. [Google Scholar] [CrossRef]
- Ghosh, D.; Raghavan, S.C. Nonhomologous end joining: New accessory factors fine tune the machinery. Trends Genet. 2021, 37, 582–599. [Google Scholar] [CrossRef]
- Weninger, A.; Fischer, J.E.; Raschmanová, H.; Kniely, C.; Vogl, T.; Glieder, A. Expanding the CRISPR/Cas9 toolkit for Pichia pastoris with efficient donor integration and alternative resistance markers. J. Cell. Biochem. 2017, 119, 3183–3198. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Miao, L.; Huang, H.; Li, Y.; Zhu, T. High-level production of glucose oxidase in Pichia pastoris: Effects of Hac1p overexpression on cell physiology and enzyme expression. Enzyme Microb. Technol. 2020, 141, 109671. [Google Scholar] [CrossRef]
- Tang, H.; Wu, Y.; Deng, J.; Chen, N.; Zheng, Z.; Wei, Y.; Luo, X.; Keasling, J. Promoter architecture and promoter engineering in Saccharomyces cerevisiae. Metabolites 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Burgard, J.; Grünwald-Gruber, C.; Altmann, F.; Zanghellini, J.; Valli, M.; Mattanovich, D.; Gasser, B. The secretome of Pichia pastoris in fed-batch cultivations is largely independent of the carbon source but changes quantitatively over cultivation time. Microb. Biotechnol. 2019, 13, 479–494. [Google Scholar] [CrossRef]
- Marsalek, L.; Gruber, C.; Altmann, F.; Aleschko, M.; Mattanovich, D.; Gasser, B.; Puxbaum, V. Disruption of genes involved in CORVET complex leads to enhanced secretion of heterologous carboxylesterase only in protease deficient Pichia pastoris. Biotechnol. J. 2017, 12, 1600584. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.; Chen, Y.; Wang, G.; Li, X.; Fang, Z.; Huang, S.; Liu, Z.; Yan, Y.; Xu, L. Over-expression of active Candida rugosa lip1 in Pichia pastoris via high cell-density fermentation and its application to resolve racemic ibuprofen. Biocatal. Biotransform. 2016, 33, 260–269. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, Z.; Liu, X.; Gao, X.; Guo, S.; Yu, T. A novel CRISPR/Cas9 system with high genomic editing efficiency and recyclable auxotrophic selective marker for multiple-step metabolic rewriting in Pichia pastoris. Synth. Syst. Biotechnol. 2023, 8, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, R.; Gong, G.; Xu, L.; Hu, Y.; Xie, L. Medium optimization for high yield production of human serum albumin in Pichia pastoris and its efficient purification. Protein Expr. Purif. 2021, 181, 105831. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, Y.; Wang, J.; Ma, C.; Qin, J.; Feng, J.; Li, Y.; Wang, X.; Chen, K. Methylotrophy of Pichia pastoris: Current advances, applications, and future perspectives for methanol-based biomanufacturing. Acs Sustain Chem Eng. 2022, 10, 1741–1752. [Google Scholar] [CrossRef]
