Probiotic Potential of Lactic Acid Bacteria and Yeast Isolated from Cocoa and Coffee Bean Fermentation: A Review
Abstract
1. Introduction
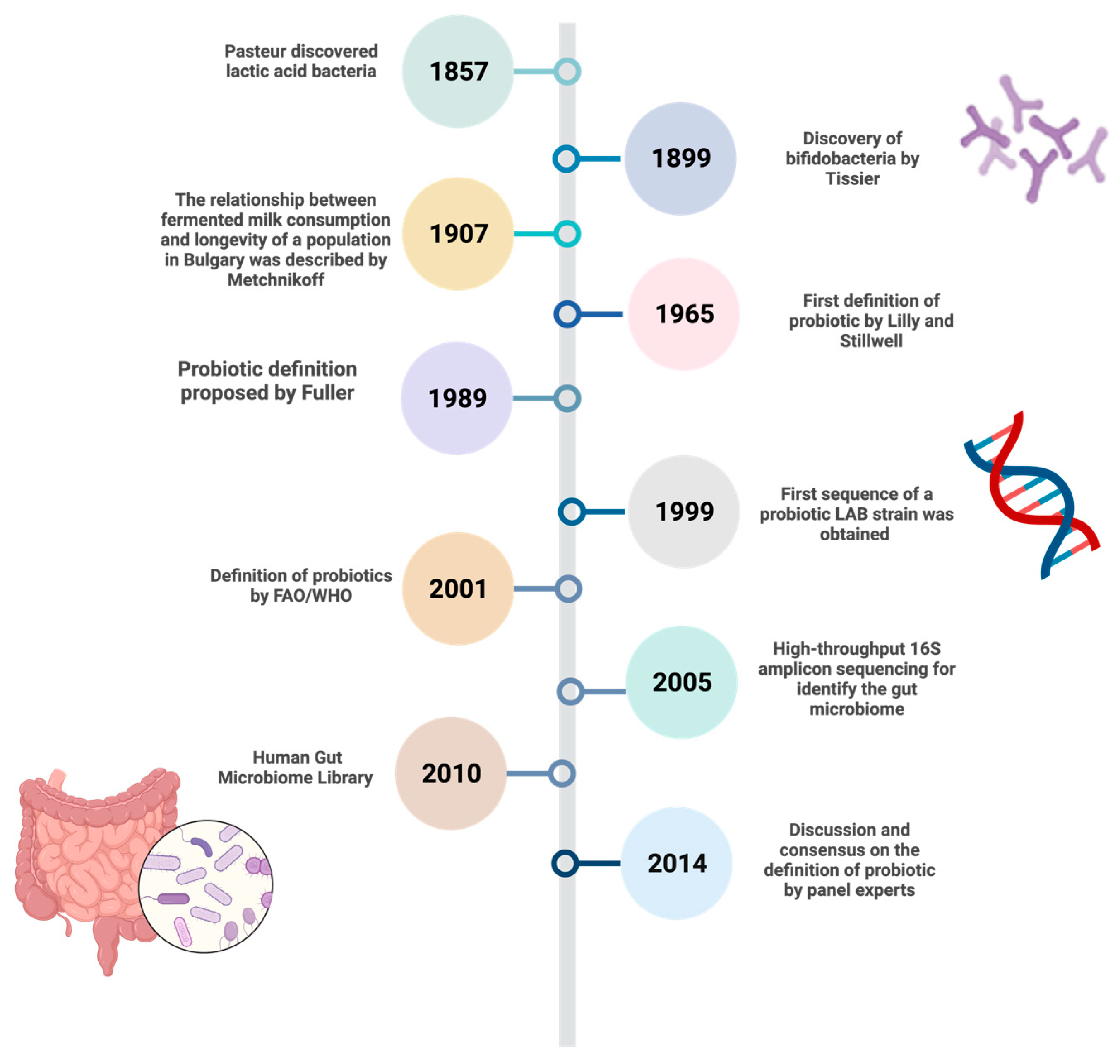
2. Historical Evolution of the Concept of Probiotic Microorganisms
3. Main Microbial Genera with Potential Probiotic Characteristics
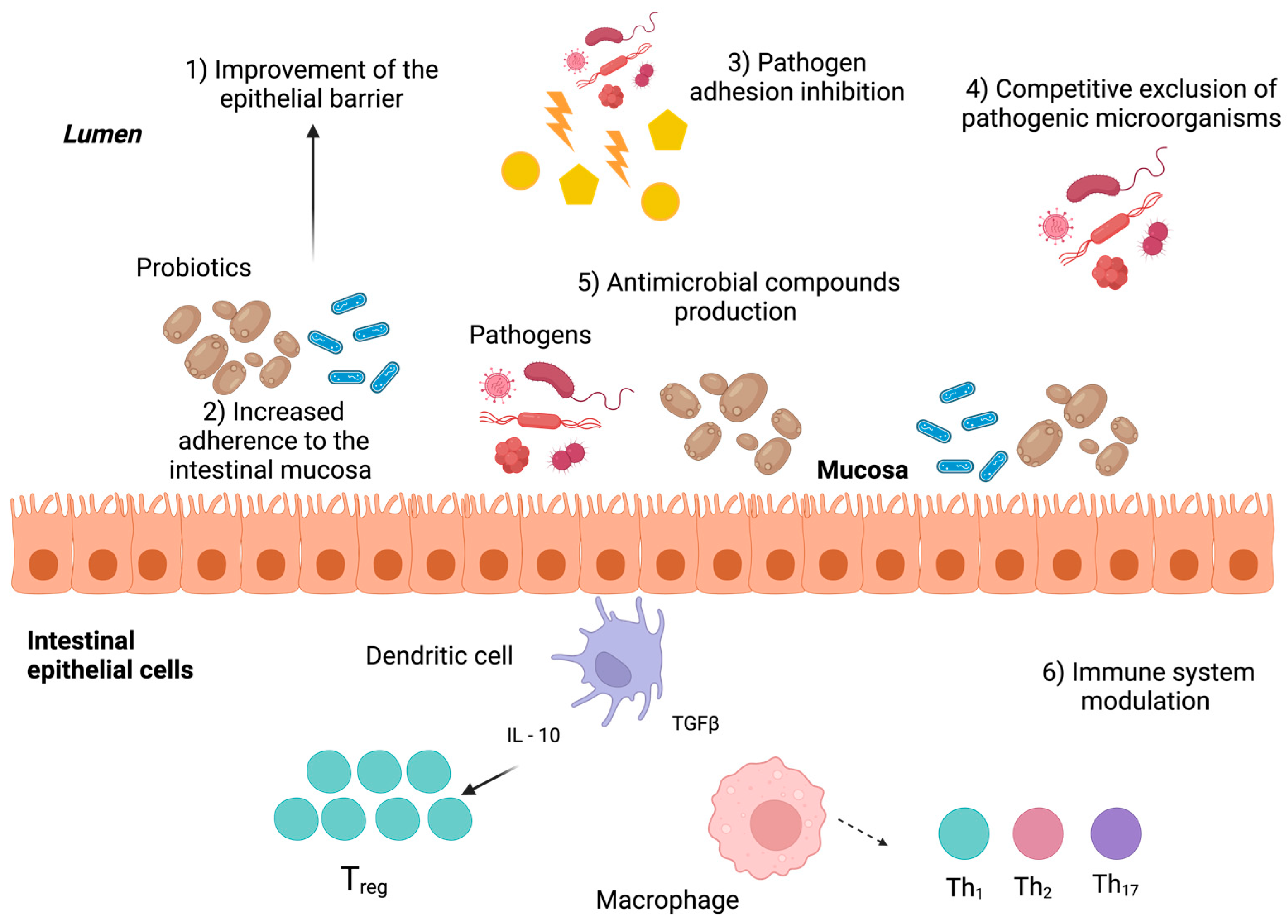
4. Beneficial Effects on Host Health Associated with Probiotic Consumption
5. Selection Criteria and Recommended Dosage of Probiotic Microorganisms
5.1. Selection Criteria for Potential Probiotic Microorganisms
5.2. Recommended Dose of Probiotic Microorganisms
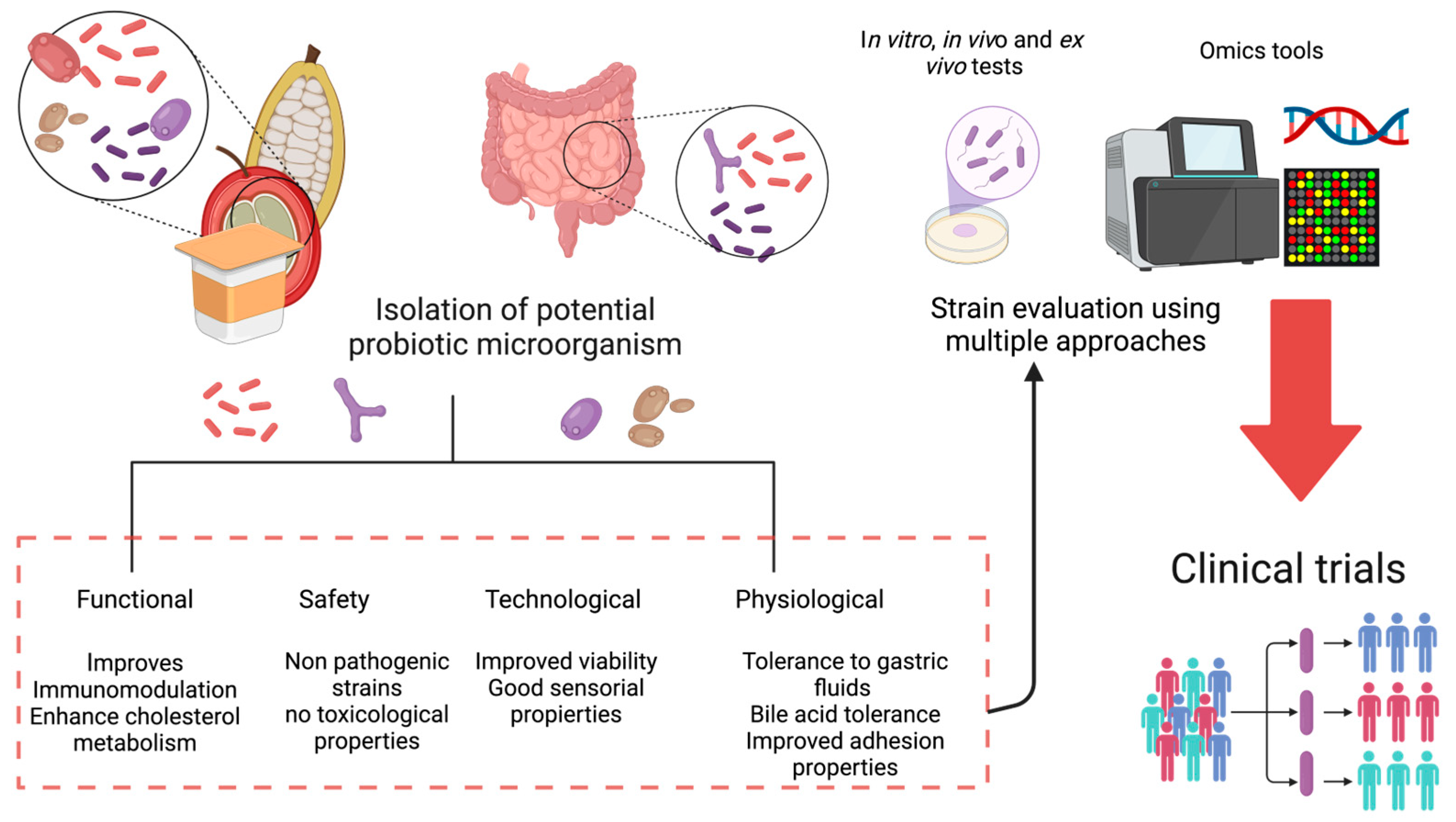
6. Sources of Probiotic Microorganisms
7. Cocoa Fermentation as a Source of Potentially Probiotic Microorganisms
7.1. Cocoa Fermentation Process
7.2. Probiotic Potential of Autochthonous Cocoa Fermentation Microorganisms
7.2.1. Probiotic Potential of Cocoa Autochthonous LAB Strains
7.2.2. Probiotic Potential of Cocoa Autochthonous Yeast Strains
8. Coffee Fermentation as a Source of Potentially Probiotic Microorganisms
8.1. Coffee Fermentation
8.2. Probiotic Potential of Autochthonous Coffee Fermentation Microorganisms
9. Potential Application and Challenges of Potential Probiotic Microorganisms Isolated from Cocoa and Coffee Fermentation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khedkar, S.; Carraresi, L.; Bröring, S. Food or Pharmaceuticals? Consumers’ Perception of Health-Related Borderline Products. PharmaNutrition 2017, 5, 133–140. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. An Introduction to Probiotics. In Probiotics: Advanced Food and Health Applications, 1st ed.; Brandelli, A., Ed.; Academic Press: London, UK, 2021; pp. 1–17. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Binns, N. Probiotics, Prebiotics and the Gut Microbiota; ILSI Europe: Brussels, Belgium, 2013. [Google Scholar]
- Edward Scott Probiotics Market Size, Trends, Industry Overview, and Leading Companies. Available online: https://www.linkedin.com/pulse/probiotics-market-edward-scott-8pbaf/ (accessed on 24 December 2024).
