Pichia kudriavzevii and Saccharomyces cerevisiae Inoculation Strategies for Cider Elaboration from Acidic Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeasts and Growth Conditions
2.2. Antagonist Activity
2.3. Laboratory-Scale Fermentations in Sterile Apple Must
2.3.1. Fermentation Trial
2.3.2. Kinetic Parameters
2.3.3. Microbiological Counts
2.3.4. Sugar Utilization
2.4. Chemical Analysis of the Ciders
2.5. Statistical Analyses
3. Results
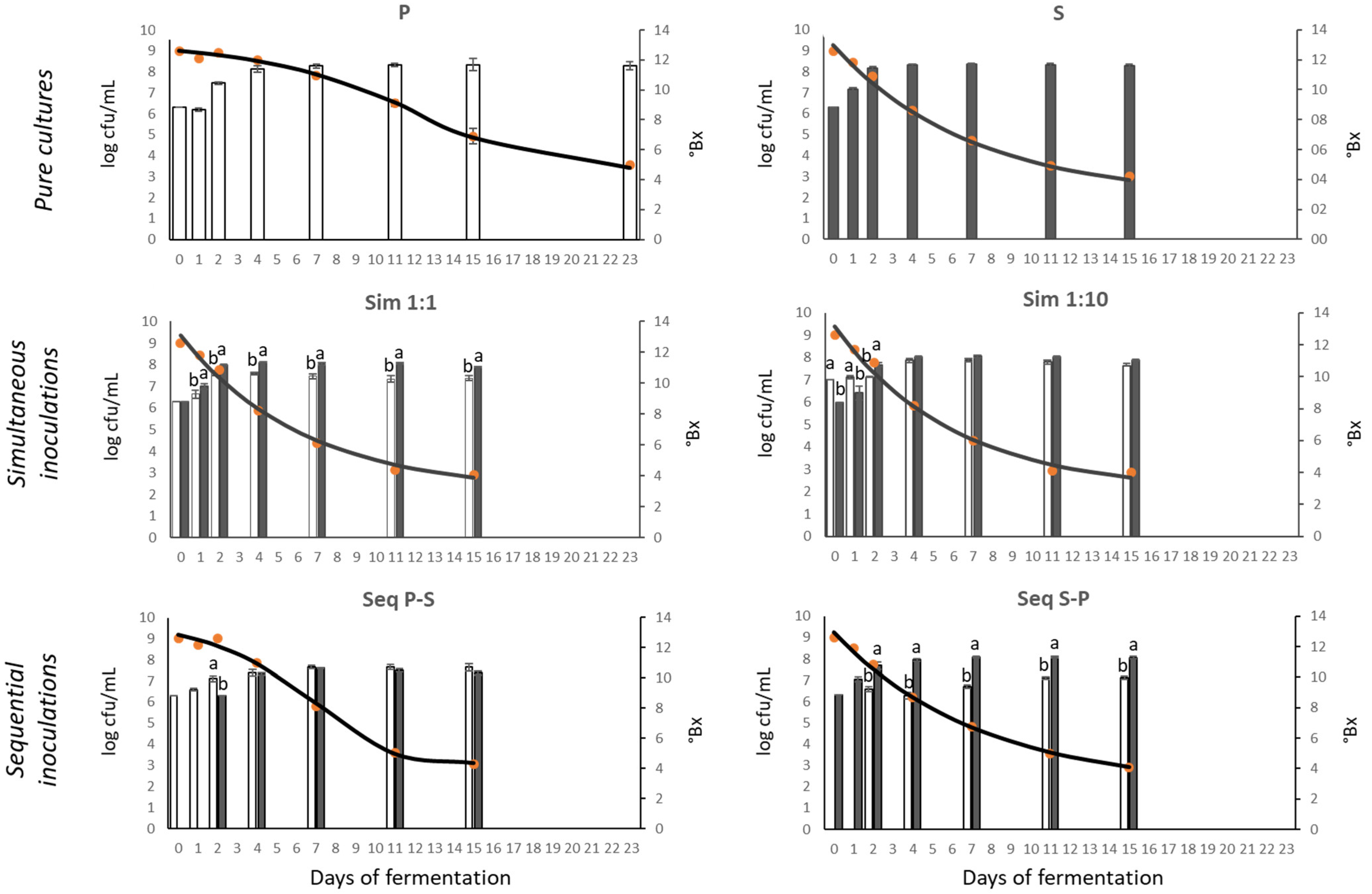
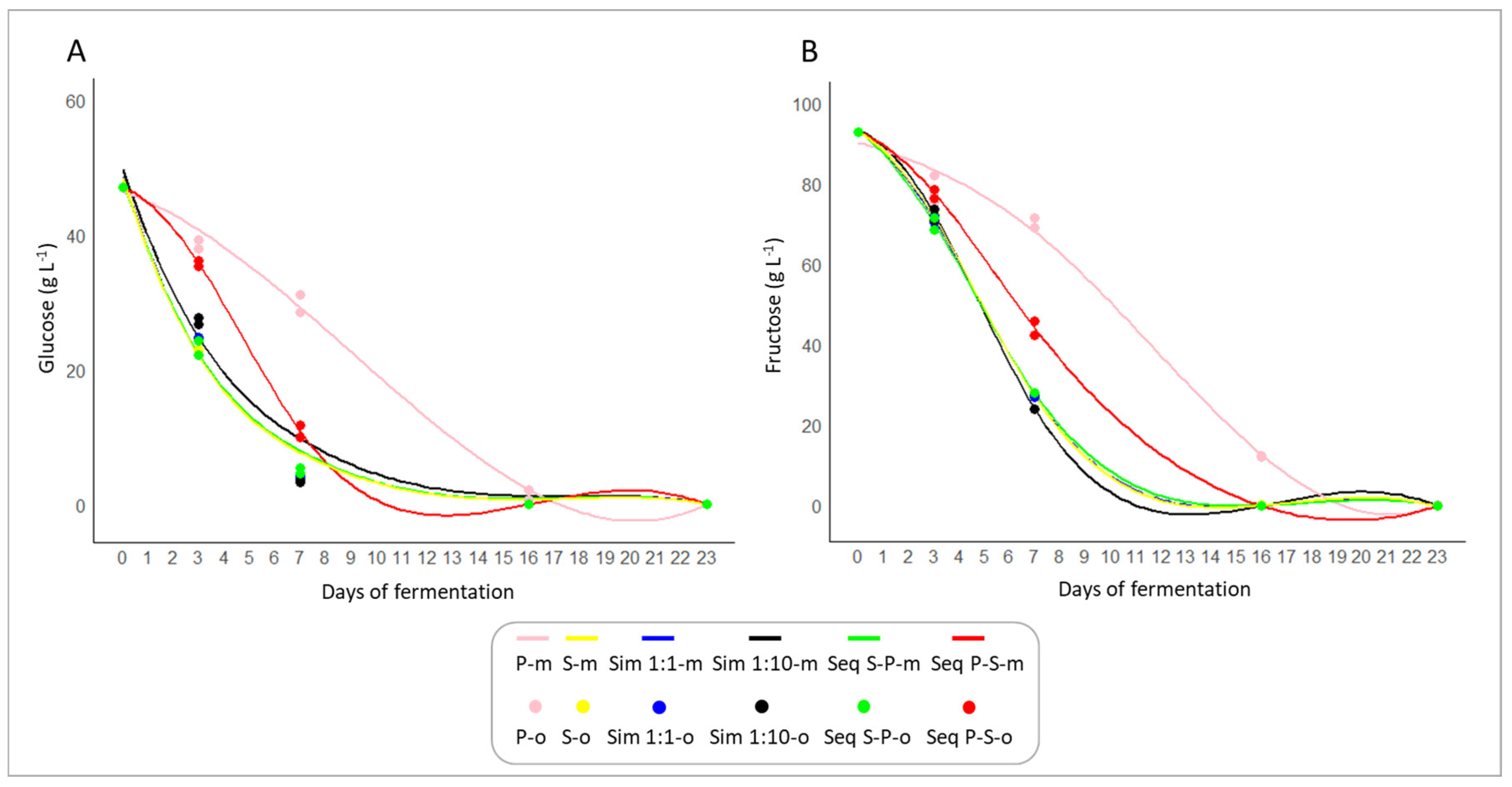
3.1. Fermentation Kinetics
3.2. Fermentation Dynamics
3.3. Antagonist Activity
3.4. Chemical Properties of Base Ciders
4. Discussion