- Bolmanis, E.; Bogans, J.; Akopjana, I.; Suleiko, A.; Kazaka, T.; Kazaks, A. Production and purification of soy leghemoglobin from Pichia pastoris cultivated in different expression media. Processes 2023, 11, 3215. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, R.; Dou, S.; Wu, Z.m.; Abdulkhani, A.; Asadollahi, M.A.; Abomohra, A.E.-F.; Sun, F. Constitutive expression of codon optimized Trichoderma reesei TrCel5A in Pichia pastoris using GAP promoter. Syst. Microbiol. Biomanuf. 2022, 2, 498–506. [Google Scholar] [CrossRef]
- Yu, F.; Li, C.; Zhang, T.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Developing a novel heme biosensor to produce high-active hemoproteins in Pichia pastoris through comparative transcriptomics. Metab. Eng. 2024, 84, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wang, C.; Chen, S.; Lian, R.; Wang, W.; Fu, L.; Wang, Y. Immunological disturbance effect of exogenous histamine towards key immune cells. Food Sci. Hum. Wellness. 2024, 13, 1856–1863. [Google Scholar] [CrossRef]
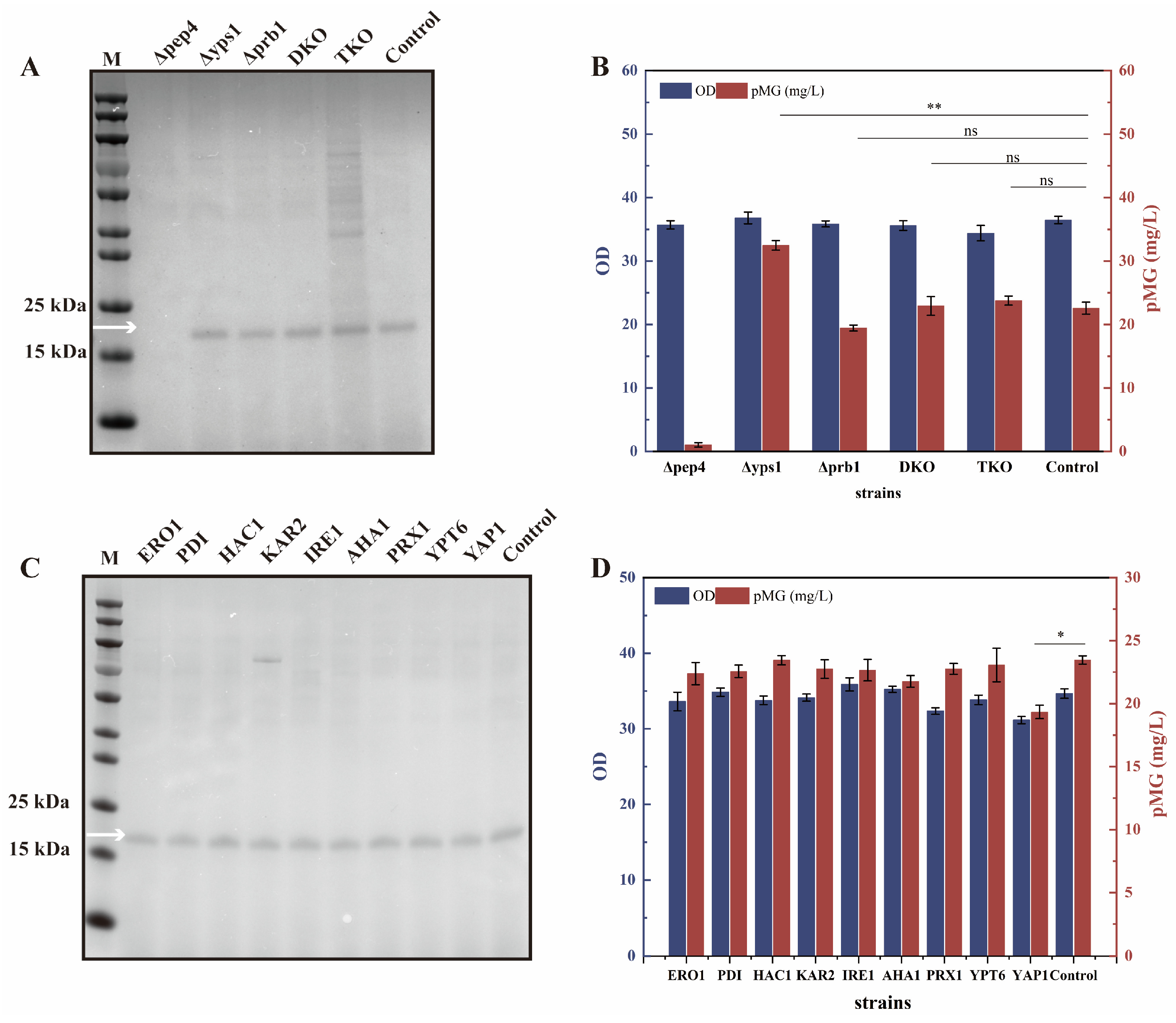


| Gene Name | Gene/Protein ID | Annotation |
|---|---|---|
| ERO1 | PAS_chr1_0011 | Endoplasmic reticulum oxidoreductase |
| PDI | PAS_chr1_0160 | Protein disulfide isomerase |
| HAC1 | PAS_chr1_0381 | Suppressor homologous to ATF/CREB1 |
| KAR2 | PAS_chr2_0140 | Immunoglobulin-binding protein |
| IRE1 | PAS_chr2_0202 | Endoplasmic reticulum stress transducer |
| AHA1 | PAS_chr3_0170 | Activator of Hsp90 ATPase |
| PRX1 | PAS_chr3_0906 | Thioredoxin-linked peroxidase |
| YPT6 | PAS_chr4_0165 | GTPase |
| YAP1 | PAS_chr4_0601 | Transcription factor response to oxidative stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Wang, Y.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Modifications of Constitutive Promoter to Large-Scale Synthesize Porcine Myoglobin in Komagataella phaffii. Fermentation 2025, 11, 49. https://doi.org/10.3390/fermentation11020049
Sun D, Wang Y, Zhou J, Li J, Chen J, Du G, Zhao X. Modifications of Constitutive Promoter to Large-Scale Synthesize Porcine Myoglobin in Komagataella phaffii. Fermentation. 2025; 11(2):49. https://doi.org/10.3390/fermentation11020049
Chicago/Turabian StyleSun, Danni, Yunpeng Wang, Jingwen Zhou, Jianghua Li, Jian Chen, Guocheng Du, and Xinrui Zhao. 2025. "Modifications of Constitutive Promoter to Large-Scale Synthesize Porcine Myoglobin in Komagataella phaffii" Fermentation 11, no. 2: 49. https://doi.org/10.3390/fermentation11020049
APA StyleSun, D., Wang, Y., Zhou, J., Li, J., Chen, J., Du, G., & Zhao, X. (2025). Modifications of Constitutive Promoter to Large-Scale Synthesize Porcine Myoglobin in Komagataella phaffii. Fermentation, 11(2), 49. https://doi.org/10.3390/fermentation11020049