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Quarella, S.; Lovrovich, P.; Scalabrin, S.; Campedelli, I.; Backovic, A.; Gatto, V.; Cattonaro, F.; Turello, A.; Torriani, S.; Felis, G.E. Draft genome sequence of the probiotic yeast Kluyveromyces marxianus fragilis B0399. Genome Announc. 2016, 4, 1128. [Google Scholar] [CrossRef]
- Maccaferri, S.; Klinder, A.; Brigidi, P.; Cavina, P.; Costabile, A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 Cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Environ. Microbiol. 2012, 78, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodríguez-Bueno, C.P.; Abreu y Abreu, A.T.; Guarner, F.; Guno, M.J.V.; Pehlivanoğlu, E.; Perez, M. Bacillus clausii for gastrointestinal disorders: A narrative literature review. Adv. Ther. 2022, 39, 4854–4874. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Abreu Y Abreu, A.T.; Marzet, C.B.; Calatayud, G.Á.; Perez, M.I.I.I.; Castro, A.P.M. Current progress and future perspectives on the use of Bacillus clausii. Microorganisms 2022, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Garrote, A.; Bonet, R. Probióticos. Farm. Prof. 2017, 31, 13–16. [Google Scholar]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Zielińska, D.; Kolozyn-Krajewska, D.; Laranjo, M. Food-origin lactic acid bacteria may exhibit probiotic properties: Review. Biomed. Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef] [PubMed]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 1–11. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Use of characterized microorganisms in fermentation of non-dairy-based substrates to produce probiotic food for gut-health and nutrition. Fermentation 2023, 9, 1. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef]
- Figueroa-Hernández, C.; Mota-Gutierrez, J.; Ferrocino, I.; Hernández-Estrada, Z.J.; González-Ríos, O.; Cocolin, L.; Suárez-Quiroz, M.L. The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 2019, 301, 41–50. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Nandha, M.C.; Shukla, R.M. Exploration of probiotic attributes in lactic acid bacteria isolated from fermented Theobroma cacao L. fruit using in vitro techniques. Front. Microbiol. 2023, 14, 1274636. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, Q.; Wang, H.; Fang, G.; Li, Y.; Zhang, J.; Liu, K. Microbial characteristics and functions in coffee fermentation: A review. Fermentation 2025, 11, 5. [Google Scholar] [CrossRef]
- Carvalho Ferreira, J.L.; de Souza Gomes, M.; Maciel de Oliveira, L.; Diniz Santos, L. Coffee fermentation process: A review. Food Res. Int. 2023, 169, 112793. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Coffee fermentation: Expedition from traditional to controlled process and perspectives for industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Bruno, L.M.; Lima, J.R.; Wurlitzer, N.J.; Rodrigues, T.C. Non-dairy cashew nut milk as a matrix to deliver probiotic bacteria. Food Sci. Technol. 2020, 40, 604–607. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar] [CrossRef]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Forestier, C.; De Champs, C.; Vatoux, C.; Joly, B. Probiotic activities of Lactobacillus casei rhamnosus: In vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 2001, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, J.; Yan, L.; Chen, W.; Liu, X.; Zhang, H. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT 2009, 42, 1640–1646. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef]
- Divyashree, S.; Anjali, P.G.; Somashekaraiah, R.; Sreenivasa, M.Y. Probiotic properties of Lactobacillus casei–MYSRD 108 and Lactobacillus plantarum-MYSRD 71 with potential antimicrobial activity against Salmonella paratyphi. Biotechnol. Rep. 2021, 32, e00672. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Rivera-Espinoza, Y.; Reyes Méndez, A.I.; Hernández-Sánchez, H. In vitro evaluation of the probiotic potential of halotolerant Lactobacilli isolated from a ripened tropical Mexican cheese. Probiotics Antimicrob. Proteins 2013, 5, 239–251. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Cebeci, A.; Gürakan, C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003, 20, 511–518. [Google Scholar] [CrossRef]
- Echegaray, N.; Yilmaz, B.; Sharma, H.; Kumar, M.; Pateiro, M.; Ozogul, F.; Lorenzo, J.M. A novel approach to Lactiplantibacillus plantarum: From probiotic properties to the omics insights. Microbiol. Res. 2023, 268, 127289. [Google Scholar] [CrossRef]
- Surve, S.; Shinde, D.B.; Kulkarni, R. Isolation, characterization and comparative genomics of potentially probiotic Lactiplantibacillus plantarum strains from Indian foods. Sci. Rep. 2022, 12, 1940. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Abdelazez, A.; Abdelmotaal, H.; Evivie, S.E.; Melak, S.; Jia, F.F.; Khoso, M.H.; Zhu, Z.T.; Zhang, L.J.; Sami, R.; Meng, X.C. Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on type I diabetes in vivo. Biomed. Res. Int. 2018, 2018, 7356173. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Kunduhoglu, B.; Hacioglu, S. Probiotic potential and gluten hydrolysis activity of Lactobacillus brevis KT16-2. Probiotics Antimicrob. Proteins 2021, 13, 720–733. [Google Scholar] [CrossRef]
- Pourbaferani, M.; Modiri, S.; Norouzy, A.; Maleki, H.; Heidari, M.; Alidoust, L.; Derakhshan, V.; Zahiri, H.S.; Noghabi, K.A. A newly characterized potentially probiotic strain, Lactobacillus brevis MK05, and the toxicity effects of its secretory proteins against MCF-7 breast cancer cells. Probiotics Antimicrob. Proteins 2021, 13, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Carbonne, C.; Chadi, S.; Kropp, C.; Molimard, L.; Chain, F.; Langella, P.; Martin, R. Ligilactobacillus salivarius CNCM I-4866, a potential probiotic candidate, shows anti-inflammatory properties in vitro and in vivo. Front. Microbiol. 2023, 14, 1270974. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Madi, A.; Prévost, H.; Feuilloley, M.; Manai, M.; Dousset, X.; Connil, N. In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 2012, 18, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Manai, M.; Kergourlay, G.; Prévost, H.; Connil, N.; Chobert, J.M.; Dousset, X. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013, 36, 296–304. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yang, R.S.; Lin, Y.C.; Xin, W.G.; Zhou, H.Y.; Wang, F.; Zhang, Q.L.; Lin, L.B. Assessment of the safety and probiotic characteristics of Lactobacillus salivarius CGMCC20700 based on whole-genome sequencing and phenotypic analysis. Front. Microbiol. 2023, 14, 1120263. [Google Scholar] [CrossRef]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus. Future Microbiol. 2010, 5, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-Month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM Patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Yerlikaya, O. Probiotic potential and biochemical and technological properties of Lactococcus lactis ssp. lactis strains isolated from raw milk and kefir grains. J. Dairy. Sci. 2019, 102, 124–134. [Google Scholar] [CrossRef]
- Mileriene, J.; Aksomaitiene, J.; Kondrotiene, K.; Asledottir, T.; Vegarud, G.E.; Serniene, L.; Malakauskas, M. Whole-genome sequence of Lactococcus lactis subsp. lactis ll16 confirms safety, probiotic potential, and reveals functional traits. Microorganisms 2023, 11, 1034. [Google Scholar] [CrossRef]
- Jaskulski, I.B.; Uecker, J.; Bordini, F.; Moura, F.; Gonçalves, T.; Chaves, N.G.; Camargo, F.; Grecco, F.B.; Fiorentini, Â.M.; da Silva, W.P.; et al. In vivo action of Lactococcus lactis subsp. lactis isolate (R7) with probiotic potential in the stabilization of cancer cells in the colorectal epithelium. Process Biochem. 2020, 91, 165–171. [Google Scholar] [CrossRef]
- Lee, N.K.; Han, K.J.; Son, S.H.; Eom, S.J.; Lee, S.K.; Paik, H.D. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT 2015, 64, 1036–1041. [Google Scholar] [CrossRef]
- Kondrotiene, K.; Lauciene, L.; Andruleviciute, V.; Kasetiene, N.; Serniene, L.; Sekmokiene, D.; Malakauskas, M. Safety assessment and preliminary in vitro evaluation of probiotic potential of Lactococcus lactis strains naturally present in raw and fermented milk. Curr. Microbiol. 2020, 77, 3013–3023. [Google Scholar] [CrossRef]
- Madana, S.T.; Sathiavelu, M. Probiotic evaluation, adherence capability and safety assessment of Lactococcus lactis strain isolated from an important herb “Murraya Koenigii”. Sci. Rep. 2024, 14, 15565. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hwang, U.S.; Choi, H.; Park, Y.S. Immunostimulatory activity of Lactococcus lactis subsp. lactis CAB701 isolated from Jeju Cabbage. Microorganisms 2023, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F. A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SAR S-CoV-2. Minerva Med. 2020, 11, 281–283. [Google Scholar] [CrossRef]