4.1. Fermentation Kinetics and Sugar Utilization
4.2. Malic Acid Consumption
4.3. Viable Cell Counts and Antagonistic Activity
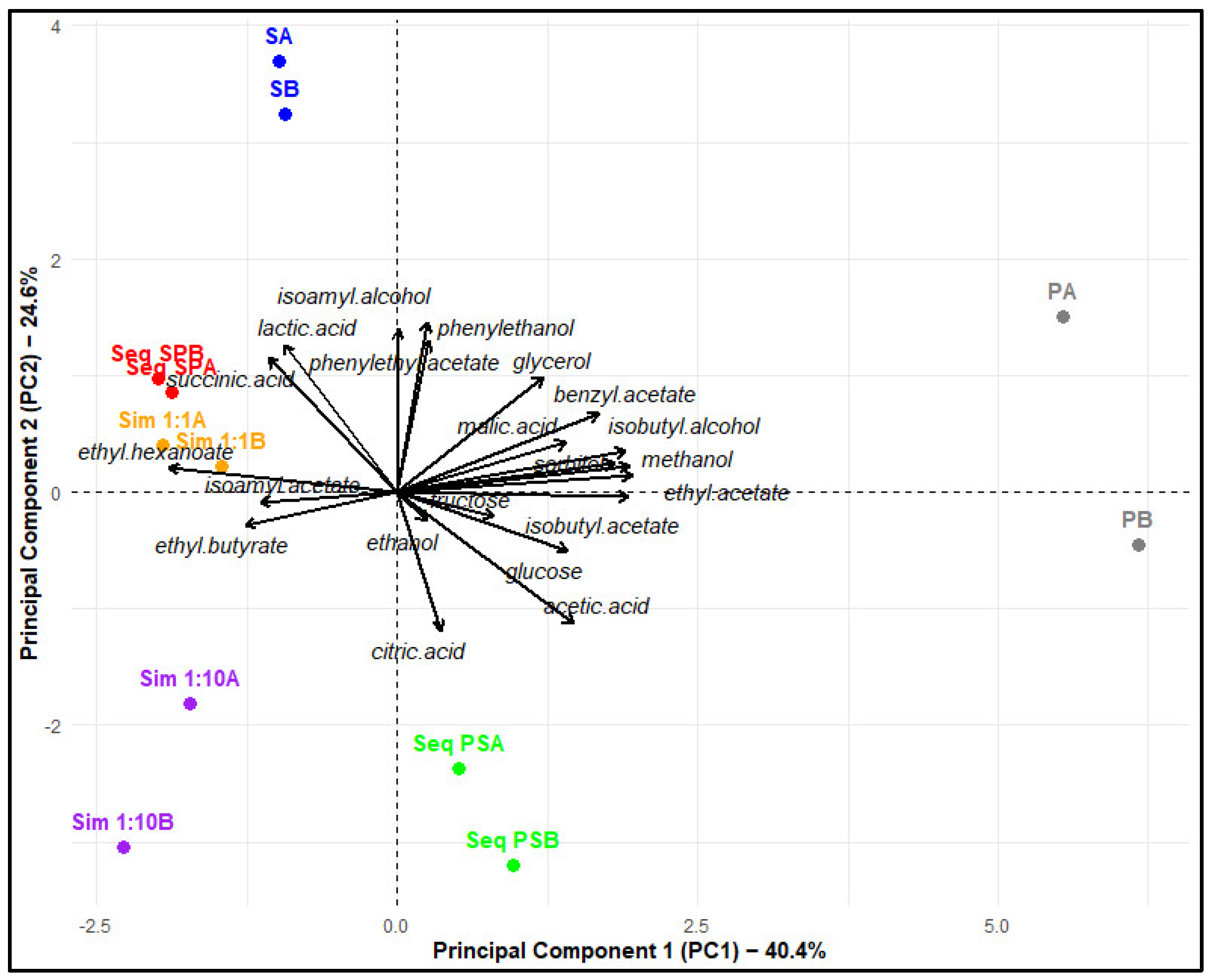
4.4. Physicochemical and Aromatic Composition of Ciders
4.4.1. Considerations of Simultaneous Fermentations
4.4.2. Considerations of Sequential Fermentations
4.4.3. Considerations of Pure Fermentations
4.4.4. Selection of the Inoculation Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AICV Cider Trends. 2022. Available online: https://aicv.org/en/publications (accessed on 26 December 2024).
- Argentine Ministry of Agriculture, Livestock and Fisheries. Cider Value Chain Report in Argentina. 2022. Available online: https://www.argentina.gob.ar/sites/default/files/cadenasagroalimentarias-febrero2020.pdf (accessed on 26 December 2024).
- Uthurry, C.A.; Susca, M.J.; Fontanini, J.M.; Gresia, J.A.; Bezic, C.A.; Caponi, A.M.; Franchi, M.L. Physicochemical and sensorial characterisation of Argentine ciders. J. Inst. Brew. 2019, 125, 433–442. [Google Scholar] [CrossRef]
- Argentine Ministry of Agriculture, Livestock and Fisheries. Variedades no Tradicionales de Manzana para la Elaboración de Sidra. 2024. Available online: https://www.argentina.gob.ar/noticias/variedades-no-tradicionales-de-manzana-para-la-elaboracion-de-sidra (accessed on 26 December 2024).
