Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Pretreatment
2.2. Physicochemical Characterization of Creosote Bush
2.3. Micro-Organism and Culture Medium
2.4. SSF Conditions and Bioactive Compounds Recovery (Box–Behnken Design)
2.5. HPLC-ESI-MS Analysis
2.6. Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Creosote Bush Leaves
3.2. Solid State Fermentation Conditions and Bioactive Compound Recovery (Box–Behnken Design)
3.3. HPLC-MS Analysis of Compounds in Fermented Extracts of Creosote Bush Leaves
3.4. Antioxidant Activity of Fermentation Extracts from Creosote Bush Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSF | Solid state fermentation |
| NDGA | Nordihydroguaiaretic acid |
| EA | Ellagic acid |
| WAC | Water absorption capacity |
| HPLC-ESI-MS | High-performance liquid chromatography electrospray ionization mass spectrometry |
| HT | Hydrolyzable tannins |
| CT | Condensed tannins |
References
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Kwok, A.L.X.; Balasooriya, H.; Ng, K. Efficacy of ellagic acid and ellagitannins on diabetes mellitus: A meta-analysis of preclinical and clinical trials. Food Biosci. 2023, 53, 102573. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Santini, A.; Marletta, L. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Guan, X.L.; Li, J.; Zhang, Y.J.; He, R.J.; Huang, Y.; Liu, B.M.; Zhou, D.X.; Deng, S.P.; Chen, H.C.; et al. Antimicrobial lignans derived from the roots of Streblus asper. Phytochem. Lett. 2016, 18, 226–231. [Google Scholar] [CrossRef]
- Vollaro, A.; Catania, M.R.; Iesce, M.R.; Sferruzza, R.; D’Abrosca, B.; Donnarumma, G.; De Filippis, A.; Cermola, F.; Della Greca, M.; Buommino, E. Antimicrobial and anti-biofilm properties of novel synthetic lignan-like compounds. New Microbiol. 2019, 42, 21–28. [Google Scholar] [PubMed]
- Reyes-Melo, K.Y.; Galván-Rodrigo, A.A.; Martínez-Olivo, I.E.; Núñez-Mojica, G.; Ávalos-Alanís, F.G.; García, A.; del Rayo Camacho-Corona, M. Larrea tridentata and its Biological Activities. Curr. Top. Med. Chem. 2021, 21, 2352–2364. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Waseem, M.; Majeed, Y.; Nadeem, T.; Naqvi, L.H.; Khalid, M.A.; Sajjad, M.M.; Sultan, M.; Khan, M.U.; Khayrullin, M.; Shariati, M.A.; et al. Conventional and advanced extraction methods of some bioactive compounds with health benefits of food and plant waste: A comprehensive review. Food Front. 2023, 4, 1681–1701. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green. Sustain. Chem. 2021, 27, 100407. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Cantún, D.; Ramos-Cassellis, M.E.; Marín-Castro, M.A.; Castelan-Vega, R.D.C. Secondary metabolites and antioxidant activity of the solid-state fermentation in apple (Pirus malus L.) and agave mezcalero (Agave angustifolia H.) bagasse. J. Fungi 2020, 6, 137. [Google Scholar] [CrossRef]
- Di Fidio, N.; Carmassi, L.; Kasmiarti, G.; Fulignati, S.; Licursi, D.; Galleti, A.N.R.; Antonetti, C. Chemical and enzymatic hydrolysis of waste wheat bran to sugars and their simultaneous biocatalytic conversion to valuable carotenoids and lipids. Catal. Today 2024, 442, 114941. [Google Scholar] [CrossRef]
- Di Fidio, N.; Tozzi, F.; Martinelli, M.; Licursi, D.; Fulignati, S.; Antonetti, C.; Raspolli Galleti, A.M. Sustainable Valorisation and Efficient Downstream Processing of Giant Reed by High-Pressure Carbon Dioxide Pretreatment. ChemPlusChem 2022, 87, e202200189. [Google Scholar] [CrossRef]
- D’Orsi, R.; Di Fidio, N.; Antonetti, C.; Raspolli Galletti, A.M.; Operamolla, A. Isolation of pure lignin and highly digestible cellulose from defatted and steam-exploded Cynara cardunculus. ACS Sustain. Chem. Eng. 2023, 11, 1875–1887. [Google Scholar] [CrossRef]
- Hernández-Baez, I.; García-López, J.C.; Espinosa-Reyes, G.; Lee-Rangel, H.A.; Faz-Colunga, D.Á.; Pinos-Rodríguez, J.M. Biomasa de gobernadora (Larrea tridentata) como forraje para borregos. Rev. Chapingo Ser. Zonas Áridas 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Sagaste, C.A.; Montero, G.; Coronado, M.A.; Ayala, J.R.; León, J.Á.; García, C.; Rojano, B.A.; Rosales, S.; Montes, D.G. Creosote Bush (Larrea tridentata) extract assessment as a green antioxidant for biodiesel. Molecules 2019, 24, 1786. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Avila-Ramos, F.; Aquino-Torres, E.; Prieto-Méndez, J.; Hetta, H.F.; Zaragoza-Bastida, A. Bactericidal activity of Larrea tridentata hydroalcoholic extract against phytopathogenic bacteria. Agronomy 2021, 11, 957. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda, L.; Morlett-Chávez, J.; Meléndez-Renteria, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger GH1 to enhance polyphenolic content and antioxidative activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Boondaeng, A.; Keabpimai, J.; Trakunjae, C.; Vaithanomsat, P.; Srichola, P.; Niyomvong, N. Cellulase production under solid-state fermentation by Aspergillus sp. IN5: Parameter optimization and application. Heliyon 2024, 10, e26601. [Google Scholar] [CrossRef] [PubMed]