- Burton, J.P.; Cowley, S.; Simon, R.R.; McKinney, J.; Wescombe, P.A.; Tagg, J.R. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: A randomized, placebo-controlled, double-blind study. Food Chem. Toxicol. 2011, 49, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Divyashri, G.; Krishna, G.; Muralidhara; Prapulla, S.G. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: In vitro and in vivo evidence. J. Med. Microbiol. 2015, 64, 1527–1540. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Sanni, A.; Tan, H. Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk. J. Appl. Microbiol. 2013, 114, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, H.; Di Cerbo, A.; Zulfiqar, F.; Sabia, C.; Nawaz, A.; Siddiqui, F.M.; Aqeel, M.; Ghazanfar, S. Probiotic characterization and population diversity analysis of gut-associated Pediococcus acidilactici for its potential use in the dairy industry. Appl. Sci. 2021, 11, 9586. [Google Scholar] [CrossRef]
- Damodharan, K.; Lee, Y.S.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 2015, 6, 768. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Daliri, E.B.M.; Oh, D.H. Screening for potential probiotic bacteria from Korean fermented soybean paste: In vitro and Caenorhabditis elegans model testing. LWT 2018, 88, 132–138. [Google Scholar] [CrossRef]
- Vasiee, A.; Falah, F.; Behbahani, B.A.; Tabatabaee-yazdi, F. Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: Interaction with pathogenic bacteria and the enteric cell line Caco-2. J. Biosci. Bioeng. 2020, 130, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, E.; Colom, J.; Simon, A.; Mazhar, S.; García-Lainez, G.; Llopis, S.; Gonzalez, N.; Enrique-López, M.; Álvarez, B.; Martorell, P.; et al. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms 2023, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Bashir, S.; Imran, M. Probiotic characterization of Bacillus species strains isolated from an artisanal fermented milk product Dahi. Folia Microbiol. 2023, 68, 757–769. [Google Scholar] [CrossRef]
- Golnari, M.; Bahrami, N.; Milanian, Z.; Rabbani Khorasgani, M.; Asadollahi, M.A.; Shafiei, R.; Fatemi, S.S.A. Isolation and characterization of novel Bacillus strains with superior probiotic potential: Comparative analysis and safety evaluation. Sci. Rep. 2024, 14, 1457. [Google Scholar] [CrossRef] [PubMed]
- Penaloza-Vazquez, A.; Ma, L.M.; Rayas-Duarte, P. Isolation and characterization of Bacillus spp. strains as potential probiotics for poultry. Can. J. Microbiol. 2019, 65, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Yu, R.; Feng, X.; Chen, L.; Zeng, Z.; Khaskheli, G.B.; Ma, H.; Chen, S. Characterization and in vitro properties of potential probiotic Bifidobacterium strains isolated from breast-fed infant feces. Ann. Microbiol. 2016, 66, 1027–1037. [Google Scholar] [CrossRef]
- Karbaschian, Z.; Mokhtari, Z.; Pazouki, A.; Kabir, A.; Hedayati, M.; Moghadam, S.S.; Mirmiran, P.; Hekmatdoost, A. Probiotic supplementation in morbid obese patients undergoing One Anastomosis Gastric Bypass-Mini Gastric Bypass (OAGB-MGB) Surgery: A randomized, double-blind, placebo-controlled, clinical trial. Obes. Surg. 2018, 28, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Zavaglia, A.G.; Kociubinski, G.; Pérez, P.; De Antoni, G. Isolation and characterization of Bifidobacterium strains for probiotic formulation. J. Food Prot. 1998, 61, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Ruas-Madiedo, P.; Margolles, A.; Solís, G.; Salminen, S.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. Int. J. Food Microbiol. 2011, 149, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, H.; Zhu, Z.; Li, Q. The complete genome sequence of Bifidobacterium longum LTBL16, a potential probiotic strain from healthy centenarians with strong antioxidant activity. Genomics 2020, 112, 769–773. [Google Scholar] [CrossRef]
- Reyes-Castillo, P.A.; González-Vázquez, R.; Torres-Maravilla, E.; Bautista-Hernández, J.I.; Zúñiga-León, E.; Leyte-Lugo, M.; Mateos-Sánchez, L.; Mendoza-Pérez, F.; Gutiérrez-Nava, M.A.; Reyes-Pavón, D.; et al. Bifidobacterium longum LBUX23 isolated from feces of a newborn; potential probiotic properties and genomic characterization. Microorganisms 2023, 11, 1648. [Google Scholar] [CrossRef]
- Fu, L.; Song, J.; Wang, C.; Fu, S.; Wang, Y. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory T cells and alterations in gut microbiota. Front. Immunol. 2017, 8, 1536. [Google Scholar] [CrossRef]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of Daily Consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Van Maren, W.W.C.; Van Bergenhenegouwen, J.; Hameetman, M.; Nierkens, S.; Jacobs, C.; De Jong, D.J.; Joosten, L.A.B.; Van’t Land, B.; Garssen, J.; et al. Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin. Vaccine Immunol. 2011, 18, 621–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Šarić, L.; Tomičić, R. Novel insights in the application of probiotic yeast Saccharomyces boulardii in dairy products and health promotion. Foods 2024, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Tomar, R.; Ganesan, K.; Prasad, G.S.; Subramanian, S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Alian Samakkhah, S.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef]
- Van der Aa Kühle, A.; Skovgaard, K.; Jespersen, L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2005, 101, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Motey, G.A.; Johansen, P.G.; Owusu-Kwarteng, J.; Ofori, L.A.; Obiri-Danso, K.; Siegumfeldt, H.; Larsen, N.; Jespersen, L. Probiotic potential of Saccharomyces cerevisiae and Kluyveromyces marxianus isolated from West African spontaneously fermented cereal and milk products. Yeast 2020, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fadda, M.E.; Mossa, V.; Deplano, M.; Pisano, M.B.; Cosentino, S. In vitro screening of Kluyveromyces strains isolated from Fiore Sardo cheese for potential use as probiotics. LWT 2017, 75, 100–106. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; Kosmerl, E.; Jiménez-Flores, R.; Alvarez, V.B. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus BDGO-YM6. Front. Microbiol. 2023, 14, 1236634. [Google Scholar] [CrossRef] [PubMed]
- Romanin, D.E.; Llopis, S.; Genovés, S.; Martorell, P.; Ramón, V.D.; Garrote, G.L.; Rumbo, M. Probiotic yeast Kluyveromyces marxianus CIDCA 8154 shows anti-inflammatory and anti-oxidative stress properties in in vivo models. Benef Microbes 2016, 7, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Yari Khosroushahi, A. Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. J. Funct. Foods 2017, 34, 408–421. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, s49–s66. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and gut dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Suarez-Lepe, J.A.; Loira, I.; Palomero, F.; Morata, A. Dairy and nondairy-based beverages as a vehicle for probiotics, prebiotics, and symbiotics: Alternatives to health versus disease binomial approach through food. In Milk-Based Beverages, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2019; Volume 9, pp. 473–520. ISBN 9780128155042. [Google Scholar]
- Vandenplas, Y.; Huys, G.; Daube, G. Probiotics: An update. J. Pediatr. 2015, 91, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in disease prevention and treatment. J. Clin. Pharmacol. 2018, 58, S164–S179. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The potential impact of probiotics on human health: An update on their health-promoting properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Ślizewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Tamayo, C. Clinical research on probiotics: The interface between science and regulation. Clin. Infect. Dis. 2008, 46, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “Probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Font de Valdez, G.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in functional foods: Evaluation of survival and approaches to improve viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Castro-López, C.; García, H.S.; Guadalupe Martínez-Ávila, G.C.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Genomics-based approaches to identify and predict the health-promoting and safety activities of promising probiotic strains—A probiogenomics review. Trends Food Sci. Technol. 2021, 108, 148–163. [Google Scholar] [CrossRef]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H. World Gastroenterology Organisation Global Guidelines-Probiotics and Prebiotics. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf (accessed on 26 December 2024).
