Bioprocessing of Jackfruit Seeds (Artocarpus heterophyllus Lam.) for Protein Enrichment in Semi-Solid State: Potential for Animal Feed Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fermentation Process
2.3. Factorial Design
2.4. Statistical Analysis
3. Results and Discussions
3.1. Water Content and Water Activity
3.2. Fixed Mineral Residues and Total Soluble Solids
3.3. Protein Content
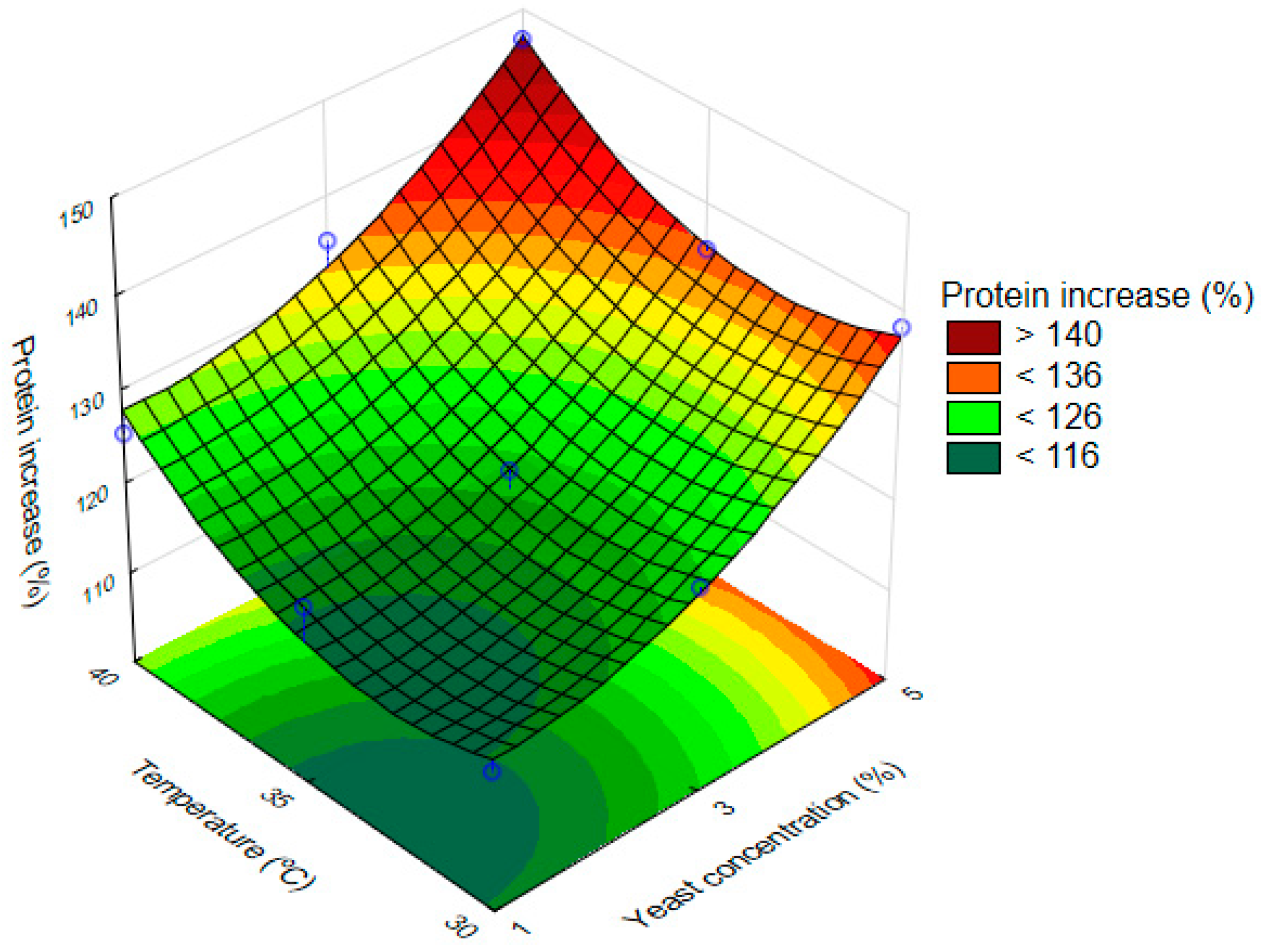
3.4. Optimization of the Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prokic, D.; Stepanov, J.; Curcic, L.; Stojic, N.; Pucarevic, M. The role of circular economy in food waste management in fulfilling the United Nations’ sustainable development goals. Acta Univ. Sapientiae Aliment. 2022, 15, 51–66. [Google Scholar]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin TB, M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- de Oliveira Sousa Wanderley, R.; de Figueirêdo, R.M.F.; de Melo Queiroz, A.J.; dos Santos, F.S.; de França Silva, A.P.; Paiva, Y.F.; Moura, H.V.; de Vilela Silva, E.T.; de Brito Araújo Carvalho, A.J.; dos Santos Lima, M.; et al. Effect of drying temperature on antioxidant activity, phenolic compound profile and hygroscopic behavior of pomegranate peel and seed flours. LWT 2023, 189, 115514. [Google Scholar]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar]
- Kaur, J.; Singh, Z.; Shah, H.M.S.; Mazhar, M.S.; Hasan, M.U.; Woodward, A. Insights into phytonutrient profile and postharvest quality management of jackfruit: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 6756–6782. [Google Scholar]