- González Flores, M.; Rodríguez, M.E.; Oteiza, J.M.; Barbagelata, R.J.; Lopes, C.A. Physiological characterization of Saccharomyces uvarum and Saccharomyces eubayanus from Patagonia and their potential for cidermaking. Int. J. Food Microbiol. 2017, 249, 9–17. [Google Scholar] [CrossRef]
- González Flores, M.; Rodríguez, M.E.; Origone, A.C.; Oteiza, J.M.; Querol, A.; Lopes, C.A. Saccharomyces uvarum isolated from Patagonian ciders shows excellent fermentative performance for low temperature cidermaking. Food Res. Int. 2019, 126, 108656. [Google Scholar] [CrossRef] [PubMed]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the physicochemical composition and microbial diversity in apple juice fermentation process: A review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, P.T.; Hu, C.Y.; Tian, D.; Deng, H.; Meng, Y.H. Screening low-methanol and high-aroma produced yeasts for cider fermentation by transcriptive characterization. Front. Microbiol. 2022, 13, 1042613. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT—Food Sci. Technol. 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Pando Bedriñana, R.; Picinelli Lobo, A.; Rodríguez Madrera, R.; Suárez Valles, B. Characteristics of Ice Juices and Ciders made by cryo-extraction with different cider apple varieties and yeast strains. Food Chem. 2019, 292, 125831. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT—Food Sci. Technol. 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Zhang, S.; Ji, C.; Liang, H. Chemical Composition and Flavor Characteristics of Cider Fermented with Saccharomyces cerevisiae and Non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Hu, L.; Chen, X.; Lin, R.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguyen, T.T.H.; Jin, J.; Lim, J.; Lee, J.; Piao, M.; Mok, I.K.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. [Google Scholar] [CrossRef]
- Li, R.-R.; Xu, M.; Zheng, J.; Liu, Y.-J.; Sun, C.-H.; Wang, H.; Guo, X.-W.; Xiao, D.-G.; Wu, X.-L.; Chen, Y.-F. Application Potential of Baijiu Non-Saccharomyces Yeast in Winemaking Through Sequential Fermentation With Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 902597. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Simonato, B.; Zapparoli, G. Yeast species diversity in apple juice for cider production evidenced by culture-based method. Folia Microbiol. 2018, 63, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, R.; Aimaier, A.; Sun, J.; Pu, X.; Shi, X.; Cheng, W.; Wang, B. Adjustment of impact phenolic compounds, antioxidant activity and aroma profile in Cabernet Sauvignon wine by mixed fermentation of Pichia kudriavzevii and Saccharomyces cerevisiae. Food Chem. X 2023, 18, 100685. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, X.; Hu, L.; Zhang, X. Effects of Crude β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, and Metschnikowia pulcherrima on the Flavor Complexity and Characteristics of Wines. Microorganisms 2020, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-X.; Wang, G.-Q.; Aihaiti, A. Combined Indigenous Yeast Strains Produced Local Wine from Over Ripe Cabernet Sauvignon Grape in Xinjiang. World J. Microbiol. Biotechnol. 2020, 36, 122. [Google Scholar] [CrossRef] [PubMed]
- del Monaco, S.M.; Barda, N.B.; Rubio, N.C.; Caballero, A.C. Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 2013, 117, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hong, Y.-A.; Park, H.-D. Co-fermentation of grape must by Issatchenkia orientalis and Saccharomyces cerevisiae reduces the malic acid content in wine. Biotechnol. Lett. 2008, 30, 1633–1638. [Google Scholar] [CrossRef]
- Seo, S.-H.; Rhee, C.; Park, H.-D. Degradation of malic acid by Issatchenkia orientalis KMBL 5774, an acidophilic yeast strain isolated from Korean grape wine pomace. J. Microbiol. 2007, 45, 521–527. [Google Scholar] [PubMed]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological management of acidity in wine industry: A review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Tratado de Enología: Microbiología Del Vino Vinificaciones, 2nd ed.; Hemisferio Sur: Buenos Aires, Argentina, 2003; pp. 154–196. [Google Scholar]
- Cerri, M.L.; Gomes, T.A.; Carraro, M.d.M.; Wojeicchowski, J.P.; Demiate, I.M.; Lacerda, L.G.; Alberti, A.; Nogueira, A. Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation 2023, 9, 1017. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasson, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Sánchez, A.; de Revel, G.; Antalick, G.; Herrero, M.; García, L.A.; Díaz, M. Influence of controlled inoculation of malolactic fermentation on the sensory properties of industrial cider. J. Ind. Microbiol. Biotechnol. 2014, 41, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic Fermentation: New Approaches to Old Problems. Microorganisms 2022, 10, 2363. [Google Scholar] [CrossRef]
- Mazzucco, M.B.; Rodríguez, M.E.; Caballero, A.C.; Lopes, C.A. Differential consumption of malic acid and fructose in apple musts by Pichia kudriavzevii strains. J. Appl. Microbiol. 2024, 135. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Sangorrín, M.P. Optimization of killer assays for yeast selection protocols. Rev. Argent. Microbiol. 2010, 42, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Use of dinitrosalicylic acid agent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Bautista-Gallego, J.; Durán-Quintana, M.C.; Garrido-Fernández, A. Effects of ascorbic acid, sodium metabisulfite and sodium chloride on freshness retention and microbial growth during the storage of Manzanilla-Aloreña cracked table olives. LWT—Food Sci. Technol. 2008, 42, 551–560. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeast. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef] [PubMed]
- RStudio Version 4.4.2; R Core Team: Vienna, Austria, 2022.