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, Jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Hong, J.Y.; Yi, S.H.; Hong, S.P.; Lee, J.E.; Paik, H.D. Bioactive compounds of probiotic Saccharomyces cerevisiae strains isolated from cucumber Jangajji. J. Funct. Foods 2019, 58, 324–329. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Yu, H.S.; Jang, H.J.; Lee, N.K.; Paik, H.D. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from Kimchi. LWT 2019, 112, 108229. [Google Scholar] [CrossRef]
- Touret, T.; Oliveira, M.; Semedo-Lemsaddek, T. Putative probiotic lactic acid bacteria isolated from Sauerkraut Fermentations. PLoS ONE 2018, 13, e0203501. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Liu, J.; Liu, Y.; Wang, Y.; Xiao, Y.; Gao, B.; Zhu, D. In vitro probiotic characterization of Lactobacillus strains from fermented tangerine vinegar and their cholesterol degradation activity. Food Biosci. 2021, 39, 100843. [Google Scholar] [CrossRef]
- Boricha, A.A.; Shekh, S.L.; Pithva, S.P.; Ambalam, P.S.; Manuel Vyas, B.R. In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 2019, 106, 201–208. [Google Scholar] [CrossRef]
- Khushboo; Karnwal, A.; Malik, T. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 2023, 14, 1170725. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Gonçalves dos Santos, M.T.P.; Galván, A.I.; Merchán, A.V.; González, E.; Córdoba, M.d.G.; Benito, M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: Probiotic characteristics and prebiotic metabolism. LWT 2019, 114, 108388. [Google Scholar] [CrossRef]
- Abouloifa, H.; Rokni, Y.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; Ben Salah, R.; Chihib, N.E.; Saalaoui, E.; Asehraou, A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob. Proteins 2020, 12, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Dushku, E.; Ioannou, A.; Staikou, A.; Yiangou, M. Probiotic properties and immunomodulatory activity of gastrointestinal tract commensal bacterial strains isolated from the edible farmed snail Cornu aspersum maxima. Fish. Shellfish. Immunol. 2019, 92, 792–801. [Google Scholar] [CrossRef]
- Nath, S.; Sikidar, J.; Roy, M.; Deb, B. In vitro screening of probiotic properties of Lactobacillus plantarum isolated from fermented milk product. Food Qual. Saf. 2020, 4, 213–223. [Google Scholar] [CrossRef]
- Liu, C.; Xue, W.J.; Ding, H.; An, C.; Ma, S.J.; Liu, Y. Probiotic potential of Lactobacillus strains isolated from fermented vegetables in Shaanxi, China. Front. Microbiol. 2022, 12, 774903. [Google Scholar] [CrossRef]
- De Almeida, M.E.; Pessoa, W.F.B.; Melgaço, A.C.C.; Ramos, L.P.; Rezende, R.P.; Romano, C.C. In vitro selection and characterization of probiotic properties in eight Lactobacillus strains isolated from Cocoa fermentation. An. Acad. Bras. Cienc. 2022, 94, 1–15. [Google Scholar] [CrossRef]
- Das Neves Selis, N.; de Oliveira, H.B.M.; Leão, H.F.; dos Anjos, Y.B.; Sampaio, B.A.; Correia, T.M.L.; Almeida, C.F.; Pena, L.S.C.; Reis, M.M.; Brito, T.L.S.; et al. Lactiplantibacillus plantarum strains isolated from spontaneously fermented cocoa exhibit potential probiotic properties against Gardnerella vaginalis and Neisseria gonorrhoeae. BMC Microbiol. 2021, 21, 198. [Google Scholar] [CrossRef]
- Abdel Tawab, F.I.; Abd Elkadr, M.H.; Sultan, A.M.; Hamed, E.O.; El-Zayat, A.S.; Ahmed, M.N. Probiotic potentials of lactic acid bacteria isolated from Egyptian fermented food. Sci. Rep. 2023, 13, 16601. [Google Scholar] [CrossRef]
- Vera-Pingitore, E.; Jimenez, M.E.; Dallagnol, A.; Belfiore, C.; Fontana, C.; Fontana, P.; von Wright, A.; Vignolo, G.; Plumed-Ferrer, C. Screening and characterization of potential probiotic and starter bacteria for plant fermentations. LWT 2016, 71, 288–294. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Manzoor, A.; Tayyeb, A. Functional probiotic attributes and gene encoding plantaracin among variant Lactobacillus plantarum strains. Microb. Pathog. 2019, 131, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, H.; Shokri, R.; Nami, Y.; Khandaghi, J.; Panahi, B. Potential probiotic characterization of lactic acid bacteria isolated from Duimaj, an Iranian traditional snack food, using biochemical, molecular and computational approaches. LWT 2023, 184, 115091. [Google Scholar] [CrossRef]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Grigoryan, S.; Bazukyan, I.; Trchounian, A. Aggregation and adhesion activity of Lactobacilli isolated from fermented products in vitro and in vivo: A potential probiotic strain. Probiotics Antimicrob. Proteins 2018, 10, 269–276. [Google Scholar] [CrossRef]
- Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian Food Products. Int. J. Microbiol. 2019, 2019, 7179514. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of diversity and probiotic efficiency of the autochthonous lactic acid bacteria in the fermentation of selected raw fruit and vegetable juices. Front. Microbiol. 2018, 9, 2539. [Google Scholar] [CrossRef]
- Amenu, D.; Bacha, K. Probiotic potential and safety analysis of lactic acid bacteria isolated from Ethiopian traditional fermented foods and beverages. Ann. Microbiol. 2023, 73, 37. [Google Scholar] [CrossRef]
- Bindu, A.; Lakshmidevi, N. Identification and in vitro evaluation of probiotic attributes of lactic acid bacteria isolated from fermented food sources. Arch. Microbiol. 2021, 203, 579–595. [Google Scholar] [CrossRef]
- Bin Masalam, M.S.; Bahieldin, A.; Alharbi, M.G.; Al-Masaudi, S.; Al-Jaouni, S.K.; Harakeh, S.M.; Al-Hindi, R.R. Isolation, molecular characterization and probiotic potential of lactic acid bacteria in Saudi raw and fermented milk. Evid. Based Complement. Alternat. Med. 2018, 2018, 7970463. [Google Scholar] [CrossRef]