- Zhang, X.; Zhu, K.; Xie, J.; Chen, Y.; Tan, L.; Liu, S.; Dong, R.; Zheng, Y.; Yu, Q. Optimization and identification of non-extractable polyphenols in the dietary fiber of jackfruit (Artocarpus heterophyllus Lam.) pulp released by alkaline, acid and enzymatic hydrolysis: Content, composition and antioxidant activities. LWT 2021, 138, 110400. [Google Scholar]
- Dhani, S.; Ngobese, N.Z.; Sharma, S.; Jaiswal, A.K. A comprehensive review on nutritional composition, health benefits, and industrial applications of Jackfruit Seeds. J. Agric. Food Res. 2025, 19, 101692. [Google Scholar]
- Waghmare, R.; Memon, N.; Gat, Y.; Gandhi, S.; Kumar, V.; Panghal, A. Jackfruit seed: An accompaniment to functional foods. Braz. J. Food Technol. 2019, 22, e2018207. [Google Scholar] [CrossRef]
- Wanapat, M.; Suriyapha, C.; Dagaew, G.; Prachumchai, R.; Phupaboon, S.; Sommai, S.; Matra, M. The recycling of tropical fruit peel waste-products applied in feed additive for ruminants: Food manufacturing industries, phytonutrient properties, mechanisms, and future applications. J. Agric. Food Res. 2024, 17, 101234. [Google Scholar]
- Sundarraj, A.A.; Ranganathan, T.V. Extraction and characterization of cellulose from jackfruit (Artocarpus integer) peel. J. Exp. Biol. Agric. Sci. 2018, 6, 414–424. [Google Scholar]
- Swami, S.B.; Thakor, N.J.; Haldankar, P.M.; Kalse, S.B. Jackfruit and Its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 565–576. [Google Scholar] [CrossRef]
- Hong, R.; Ting, L.; Huijie, W. Optimization of extraction condition for phytic acid from peanut meal by response surface methodology. Resour.-Effic. Technol. 2017, 3, 226–231. [Google Scholar]
- Li, S.; Li, C.; Chen, S.; Wang, X.; Liu, J.; Deng, X.; Cai, H.; Liu, G. Effects of Solid-State Fermentation on the Standardized Ileal Digestibility of Amino Acids and Apparent Metabolizable Energy in Peanut Meal Fed to Broiler Chickens. Fermentation 2023, 9, 346. [Google Scholar] [CrossRef]