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Saayman, M.; Viljoen-Bloom, M. The biochemistry of malic acid metabolism by wine yeasts—A review. S. Afr. J. Enol. Vitic. 2006, 27, 2. [Google Scholar] [CrossRef]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr. Genet. 2003, 43, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Su, J.; Wang, T.; Wang, Y.; Li, Y.-Y.; Li, H. The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl. Microbiol. Biotechnol. 2014, 98, 1–12. [Google Scholar] [CrossRef]
- Yéramian, N.; Chaya, C.; Suárez-Lepe, J.A. L-(-)-malic acid production by Saccharomyces spp. during the alcoholic fermentation of wine. J. Agric. Food Chem. 2007, 55, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, A.M.; Taggart, N.T.; Lee, M.D.; Boyer, J.M.; Rowley, P.A. The prevalence of killer yeasts and double-stranded RNAs in the budding yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- del Mónaco, S.; Rodríguez, M.E.; Lopes, C.A. Pichia kudriavzevii as a representative yeast of North Patagonian winemaking terroir. Int. J. Food Microbiol. 2016, 230, 31–39. [Google Scholar] [CrossRef]
- Bleve, G.; Grieco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef]
- Kottoh, I.D.; Chen, O.; Wang, W.; Yi, L.; Deng, L.; Zeng, K. Evaluation of yeast isolates from kimchi with antagonistic activity against green mold in citrus and elucidating the action mechanisms of three yeasts: P. kudriavzevii, K. marxianus, and Y. lipolytica. Postharvest Biol. Technol. 2021, 182, 111495. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Abo Elyousr, K.A.M.; Ismail, I.M. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biocontrol 2020, 151, 104239. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, K.; Chen, H.; Li, Z. Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods 2023, 12, 3501. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Argentine Food Code. Argentine Ministry of Health, National Administration of Drugs, Foods, and Medical Technology (ANMAT). Chapter XIII, Article 1085 and 1087. 2023. Available online: http://www.argentina.gob.ar/sites/default/files/anmat_caa_capitulo_xiii.pdf (accessed on 26 December 2024).
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vit. 2000, 21 (Special Issue), 97–129. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-K.; Wang, J.; Chen, F.-S.; Zhang, X.-Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT—Food Sci. Technol. 2019, 116, 108477. [Google Scholar] [CrossRef]
| Code | Inoculation Strategy | Inoculum (Cells/mL) | |
|---|---|---|---|
| S. cerevisiae ÑIF8 | P. kudriavzevii NPCC1651 | ||
| P | Pure culture | - | 2 × 106 |
| S | Pure culture | 2 × 106 | - |
| Sim 1:1 | Simultaneous 1:1 | 1 × 106 | 1 × 106 |
| Sim 1:10 | Simultaneous 1:10 | 1 × 106 | 1 × 107 |
| Seq P-S | Sequential | 1 × 106 (48 h) * | 1 × 106 |