- Banik, A.; Mondal, J.; Rakshit, S.; Ghosh, K.; Sha, S.P.; Halder, S.K.; Ghosh, C.; Mondal, K.C. Amelioration of cold-induced gastric injury by a yeast probiotic isolated from traditional fermented foods. J. Funct. Foods 2019, 59, 164–173. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Obaid, R.S.; Olaimat, A.N.; Liu, S.Q.; Ayyash, M.M. In vitro characterization and identification of potential probiotic yeasts isolated from fermented dairy and non-dairy food products. J. Fungi 2022, 8, 544. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Evaluation of physiological properties of yeast strains isolated from olive oil and their in vitro probiotic trait. Food Microbiol. 2019, 78, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Helmy, E.A.; Soliman, S.A.; Abdel-Ghany, T.M.; Ganash, M. Evaluation of potentially probiotic attributes of certain dairy yeast isolated from buffalo sweetened Karish cheese. Heliyon 2019, 5, e01649. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.A.; Cristina de Souza, A.; Ferreira, I.; Melo, D.S.; Lopes, L.A.A.; Magnani, M.; Schwan, R.F.; Dias, D.R. Probiotic properties of yeasts isolated from Brazilian fermented table olives. J. Appl. Microbiol. 2021, 131, 1983–1997. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Cenzi, G.; Melo, D.S.; Dias, D.R.; Schwan, R.F. Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics Antimicrob. Proteins 2020, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Weckx, S. The functional role of lactic acid bacteria in cocoa bean fermentation. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R., Vignolo, G.M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 248–278. ISBN 9781118868409. [Google Scholar]
- Huerta-Conde, J.A.; Schorr-Galindo, S.; Figueroa-Hernández, C.; Hernández-Estrada, Z.J.; Suárez-Quiroz, M.L.; González-Rios, O. Isolation of autochthonous microorganisms to formulate a defined inoculum for small-scale cocoa fermentation. Rev. Mex. Ing. Quim. 2021, 20, 239–256. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as producers of flavor precursors during cocoa bean fermentation and their relevance as starter cultures: A review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Favero, G.R.; de Carvalho, J.C.; Goés-Neto, A.; Rogez, H.; Soccol, C.R. Global cocoa fermentation microbiome: Revealing new taxa and microbial functions by next generation sequencing technologies. World J. Microbiol. Biotechnol. 2021, 37, 1–17. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Papalexandratou, Z.; Lefeber, T.; Bahrim, B.; Lee, O.S.; Daniel, H.M.; De Vuyst, L. Hanseniaspora opuntiae, Saccharomyces cerevisiae, Lactobacillus fermentum, and Acetobacter pasteurianus Predominate during well-performed Malaysian cocoa bean box fermentations, underlining the importance of these microbial species for a successful cocoa. Food Microbiol. 2013, 35, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lefeber, T.; Janssens, M.; Camu, N.; De Vuyst, L. Kinetic analysis of strains of lactic acid bacteria and acetic acid bacteria in cocoa pulp simulation media toward development of a starter culture for cocoa bean fermentation. Appl. Environ. Microbiol. 2010, 76, 7708–7716. [Google Scholar] [CrossRef] [PubMed]
- Lefeber, T.; Janssens, M.; Moens, F.; Gobert, W.; De Vuyst, L. Interesting starter culture strains for controlled cocoa bean fermentation revealed by simulated cocoa pulp fermentations of cocoa-specific lactic acid bacteria. Appl. Environ. Microbiol. 2011, 77, 6694–6698. [Google Scholar] [CrossRef]
- Adler, P.; Bolten, C.J.; Dohnt, K.; Hansen, C.E.; Wittmann, C. Core fluxome and metafluxome of lactic acid bacteria under simulated cocoa pulp fermentation conditions. Appl. Environ. Microbiol. 2013, 79, 5670–5681. [Google Scholar] [CrossRef]
- Adler, P.; Frey, L.J.; Berger, A.; Bolten, C.J.; Hansen, C.E.; Wittmann, C. The key to acetate: Metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl. Environ. Microbiol. 2014, 80, 4702–4716. [Google Scholar] [CrossRef]
- Moens, F.; Lefeber, T.; De Vuyst, L. Oxidation of metabolites highlights the microbial interactions and role of Acetobacter pasteurianus during cocoa bean fermentation. Appl. Environ. Microbiol. 2014, 80, 1848–1857. [Google Scholar] [CrossRef]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef]
- Bastos, V.S.; Santos, M.F.; Gomes, L.P.; Leite, A.M.; Flosi Paschoalin, V.M.; Del Aguila, E.M. Analysis of the cocobiota and metabolites of Moniliophthora perniciosa -Resistant Theobroma Cacao beans during spontaneous fermentation in southern Brazil. J. Sci. Food Agric. 2018, 98, 4963–4970. [Google Scholar] [CrossRef]
- Batista, N.N.; Ramos, C.L.; Ribeiro, D.D.; Pinheiro, A.C.M.; Schwan, R.F. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT 2015, 63, 221–227. [Google Scholar] [CrossRef]
- Foong, Y.J.; Lee, S.T.; Ramli, N.; Tan, Y.N.; Ayob, M.K. Incorporation of potential probiotic Lactobacillus plantarum isolated from fermented cocoa beans into dark chocolate: Bacterial viability and physicochemical properties analysis. J. Food Qual. 2013, 36, 164–171. [Google Scholar] [CrossRef]
- Melo, T.A.; Dos Santos, T.F.; Pereira, L.R.; Passos, H.M.; Rezende, R.P.; Romano, C.C. Functional profile evaluation of Lactobacillus fermentum TCUESC01: A new potential probiotic strain isolated during cocoa fermentation. Biomed. Res. Int. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.G.T.; Melo, D.d.S.; Ramos, C.L.; Moreira, S.I.; Alves, E.; Schwan, R.F. Yeasts isolated from Brazilian fermented foods in the protection against infection by pathogenic food bacteria. Microb. Pathog. 2020, 140, 103969. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Costa, K.; Acurcio, L.B.; Sandes, S.H.C.; Cassali, G.D.; Uetanabaro, A.P.T.; Costa, A.M.; Nicoli, J.R.; Neumann, E.; Porto, A.L.F. In vitro and in vivo evaluation of two potential probiotic lactobacilli isolated from cocoa fermentation (Theobroma cacao L.). J. Funct. Foods 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Pessoa, W.F.B.; Melgaço, A.C.C.; De Almeida, M.E.; Ramos, L.P.; Rezende, R.P.; Romano, C.C. In vitro activity of Lactobacilli with probiotic potential isolated from cocoa fermentation against Gardnerella vaginalis. Biomed. Res. Int. 2017, 2017, 3264194. [Google Scholar] [CrossRef] [PubMed]
- Teles Santos, T.; Santos Ornellas, R.M.; Borges Arcucio, L.; Messias Oliveira, M.; Nicoli, J.R.; Villela Dias, C.; Trovatti Uetanabaro, A.P.; Vinderola, G. Characterization of Lactobacilli strains derived from cocoa fermentation in the south of Bahia for the development of probiotic cultures. LWT 2016, 73, 259–266. [Google Scholar] [CrossRef]
- Wulan, R.; Astuti, R.I.; Rukayadi, Y.; Meryandini, A. Evaluation of indigenous Pichia kudriavzevii from cocoa fermentation for a probiotic candidate. Biodiversitas 2021, 22, 1317–1325. [Google Scholar] [CrossRef]