- Gregory; Chan, S. Future production of yeast biomass for sustainable proteins: A critical review. Sustain. Food Technol. 2024, 2, 1592–1609. [Google Scholar]
- Kou, H.; Zheng, J.; Ye, G.; Qiao, Z.; Zhang, K.; Luo, H.; Zou, W. Optimization of Clostridium beijerinckii semi-solid fermentation of rape straw to produce butyric acid by genome analysis. Bioresour. Bioprocess. 2024, 11, 24. [Google Scholar]
- da Silva, A.F.V.; Santos, L.A.D.; de Melo, A.H.F.; Jucá, J.F.T.; de Melo Sales Santos, A.F.; Porto, T.S. Use of Cellulase Obtained from Solid-State Fermentation of Orange and Passion Fruit Peels as an Enzymatic Pre-treatment Step for Anaerobic Digestion. Bioenergy Res. 2024, 17, 1288–1301. [Google Scholar]
- Khurshida, S.; Muchahary, S.; Samyor, D.; Sit, N.; Deka, S.C. Protein enrichment of cassava flour by Saccharomyces cerevisiae fermentation and development of a muffin. Food Meas. 2025, 19, 2438–2448. [Google Scholar] [CrossRef]
- Polyorach, S.; Wanapat, M.; Wanapat, S. Enrichment of protein content in cassava (Manihot esculenta Crantz) by supplementing with yeast for use as animal feed. Emir. J. Food Agric. 2013, 25, 142–149. [Google Scholar]
- de Sousa, A.P.M.; Campos, A.R.N.; Gomes, J.P.; de Santana, R.A.C.; de França Silva, A.P.; de Macedo, A.D.B.; Costa, J.D. Enriquecimento proteico dos resíduos da jaca por fermentação semissólida. Braz. Appl. Sci. Rev. 2020, 4, 987–997. [Google Scholar]
- IAL—Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos; IAL: São Paulo, Brazil, 2008; p. 1020. [Google Scholar]
- Ferreira, J.P.d.L.; Queiroz, A.J.d.M.; Figueirêdo, R.M.F.d.; Silva, W.P.d.; Gomes, J.P.; Santos, D.d.C.; Silva, H.A.; Rocha, A.P.T.; Paiva, A.C.C.d.; Chaves, A.D.C.G.; et al. Utilization of Cumbeba (Tacinga inamoena) Residue: Drying Kinetics and Effect of Process Conditions on Antioxidant Bioactive Compounds. Foods 2021, 10, 788. [Google Scholar] [CrossRef]