| Seq S-P | Sequential | 1 × 106 | 1 × 106 (48 h) * |
| Kinetic Parameters | Kinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|
| P | Seq P-S | S | Sim 1:1 | Sim 1:10 | Seq S-P | ||
| * Sigmoid Model | ** Exponential Model | ||||||
| A (°Brix) | 4.78 ± 0.30 a | 4.38 ± 0.04 a | D (Brix) | 2.80 ± 0.15 a | 2.94 ± 0.05 a | 2.82 ± 0.01 a | 2.77 ± 0.05 a |
| C | 8.46 ± 0.32 b | 9.53 ± 0.06 a | S | 10.20 ± 0.15 a | 10.17 ± 0.06 a | 10.34 ± 0.01 a | 10.19 ± 0.06 a |
| K (°Brix.h − 1) | 0.008 ± 0.0012 b | 0.012 ± 0.0001 a | K (°Brix.h − 1) | 0.0060 ± 3 × 10−4 b | 0.0066 ± 9 × 10−5 a | 0.0069 ± 6 × 10−5 a | 0.0056 ± 2 × 10−4 b |
| M (h) | 316.3 ± 33.4 a | 178.7 ± 1.0 b | |||||
| Glucose Consumption | |||||||
|---|---|---|---|---|---|---|---|
| Sigmoid Decay Model * | P | Seq P-S | Exponential Decay Model ** | S | Sim 1:1 | Sim 1:10 | Seq S-P |
| A (g/L glucose) | 0.12 ± 0.02 | 0.08 ± 0.01 | D (g/L glucose) | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.1 ± 0.01 | 0.06 ± 0.01 |
| C | 55.7 ± 1.3 b | 55.2 ± 0.4 b | S | 48.3 ± 0.1 | 48.5 ± 0.5 | 49.7 ± 0.23 | 48.3 ± 0.3 |
| K (g/L.h − 1) | 0.007 ± 3 × 10−4 c | 0.014 ± 2 × 10−4 a | K (g/L.h − 1) | 0.0110 ± 1 × 10−5 a | 0.0108 ± 6 × 10−4 a | 0.0097 ± 1 × 10−4 b | 0.0107 ± 7 × 10−4 a |
| M (h) | 226.3 ± 16.3 a | 132.5 ± 4.9 b | |||||
| Fructose Consumption | |||||||
| Sigmoid Decay Model * | P | S | Sim 1:1 | Sim 1:10 | Seq P-S | Seq S-P | |
| A (g/L fructose) | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.02 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.01 | 0.02 ± 0.01 | |
| C | 100.3 ± 1.2 c | 111.8 ± 3.6 ab | 113.6 ± 3.9 ab | 107.6 ± 4.2 bc | 109.6 ± 3.4 bc | 120.3 ± 9.6 a | |
| K (g/L.h − 1) | 0.008 ± 7 × 10−4 c | 0.012 ± 9 × 10−4 ab | 0.012 ± 9 × 10−4 b | 0.014 ± 1 × 10−3 a | 0.010 ± 5 × 10−4 b | 0.010 ± 1 × 10−3 b | |
| M (h) | 290.6 ± 8.2 a | 139.1 ± 4.2 c | 137.3 ± 3.6 c | 138.7 ± 4.9 c | 177.1 ± 9.2 b | 131.0 ± 10.7 c | |
| Pure Culture Inoculations | Simultaneous Inoculations | Sequential Inoculations | |||||
|---|---|---|---|---|---|---|---|
| P | S | Sim 1:1 | Sim 1:10 | Seq P-S | Seq S-P | ||
| Glucose | t10 | 53.8 ± 0.7 a | 12.3 ± 0.1 d | 12.7 ± 1.2 d | 16.95 ± 1.3 c | 19.7 ± 0.9 b | 12.5 ± 1.0 d |
| t50 | 206.6 ± 8.5 a | 65.8 ± 0.1 d | 77.7 ± 3.4 bc | 121.1 ± 3.0 cd | 84.1 ± 1.4 b | 67.3 ± 3.5 cd | |
| t95 | 380.4 ± 14.6 a | 275.6 ± 0.5 c | 279.6 ± 11.7 c | 318.0 ± 4.1 b | 178.0 ± 1.8 d | 281.9 ± 13.5 c | |
| Fructose | t10 | 76.3 ± 13.1 a | 39.1 ± 1.8 b | 38.3 ± 1.5 b | 41.6 ± 2.9 b | 51.4 ± 3.3 b | 35.6 ± 2.8 b |
| t50 | 258.3 ± 6.4 a | 129.4 ± 1.4 c | 128.7 ± 0.7 c | 127.0 ± 2.0 c | 163.2 ± 4.5 b | 127.7 ± 2.2 c | |
| t95 | 436.9 ± 3.4 a | 235.9 ± 2.5 d | 237.6 ± 3.7 cd | 222.0 ± 3.2 e | 291.2 ± 1.5 b | 246.7 ± 6.2 c | |
| Pure Cultures | Simultaneous Inoculations | Sequential Inoculations | ||||