- Pessôa, M.G.; Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pastore, G.M.; Molina, G. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.F.; Melo, T.A.; Santos, D.S.; Rezende, R.P.; Dias, J.C.T.; Romano, C.C. Efficacy of oral administration of lactic acid bacteria isolated from cocoa in a fermented milk preparation: Reduction of colitis in an experimental rat model. Genet. Mol. Res. 2016, 15, 15038097. [Google Scholar] [CrossRef] [PubMed]
- Saito, V.S.T.; dos Santos, T.F.; Vinderola, C.G.; Romano, C.; Nicoli, J.R.; Araújo, L.S.; Costa, M.M.; Andrioli, J.L.; Uetanabaro, A.P.T. Viability and resistance of Lactobacilli isolated from cocoa fermentation to simulated gastrointestinal digestive steps in soy yogurt. J. Food Sci. 2014, 79, M208–M213. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.Z.d.S.; Passos, M.R.; Silva de Macêdo Neres, N.; Almeida, R.S.; Pita, L.S.; Santos, I.A.; Santana Silveira, P.H.; Reis, M.M.; Santos, I.P.; de Oliveira Negrão Ricardo, L.; et al. Antimicrobial Activity of Lactobacillus fermentum TcUESC01 against Streptococcus mutans UA159. Microb. Pathog. 2020, 142, 104063. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Leandro, E.; Ginani, V.C.; de Alencar, E.R.; Pereira, O.G.; Rose, E.C.P.; do Vale, H.M.M.; Pratesi, R.; Hecht, M.M.; Cavalcanti, M.H.; Tavares, C.S.O. Isolation, identification, and screening of lactic acid bacteria with probiotic potential in silage of different species of forage plants, cocoa beans, and artisanal salami. probiotics Antimicrob. Proteins 2021, 13, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Cassimiro, D.M.d.J.; Batista, N.N.; Fonseca, H.C.; Oliveira Naves, J.A.; Coelho, J.M.; Bernardes, P.C.; Dias, D.R.; Schwan, R.F. Wet Fermentation of Coffea canephora by lactic acid bacteria and yeasts using the self-induced anaerobic fermentation (SIAF) method enhances the coffee quality. Food Microbiol. 2023, 110, 104161. [Google Scholar] [CrossRef]
- Nasanit, R.; Satayawut, K. Microbiological study during coffee fermentation of Coffea arabica var. Chiangmai 80 in Thailand. Kasetsart J.-Nat. Sci. 2015, 49, 32–41. [Google Scholar]
- Zavišić, G.; Ristić, S.; Petričević, S.; Janković, D.; Petković, B. Microbial contamination of food: Probiotics and postbiotics as potential biopreservatives. Foods 2024, 13, 2487. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Shahryari, S.; Kharazmi, M.S.; Jafari, S.M. Food applications of probiotic yeasts; focusing on their techno-functional, postbiotic and protective capabilities. Trends Food Sci. Technol. 2022, 128, 278–295. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Penha Rodrigues Pereira, E.; Silva da Graça, J.; Manfrinato Ferreira, B.; Fasura Balthazar, C.; Xavier-Santos, D.; França Bezerril, F.; Magnani, M.; Sant’Ana, A.S. What are the main obstacles to turning foods healthier through probiotics incorporation? A review of functionalization of foods by probiotics and bioactive metabolites. Food Res. Int. 2024, 176, 113785. [Google Scholar] [CrossRef] [PubMed]
| Microbial Genus | Main Species | Probiotic Strains Validated Examples | References |
|---|---|---|---|
| Lactobacillus | Lb. acidophilus, Lb. helveticus, Lb. crispatus, Lb. johnsonii, Lb. delbrueckii | Lb. johnsonii NCC 533, Lb. helveticus R0052, Lb. acidophilus LA-05 | [38,39] |
| Limosilactobacillus | Lim. fermentum, Lim. reuteri | Lim. fermentum ME-3, Lim. reuteri DSM17938, ATCC PTA 4659, and ATCC PTA 6475 | [40,41,42,43] |
| Lacticaseibacillus | Lcb. casei, Lcb. paracasei, Lcb. rhamnosus | Lcb. casei Shirota, Lcb. casei DN-114001, Lcb. rhamnosus GG Lcb. rhamnosus SD4 and SD11 | [44,45,46,47,48] |
| Lactiplantibacillus | Lpb. plantarum | Lpb. plantarum 299v | [49,50,51,52,53,54,55] |
| Levilactobacillus | Lev. brevis | Lev. brevis MK05 | [49,56,57,58,59] |
| Ligilactobacillus | Lig. salivarius | Lig. salivarius UCC118 | [60,61,62,63,64] |
| Lactococcus | L. lactis | L. lactis subsp. lactis CAB701, L. lactis MKL8 | [65,66,67,68,69,70,71,72] |
| Streptococcus | S. salivarius | S. salivarius LAB813 | [73,74] |
| Enterococcus | E. faecium E. faecalis | E. faecium SF68 | [75,76,77,78] |
| Pediococcus | P. acidilactici | P. acidilactici DSM 16342, GR1 and LB-3 | [79,80,81,82] |
| Bacillus | B. clausii B. coagulans | B. coagulans BC30, B. coagulans Unique IS-2 | [83,84,85,86] |
| Bifidobacterium | B. lactis, B. longum, B. infantis, B. bifidum, B. breve, B. adolescentis, B. animalis, B. thermophilum | B. infantis 35624, B. lactis Bi-07, B. lactis Bl-04, B. lactis HN019, B. lactis BB-12, B. breve M-16V | [87,88,89,90,91,92,93,94,95,96] |
| Saccharomyces | S. boulardii | S. boulardii CNCM I-745 | [97,98,99,100,101,102] |
| Kluyveromyces | K. marxianus fragilis | K. marxianicus CBS 6936 | [102,103,104,105,106] |
| Food Source of Isolation | Potentially Probiotic Microorganism | References |
|---|---|---|
| Fermented chili and pear | Lpb. pentosus | [134] |
| Pickles, milk, curd, wheat dough | Lb. acidophilus, Lb. delbrueckii | [135] |
| Artisanal Serpa cheese | Lpb. plantarum, Lpb. pentosus, Lev. brevis | [136] |
| Green olives from Morocco | [137] | |
| Edible cultivated snail Cornu aspersum maxima | Lpb. plantarum | [138] |
| Fermented milk products | [139] | |
| Pickled Chinese cabbage, carrots, and cowpea | [140] | |
| Tangerine vinegar | [133] | |
| Cocoa fermentation | [141,142] | |
| Soybean, pickles, plant-based meat, soy sausage | [143] | |
| Fermented quinoa drink | [144] | |
| Traditional fermented cereal foods from China | [145] | |
| Rotten fruits and vegetables such as apples, grapes, strawberries, tomatoes, cauliflower, and cucumber | [146] | |
| Duimaj-traditional snack food from Iran | Lpb. plantarum, Lpb. pentosus | [147] |
| Harbin sausages | Lev. brevis, Lat. curvatus, Lim. Fermentum, Pediococus pentosaceus | [148] |
| Jangajji-traditional Korean fermented food | Lpb. paraplantarum | [128] |
| Kimchi-traditional Korean fermented food | Weissella cibaria | [131] |
| Chanakh cheese from Armenia | Lcb. rhamnosus, Lb. helveticus, Lb. acidophilus | [149] |
| Teff dough, Kocho, and Ergo- traditional Ethiopian fermented food | Lcb. paracasei, Lpb. plantarum | [150] |
| Vegetable and fruit juices | Leuconostoc mesenteroides, Pediococcus pentosaceus | [151] |
| Traditional Ethiopian foods and beverages (Bulla, Kotcho, Ergo, Bukuri) | Pediococcus pentosaceus, P. acidilactici, L. lactis | [152] |
| Fermented cereal-based foods, fermented pulses, and fermented milk products | Enterococcus durans, Enterobacter faecium, Lpb. plantarum, Lim. fermentum | [153] |