- Alcântara, S.R.; Almeida, F.d.A.C.; da Silva, F.L.H.; Gomes, J.P. Isotermas de adsorção do pedúnculo seco do caju. Rev. Bras. De Eng. Agrícola E Ambient. 2009, 13, 81–87. [Google Scholar] [CrossRef]
- Costa, A.R.; Fernandes, H.; Salgado, J.M.; Belo, I. Solid State and Semi-Solid Fermentations of Olive and Sunflower Cakes with Yarrowia lipolytica: Impact of Biological and Physical Pretreatments. Fermentation 2023, 9, 734. [Google Scholar] [CrossRef]
- Figueirôa, J.A.; Menezes Novaes, G.U.; de Souza Gomes, H.; de Morais Silva, V.L.M.; de Morais Lucena, D.; Lima, L.M.R.; de Souza, S.A.; Viana, L.G.F.C.; Rolim, L.A.; da Silva Almeida, J.R.G.; et al. Opuntia ficus-indica is an excellent eco-friendly biosorbent for the removal of chromium in leather industry effluents. Heliyon 2021, 7, e07292. [Google Scholar] [CrossRef]
- Chen, A.; Si, Q.; Xu, Q.; Pan, C.; Chen, J. Evaluation of Stress Tolerance and Fermentation Performance in Commercial Yeast Strains for Industrial Applications. Foods 2025, 14, 142. [Google Scholar] [CrossRef]
- Douradinho, R.; Sica, P.; Tonoli, F.; Mattos, E.; Oliveira, M.; Pinto, A.; Mota, L.; Faria, T.; Costa, V.F.; Leite, G.; et al. Osmotic Stress Alleviation in Saccharomyces cerevisiae for High Ethanol Fermentations with Different Wort Substrates. Stresses 2023, 3, 813–826. [Google Scholar] [CrossRef]