|---|---|---|---|---|---|---|
| P | S | Sim 1:1 | Sim 1:10 | Seq P-S | Seq S-P | |
| General Compounds | ||||||
| Glucose (g L−1) | 0.20 ± 0.1 | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.16 ± 0.05 | 0.12 ± 0.09 | 0.14 ± 0.10 |
| Fructose (g L−1) | 0.16 ± 0.11 | 0.06 ± 0.05 | 0.12 ± 0.08 | 0.09 ± 0.05 | 0.16 ± 0.08 | 0.21 ± 0.12 |
| Glycerol (g L−1) | 3.54 ± 0.31 a | 3.16 ± 0.01 abc | 2.81 ± 0.02 c | 2.23 ± 0.05 d | 2.93 ± 0.02 bc | 3.28 ± 0.03 ab |
| Sorbitol (g L−1) | 6.03 ± 0.34 a | 5.50 ± 0.03 b | 5.41 ± 0.00 b | 5.24 ± 0.12 b | 5.50 ± 0.03 b | 5.42 ± 0.03 b |
| Ethanol (% v/v) | 5.65 ± 0.22 ab | 5.62 ± 0.12 ab | 5.79 ± 0.05 a | 5.63 ± 0.02 ab | 5.62 ± 0.03 ab | 5.42 ± 0.04 b |
| Methanol (% v/v) | 0.05 ± 0.01 | ND | ND | ND | ND | ND |
| pH | 3.31 ± 0.015 c | 3.33 ± 0.005 c | 3.35 ± 0.005 b | 3.39 ± 0.005 a | 3.36 ± 0.005 b | 3.35 ± 0.005 b |
| Organic Acids | ||||||
| Citric acid (g L−1) | 0.20 ± 0.01 a | 0.09 ± 0.01 b | 0.19 ± 0.01 a | 0.19 ± 0.02 a | 0.21 ± 0.03 a | 0.19 ± 0.02 a |
| Malic acid (g L−1) | 5.55 ± 0.19 a | 5.43 ± 0.08 ab | 5.13 ± 0.05 cd | 5.10 ± 0.21 cd | 5.28 ± 0.11 bc | 5.05 ± 0.05 d |
| Acetic acid (g L−1) | 0.40 ± 0.05 a | 0.07 ± 0.02 d | 0.17 ± 0.05 bc | 0.26 ± 0.03 b | 0.40 ± 0.01 a | 0.16 ± 0.00 cd |
| Lactic acid (g L−1) | 0.55 ± 0.09 c | 0.95 ± 0.01 ab | 0.81 ± 0.06 b | 0.49 ± 0.04 c | 0.54 ± 0.10 c | 1.00 ± 0.03 a |
| Succinic acid (g L−1) | 0.43 ± 0.05 b | 0.66 ± 0.01 a | 0.62 ± 0.01 a | 0.43 ± 0.02 b | 0.42 ± 0.00 b | 0.68 ± 0.01 a |
| Total acids (g L−1) | 7.13 ± 0.22 ab | 7.21 ± 0.08 a | 6.93 ± 0.1 ab | 6.47± 0.01 c | 6.85 ±0.07 b | 7.06 ± 0.04 ab |
| Higher alcohols (mg L−1) | ||||||
| 1-propanol | ND | ND | ND | ND | ND | ND |
| 1-octanol | <DL = 0.08 | <DL = 0.08 | <DL = 0.08 | <DL = 0.08 | <DL = 0.08 | <DL = 0.08 |
| 1-butanol | <DL = 1.0 | <DL = 1.0 | <DL = 1.0 | <DL = 1.0 | <DL = 1.0 | <DL = 1.0 |
| 2-phenylethanol | 7.65 ± 1.35 b | 11.75 ± 2.25 a | 5.85 ± 0.05 b | <DL c | <DL c | 6.40 ± 0.50 b |
| Benzyl alcohol | <DL = 0.07 | <DL = 0.07 | <DL = 0.07 | <DL = 0.07 | <DL = 0.07 | <DL = 0.07 |
| Isoamyl alcohol | 225 ± 15 b | 250 ± 3 a | 235 ± 5 ab | 150 ± 4 c | 125 ± 5 d | 245 ± 5 a |
| Isobutyl alcohol | 64.5 ± 5.5 a | 37.0 ± 1.5 b | 38.0 ± 2 b | 29.0 ± 0.8 b | 34.5 ± 1.5 b | 34.0 ± 6.0 b |
| Total higher alcohols | 297 ± 19.1 a | 299 ± 2.2 a | 279 ± 5.0 a | 179 ± 1.2 b | 160 ± 6.5 b | 285 ± 11.5 a |
| Esters (mg L−1) | ||||||
| Ethyl acetate | 485 ± 75 a | 220 ± 20 b | 110 ± 1 c | 160 ± 2 bc | 240 ± 30 b | 84.5 ± 0.5 c |
| 2-Phenylethyl acetate | 0.079 ± 0.014 ab | 0.089 ± 0.005 a | 0.071 ± 0.002 ab | 0.068 ± 0.006 ab | <DL = 0.05 c | 0.067 ± 0.001 b |
| Benzyl acetate | 0.555 ± 0.145 a | 0.275 ± 0.045 b | 0.185 ± 0.015 bc | 0.130 ± 0.010 bc | 0.115 ±0.005 bc | 0.101 ± 0.019 c |