| Saudi Arabia’s raw and fermented milk | Lcb. casei, Lpb. plantarum and E. faecium | [154] |
| Cocoa fermentation | Lim. fermentum, L. lactis subsp. lactis | [28] |
| Traditional fermented foods: rice-based ethnic fermented beverage, chhurpi, Khambir (wheat-based leavened bread) | S. cerevisiae | [155] |
| Shanklish (dried Labanah fermented by fungi), Jordanian green olives | Pichia kudriavzevii, Pichia sp. S cerevisiae | [156] |
| Kefir | Saccharomyces unisporus, Kluyveromyces lactis | [157] |
| Italian virgin olive oil | Candida adriatica, Candida diddensiae, Nakazawaea molendiniolei, N. wickerhamii, Wickerhamomyces anomalus, Yamadazyma terventina | [158] |
| Buffalo Karish cheese | S. cerevisiae. W. anomalus, P. kudriavzevii | [159] |
| Fermented Brazilian table olives | S. cerevisiae, P. guillermondii, C. tropicalis, Meyerozyma caribbica, Debaromyces hansenii | [160] |
| Caxiri (Brazilian indigenous beverage), kefir, and cacao fermentation | S. cerevisiae, Pichia kluyeri | [161] |
| Bacteria Strain(s) | In Vitro Properties | In Vivo Properties | Other Functional Properties | References |
|---|---|---|---|---|
| Lim. fermentum 5.2 Lpb. plantarum 6.2 Lpb. plantarum 7.1 | Surface properties (auto- aggregation and hydrophobicity), Adhesion to vaginal epithelial cells (HMVII), Antimicrobial properties against G. vaginalis | [181] | ||
| Lpb. plantarum (CH3 and CH41) and Lim. fermentum (CH58) | Tolerance to pH 2.0 and bile salt, hydrophobicity, and auto-aggregation capacity | [185] | ||
| Lpb. plantarum CH3 and CH41 | Adhesion properties to Caco-2 cells | |||
| Lim. fermentum CH58 | Antimicrobial activity against antagonistic activity against L. monocytogenes and S. aureus | |||
| Lim. fermentum fermentum TCUESC01 | Resistance to GIT simulated conditions, auto-aggregation, and susceptibility to most antibiotics tested. | Survival after 28-day storage at 4 °C in the milk matrix | [178] | |
| Lpb. plantarum TCUESC02 and Lpb. plantarum TCUESC01 strains | Antagonistic activity against pathogenic bacteria | |||
| Lim. fermentum TCUESC01and Lpb. plantarum TCUESC02 | Antagonism against six pathogenic bacteria (S. enterica var Typhimurium, E. coli, E. faecalis, L. monocytogenes, S. aureus, S. flexneri) | Antagonistic activity against enteropathogen and a lower anti-inflammatory pattern of immune response to infection | [180] | |
| Lpb. plantarum 286 | High resistance to the GIT simulation process | High antimicrobial activity against pathogenic strains (S. enterica var Typhimurium, E. coli, E. faecalis, L. monocytogenes, S. flexi | [182] | |
| L. lactis subsp. lactis CR2 and Lim. fermentum CYF3 | Tolerance to acidic conditions, bile salts No hemolytic activity No DNAse activity | Production of bioactive compounds (antioxidant activity and anti-glycemic) Exopolysaccharide (EPS) production | [28] | |
| Lpb. plantarum Lp03, Lpb. plantarum Lp289, Lpb. plantarum Lp291 | Tolerance pH < 4.5, hydrophobicity, auto-aggregation, co-aggregation, biofilm formation, and antimicrobial activity against pathogens (G. vaginalis and N. gonorrhoeae) | [142] | ||
| Lpb. plantarum 2.1, Lpb. plantarum A2 | Tolerance to acid pH, hydrophobic surface, good auto-aggregation and co-aggregation properties Inhibition of growth of pathogens (Salmonella enteritidis and E. coli EHC) | [141] | ||
| Lim. fermentum and Lpb. plantarum | Low number of leukocytes, reduced histological damage, anti-inflammatory activity in a rat model | [186] | ||
| Lpb. plantarum TCUESC02 and Lim. fermentum TCUESC01 | Tolerance to acid pH, cell viability, and resistance to GIT digestion during soy yogurt storage | [187] | ||
| Lim. fermentum TCUESC01 | Anti-adherence and bactericidal activity against planktonic cells of S. mutans | [188] | ||
| Lim. fermentum C4848.7 and Leu. pseudomesenteroides C4820.3 | No hemolysis, DNase, or gelatinase activity. No biogenic amine production, Co-aggregation, auto-aggregation and adhesion to CaCo-2 cells | [189] |
| Yeast Strain(s) | In Vitro Properties | In Vivo Properties | Other Functional Properties | References |
|---|---|---|---|---|
| K. marxianus, C. orthopsilosis, C. quercitrusa, H. uvarum, H. opuntiae and P. kluyveri, and H. uvarum | Tolerance to pH 2.0, 37 °C, bile salts, auto-aggregation, hydrophobicity, no hemolytic activity | Only strains P. kluyveri CCMA0615 and C. quercitrusa CCMA0560 showed antioxidant activity (DPPH method) and phytate hydrolysis | [161] | |
| P. kluyveri CCMA 0615 | co-aggregation capacity with E. coli EPEC, L. monocytogenes, and S. Enteridis, adhesion properties to Caco-2 cells, 50% inhibition of bacterial infection | [179] | ||
| P. kudriavzevii 2P10 | Tolerance to acidic pH, bile salts, auto-aggregation, and co-aggregation | Antioxidant activity and H2O2 oxidative stress | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Palestino, A.; Gómez-Vargas, R.; Suárez-Quiroz, M.; González-Ríos, O.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Estrada-Beltrán, A.E.; Figueroa-Hernández, C.Y. Probiotic Potential of Lactic Acid Bacteria and Yeast Isolated from Cocoa and Coffee Bean Fermentation: A Review. Fermentation 2025, 11, 95. https://doi.org/10.3390/fermentation11020095
López-Palestino A, Gómez-Vargas R, Suárez-Quiroz M, González-Ríos O, Hernández-Estrada ZJ, Castellanos-Onorio OP, Alonso-Villegas R, Estrada-Beltrán AE, Figueroa-Hernández CY. Probiotic Potential of Lactic Acid Bacteria and Yeast Isolated from Cocoa and Coffee Bean Fermentation: A Review. Fermentation. 2025; 11(2):95. https://doi.org/10.3390/fermentation11020095
Chicago/Turabian StyleLópez-Palestino, Aylin, Regina Gómez-Vargas, Mirna Suárez-Quiroz, Oscar González-Ríos, Zorba Josué Hernández-Estrada, Olaya Pirene Castellanos-Onorio, Rodrigo Alonso-Villegas, Aztrid Elena Estrada-Beltrán, and Claudia Yuritzi Figueroa-Hernández. 2025. "Probiotic Potential of Lactic Acid Bacteria and Yeast Isolated from Cocoa and Coffee Bean Fermentation: A Review" Fermentation 11, no. 2: 95. https://doi.org/10.3390/fermentation11020095
APA StyleLópez-Palestino, A., Gómez-Vargas, R., Suárez-Quiroz, M., González-Ríos, O., Hernández-Estrada, Z. J., Castellanos-Onorio, O. P., Alonso-Villegas, R., Estrada-Beltrán, A. E., & Figueroa-Hernández, C. Y. (2025). Probiotic Potential of Lactic Acid Bacteria and Yeast Isolated from Cocoa and Coffee Bean Fermentation: A Review. Fermentation, 11(2), 95. https://doi.org/10.3390/fermentation11020095