- Sousa, A.P.M.; Campos, A.R.N.; Gomes, J.P.; Santana, R.A.C.; Silva, A.P.F.; Macedo, A.D.B.; Costa, J.D. Protein enrichment of jackfruit peel waste through solid-state fermentation. Rev. Bras. Ciências Agrárias—Braz. J. Agric. Sci. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Knychala, M.M.; Boing, L.A.; Ienczak, J.L.; Trichez, D.; Stambuk, B.U. Precision Fermentation as an Alternative to Animal Protein, a Review. Fermentation 2024, 10, 315. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Rossetti Rogerio, M.F.; Bassinello, P.Z.; Oomah, B.D. The use of fermentation in the valorization of pulses by-products. Trends Food Sci. Technol. 2025, 159, 104957. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Dimou, C.; Konstantina, N.; Dimou, C.M. Designing, developing and optimizing a two-stage solid state fermentation process through valorization of cereal milling and legume by-products and waste streams for the production of novel functional protein rich type-miso nutri-powder, using Aspergillus oryzae. Biomed. J. Sci. Tech. Res. 2022, 44, 35763–35771. [Google Scholar]
- Gregório, M.; Araújo, M.; Albuquerque, A.; Rodrigues, T.; Santos, N.C.; Fonseca, M.T.; Costa, M.E.d.; Tomé, A.; Gomes, J.; Gouveia, D.; et al. Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity. Foods 2024, 13, 3551. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Wang, G.; Jing, Y.; Jiao, C.; Sun, L.; Huang, H.; Li, Y.; Zhang, J. Enhancement of Apostichopus japonicus peptide flavor through bacterial and enzyme co-fermentation (BECF) and the identification of novel antioxidant peptides in the fermented product. Food Chem. X 2025, in press. [Google Scholar]
- Jian, C.; Yang, X.; Tuccillo, F.; Hashim, M.; Cera, S.; Yan, J.-K.; Coda, R.; Maina, N.H.; Katina, K.; Wang, Y. Impact of fermentation conditions and dextran structure on the rheological and textural properties of a novel high-protein, high-fiber and low-fat plant-based cheese. Food Hydrocoll. 2025, 164, 111209. [Google Scholar]