| Isoamyl acetate | 2.05 ± 0.25 c | 2.02 ± 0.21 c | 3.23 ± 0.12 a | 2.85 ± 0.15 ab | 1.75 ± 0.05 c | 2.75 ± 0.15 b |
| Isobutyl acetate | 0.077 ± 0.006 a | 0.035 ± 0.002 b | 0.040 ± 0.005 b | 0.039 ± 0.004 b | 0.045 ± 0.005 b | 0.039 ± 0.004 b |
| Ethyl butyrate | 0.0735 ± 0.017 b | 0.0905 ± 0.008 b | 0.0895 ± 0.021 b | 0.13 ± 5 × 10−4 a | 0.08 ± 4 × 10−4 b | 0.099 ± 5 × 10−4 ab |
| Ethyl decanoate | <DL = 0.60 | <DL = 0.60 | <DL = 0.60 | <DL = 0.60 | <DL = 0.60 | <DL = 0.60 |
| Ethyl hexanoate | <DL = 0.80 | 2 ± 0.01 b | 2.15 ± 0.05 b | 2.45 ± 0.15 a | 1.05 ± 0.05 c | 2.1 ± 0.1 b |
| Ethyl lactate | ND | ND | ND | ND | ND | ND |
| Ethyl octanoate | 0.15 ± 0.03 d | 3.0 ± 1.7 abc | 4.1 ± 1.4 ab | 4.85 ± 0.45 a | 2.15 ± 0.05 bcd | 1.5 ± 0.2 cd |
| Total esters | 488.5 ± 74.9 a | 224.4 ± 20.2 b | 115.7 ± 5 c | 165.6 ± 1.3 bc | 243 ± 30 b | 89 ± 1 c |
| Total esters * | 3.53 ± 0.07 d | 4.48 ± 0.25 c | 5.74 ± 0.01 a | 5.66 ± 0.32 ab | 3.09 ± 0.01 d | 5.15 ± 0.23 b |
| Terpenes (μg L−1) | ||||||
| α-Terpineol | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 |
| Limonene | <DL = 10 | < DL = 10 | <DL = 10 | <DL = 10 | <DL = 10 | <DL = 10 |
| Linalool | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 |
| Nerol | <DL = 0.001 | <DL = 0.001 | 0.001 | <DL = 0.001 | <DL = 0.001 | <DL = 0.001 |
| Geraniol | <DL = 0.0015 | <DL = 0.0015 | <DL = 0.0015 | <DL = 0.0015 | <DL = 0.0015 | <DL = 0.0015 |
| Volatile phenols (μg L−1) | ||||||
| 4-Ethylphenol | <DL = 20.0 | <DL = 20.0 | <DL = 20.0 | <DL = 20.0 | <DL = 20.0 | <DL = 20.0 |
| 4-Ethylguaiacol | <DL = 6.0 | <DL = 6.0 | <DL = 6.0 | <DL = 6.0 | <DL = 6.0 | <DL = 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucco, M.B.; Jovanovich, M.; Rodríguez, M.E.; Oteiza, J.M.; Lopes, C.A. Pichia kudriavzevii and Saccharomyces cerevisiae Inoculation Strategies for Cider Elaboration from Acidic Apples. Fermentation 2025, 11, 79. https://doi.org/10.3390/fermentation11020079
Mazzucco MB, Jovanovich M, Rodríguez ME, Oteiza JM, Lopes CA. Pichia kudriavzevii and Saccharomyces cerevisiae Inoculation Strategies for Cider Elaboration from Acidic Apples. Fermentation. 2025; 11(2):79. https://doi.org/10.3390/fermentation11020079
Chicago/Turabian StyleMazzucco, María Belén, Milena Jovanovich, María Eugenia Rodríguez, Juan Martín Oteiza, and Christian Ariel Lopes. 2025. "Pichia kudriavzevii and Saccharomyces cerevisiae Inoculation Strategies for Cider Elaboration from Acidic Apples" Fermentation 11, no. 2: 79. https://doi.org/10.3390/fermentation11020079
APA StyleMazzucco, M. B., Jovanovich, M., Rodríguez, M. E., Oteiza, J. M., & Lopes, C. A. (2025). Pichia kudriavzevii and Saccharomyces cerevisiae Inoculation Strategies for Cider Elaboration from Acidic Apples. Fermentation, 11(2), 79. https://doi.org/10.3390/fermentation11020079