| Exp. | Yeast Concentration | Temperature | ||
|---|---|---|---|---|
| Coded Value | Real Value (%) | Coded Value | Real Value (°C) | |
| 1 | −1 | 1 | −1 | 30 |
| 2 | −1 | 1 | 0 | 35 |
| 3 | −1 | 1 | +1 | 40 |
| 4 | 0 | 3 | −1 | 30 |
| 5 | 0 | 3 | 0 | 35 |
| 6 | 0 | 3 | +1 | 40 |
| 7 | +1 | 5 | −1 | 30 |
| 8 | +1 | 5 | 0 | 35 |
| 9 | +1 | 5 | +1 | 40 |
| 10 | 0 | 3 | 0 | 35 |
| Experiments | Water Content (%) | Water Activity | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| 1 | 60.10 ± 0.40 | 42.34 ± 0.81 | 0.9883 ± 0.002 | 0.9149 ± 0.014 |
| 2 | 57.19 ± 0.25 | 33.11 ± 0.25 | 0.9883 ± 0.002 | 0.8110 ± 0.041 |
| 3 | 58.52 ± 0.25 | 39.72 ± 1.41 | 0.9881 ± 0.002 | 0.6429 ± 0.042 |
| 4 | 60.10 ± 0.40 | 43.33 ± 1.08 | 0.9883 ± 0.002 | 0.9023 ± 0.018 |
| 5 | 57.19 ± 0.25 | 33.70 ± 0.89 | 0.9881 ± 0.002 | 0.7606 ± 0.012 |
| 6 | 58.52 ± 0.25 | 33.51 ± 0.04 | 0.9858 ± 0.002 | 0.6352 ± 0.204 |
| 7 | 60.10 ± 0.40 | 44.22 ± 0.06 | 0.9883 ± 0.002 | 0.8975 ± 0.008 |
| 8 | 57.19 ± 0.25 | 30.76 ± 0.34 | 0.9883 ± 0.002 | 0.7553 ± 0.012 |
| 9 | 58.52 ± 0.25 | 32.19 ± 0.56 | 0.9881 ± 0.002 | 0.6071 ± 0.025 |
| 10 | 57.19 ± 0.25 | 32.08 ± 0.10 | 0.9881 ± 0.002 | 0.7202 ± 0.007 |
| Experiments | Fixed Mineral Waste (%) | Total Soluble Solids (°Brix) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| 1 | 3.18 ± 0.06 | 7.44 ± 0.01 | 45.36 ± 0.44 | 8.27 ± 0.52 |
| 2 | 3.20 ± 0.02 | 6.31 ± 0.07 | 36.44 ± 0.42 | 6.26 ± 0.29 |
| 3 | 2.77 ± 0.14 | 7.23 ± 0.27 | 32.79 ± 0.42 | 5.54 ± 0.17 |
| 4 | 3.78 ± 0.26 | 7.51 ± 0.21 | 43.86 ± 0.15 | 7.77 ± 0.17 |
| 5 | 3.83 ± 0.37 | 6.54 ± 0.05 | 36.91 ± 0.28 | 5.49 ± 0.70 |
| 6 | 2.96 ± 0.09 | 7.47 ± 0.34 | 35.56 ± 0.21 | 5.01 ± 0.15 |
| 7 | 3.21 ± 0.03 | 7.44 ± 0.06 | 40.85 ± 0.24 | 6.09 ± 0.75 |
| 8 | 3.18 ± 0.09 | 6.54 ± 0.05 | 36.01 ± 0.64 | 4.74 ± 0.42 |
| 9 | 3.06 ± 0.03 | 7.47 ± 0.03 | 39.85 ± 0.61 | 4.22 ± 0.17 |
| 10 | 2.83 ± 0.06 | 6.54 ± 0.07 | 36.56 ± 0.92 | 7.71 ± 0.42 |
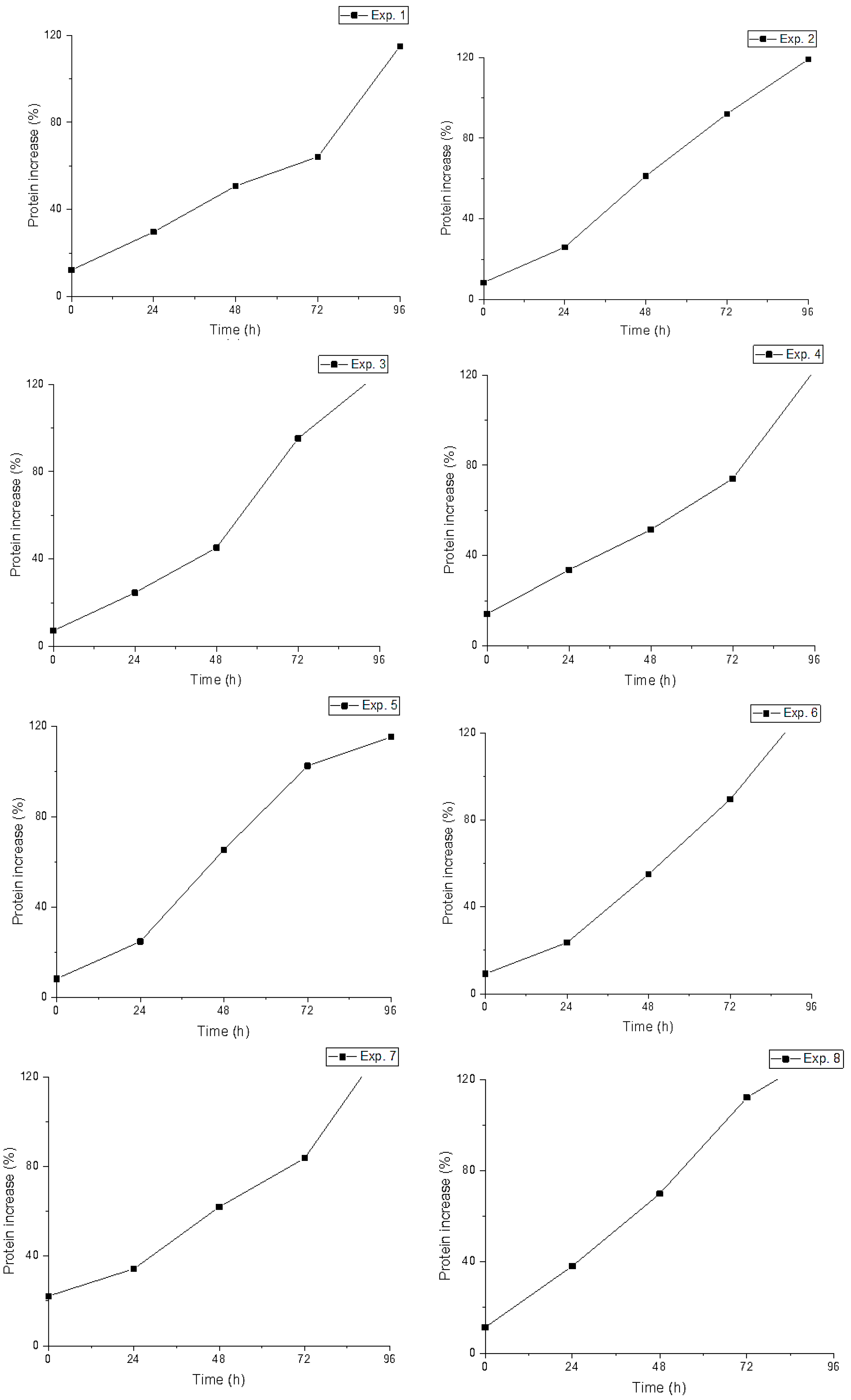
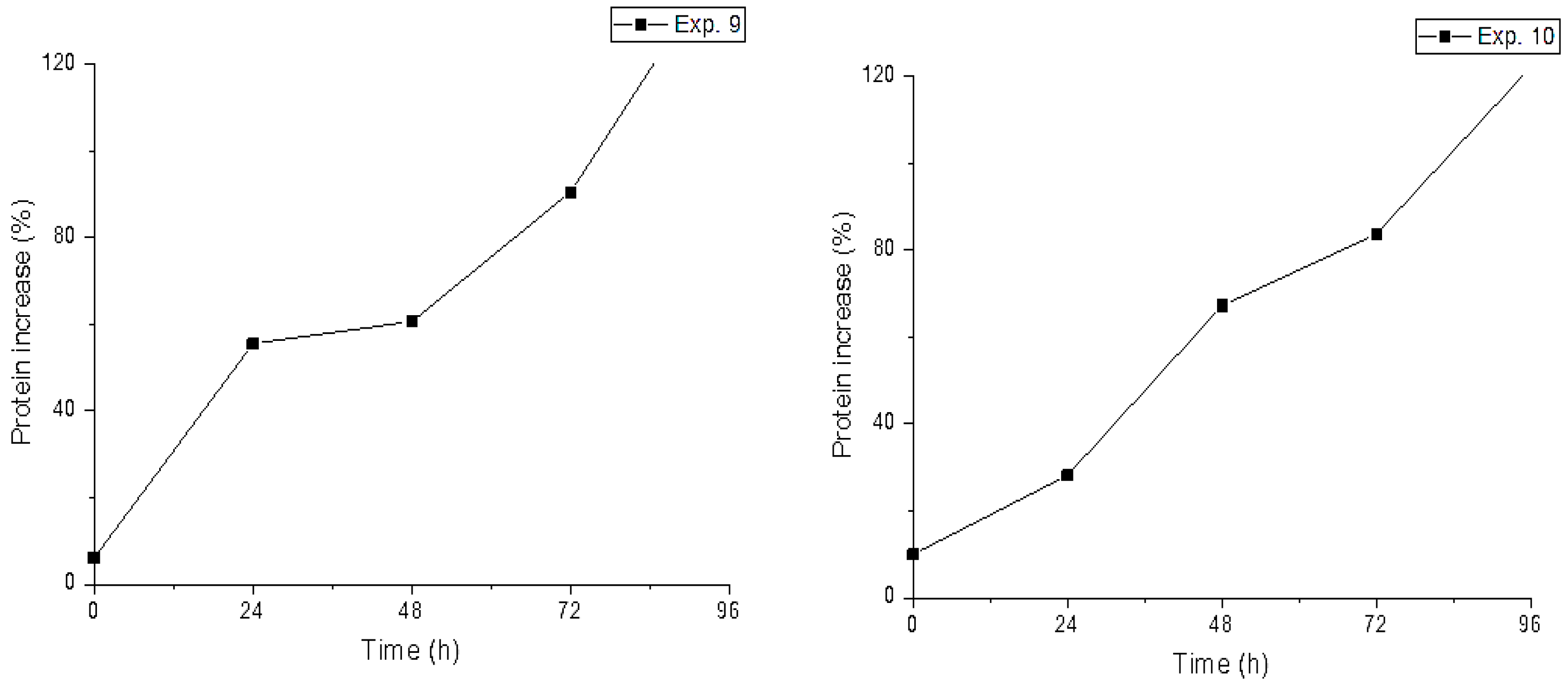
| Exp. | YC (%) | Temp (°C) | Crude Protein (%) | Protein Increase (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | ||||
| 1 | 1 | 30 | 14.5 ± 0.6 | 16.9 ± 0.7 | 19.6 ± 0.6 | 21.4 ± 0.7 | 28.5 ± 0.2 | 115.0 |
| 2 | 1 | 35 | 13.9 ± 0.6 | 16.5 ± 0.2 | 20.6 ± 1.2 | 25.1 ± 1.2 | 27.4 ± 1.5 | 119.1 |
| 3 | 1 | 40 | 13.5 ± 0.4 | 15.6 ± 0.3 | 18.6 ± 0.6 | 25.1 ± 1.5 | 29.2 ± 1.4 | 125.3 |
| 4 | 3 | 30 | 14.6 ± 0.5 | 17.4 ± 0.3 | 19.7 ± 0.2 | 22.7 ± 1.1 | 28.6 ± 0.1 | 122.2 |
| 5 | 3 | 35 | 13.9 ± 0.6 | 15.8 ± 0.6 | 21.1 ± 0.7 | 25.1 ± 0.9 | 27.9 ± 0.4 | 115.3 |
| 6 | 3 | 40 | 13.8 ± 1.1 | 15.8 ± 0.4 | 19.7 ± 0.6 | 23.8 ± 0.6 | 30.5 ± 0.9 | 135.2 |
| 7 | 5 | 30 | 15.9 ± 0.4 | 17.5 ± 0.3 | 20.8 ± 0.7 | 23.5 ± 1.1 | 31.0 ± 1.3 | 138.2 |
| 8 | 5 | 35 | 14.2 ± 0.6 | 17.7 ± 0.7 | 22.0 ± 0.5 | 27.4 ± 0.6 | 30.4 ± 0.6 | 135.1 |
| 9 | 5 | 40 | 13.4 ± 1.0 | 20.1 ± 0.7 | 19.5 ± 0.3 | 24.4 ± 0.7 | 32.1 ± 0.7 | 146.9 |
| 10 | 3 | 35 | 14.2 ± 0.8 | 16.6 ± 1.3 | 21.9 ± 0.5 | 23.8 ± 1.0 | 28.6 ± 1.1 | 122.1 |
| Fator | Quadratic Sum | Degree of Freedom | Quadratic Mean | F | p |
|---|---|---|---|---|---|
| YC (L) | 616.107 | 1 | 616.01106 | 51.01106 | 0.000836 |
| YC (Q) | 111.04 | 1 | 111.044 | 0.48002 | 0.519270 |
| T (L) | 170.667 | 1 | 170.6667 | 14.13049 | 0.013170 |
| T (Q) | 105.190 | 1 | 105.1905 | 8.70933 | 0.031843 |
| Erro | 60.390 | 5 | 12.0779 | ||
| Total SS | 1045.604 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, A.P.M.; Campos, A.R.N.; Gomes, J.P.; de Santana, R.A.C.; Queiroz, A.J.d.M.; de Figueirêdo, R.M.F.; Gregório, M.G.; Santos, N.C.; Silva, W.P.d.; Gomes, M.M.d.A.; et al. Bioprocessing of Jackfruit Seeds (Artocarpus heterophyllus Lam.) for Protein Enrichment in Semi-Solid State: Potential for Animal Feed Production. Fermentation 2025, 11, 185. https://doi.org/10.3390/fermentation11040185
de Sousa APM, Campos ARN, Gomes JP, de Santana RAC, Queiroz AJdM, de Figueirêdo RMF, Gregório MG, Santos NC, Silva WPd, Gomes MMdA, et al. Bioprocessing of Jackfruit Seeds (Artocarpus heterophyllus Lam.) for Protein Enrichment in Semi-Solid State: Potential for Animal Feed Production. Fermentation. 2025; 11(4):185. https://doi.org/10.3390/fermentation11040185
Chicago/Turabian Stylede Sousa, Ana Paula Moisés, Ana Regina Nascimento Campos, Josivanda Palmeira Gomes, Renato Alexandre Costa de Santana, Alexandre Jose de Melo Queiroz, Rossana Maria Feitosa de Figueirêdo, Mailson Gonçalves Gregório, Newton Carlos Santos, Wilton Pereira da Silva, Michael Marcos de Aquino Gomes, and et al. 2025. "Bioprocessing of Jackfruit Seeds (Artocarpus heterophyllus Lam.) for Protein Enrichment in Semi-Solid State: Potential for Animal Feed Production" Fermentation 11, no. 4: 185. https://doi.org/10.3390/fermentation11040185
APA Stylede Sousa, A. P. M., Campos, A. R. N., Gomes, J. P., de Santana, R. A. C., Queiroz, A. J. d. M., de Figueirêdo, R. M. F., Gregório, M. G., Santos, N. C., Silva, W. P. d., Gomes, M. M. d. A., Araújo, M. A., dos Santos, F. S., Adelino de Melo, B., Moura, H. V., & Paiva, Y. F. (2025). Bioprocessing of Jackfruit Seeds (Artocarpus heterophyllus Lam.) for Protein Enrichment in Semi-Solid State: Potential for Animal Feed Production. Fermentation, 11(4), 185. https://doi.org/10.3390/fermentation11040185