- Osmolovskiy, A.A.; Popova, E.A.; Kreyer, V.G.; Baranova, N.A.; Egorov, N.S. Vermiculite as a new carrier for extracellular protease production by Aspergillus spp. under solid-state fermentation. Biotechnol. Rep. 2021, 29, e00576. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Urrutia, C.; Volke-Sepulveda, T.; Figueroa-Martinez, F.; Favela-Torres, E. Solid-state fermentation enhances inulinase and invertase production by Aspergillus brasiliensis. Process Biochem. 2021, 108, 169–175. [Google Scholar] [CrossRef]
- Paz-Arteaga, S.L.; Cadena-Chamorro, E.; Serna-Cock, L.; Torres-Castañeda, H.; Pabón-Rodríguez, O.V.; Agudelo-Morales, C.E.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Torres-León, C. Dual emerging applications of solid-state fermentation (SSF) with Aspergillus niger and ultrasonic-assisted extraction (UAE) for the obtention of antimicrobial polyphenols from pineapple waste. Fermentation 2023, 9, 706. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdes, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Chávez-González, M.L. Comparative extraction study of grape pomace bioactive compounds by submerged and solid-state fermentation. J. Chem. Technol. Biotechnol. 2022, 97, 1494–1505. [Google Scholar] [CrossRef]
- Islam, S.; Miah, M.A.S.; Islam, M.F.; Bhuiyan, M.N.I.; Tisa, K.J.; Naim, M.R. Exploring the effects of spontaneous and solid-state fermentation on the physicochemical, functional and structural properties of whole wheat flour (Triticum aestivum L.). Innov. Food Sci. Emerg. Technol. 2024, 97, 103798. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Torres-Leon, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Recovery of ellagic acid from Mexican rambutan peel by solid-state fermentation-assisted extraction. Food Bioprod. Process 2022, 134, 86–94. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Li, Y.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Supplemental UV-A affects growth and antioxidants of Chinese kale baby-leaves in artificial light plant factory. Horticulturae 2021, 7, 294. [Google Scholar] [CrossRef]
- Gu, L.B.; Zhang, G.J.; Du, L.; Du, J.; Qi, K.; Zhu, X.L.; Zhang, X.Y.; Jiang, Z.H. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method. LWT 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Jančík, F.; Kubelková, P.; Loučka, R.; Jambor, V.; Kumprechtová, D.; Homolka, P.; Koukolová, V.; Tyrolová, Y.; Výborná, A. Shredlage Processing Affects the Digestibility of Maize Silage. Agronomy 2022, 12, 1164. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Di Daniele, F.; Campo, M.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Marrone, G.; Romani, A. Effect of hydrolysable tannins and anthocyanins on recurrent urinary tract infections in nephropathic patients: Preliminary data. Nutrients 2021, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Estrada-Gil, L.E.; Torres-León, C.; Chávez-González, M.L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Enhancing the Release of Ellagic Acid from Mexican Rambutan Peel Using Solid-State Fermentation. Biomass 2024, 4, 1005–1016. [Google Scholar] [CrossRef]
- Ordoñez-Torres, A.; Torres-León, C.; Hernández-Almanza, A.; Flores-Guía, T.; Luque-Contreras, D.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-microwave-assisted extraction of polyphenolic compounds from Mexican “Ataulfo” mango peels: Antioxidant potential and identification by HPLC/ESI/MS. Phytochem. Anal. 2021, 32, 495–502. [Google Scholar] [CrossRef]
- López-Cárdenas, F.; Ochoa-Reyes, E.; Baeza-Jiménez, R.; Tafolla-Arellano, J.C.; Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J. Solid-State Fermentation as a Sustainable Tool for Extracting Phenolic Compounds from Cascalote Pods. Fermentation 2023, 9, 823. [Google Scholar] [CrossRef]
- Coronado-Contreras, S.A. Evaluación Sobre la Producción de ácido Nordihidroguaiarético a Partir de la Fermentación en Estado sólido de Hojas de Larrea tridentata (Sessé & Moc. Ex DC.) por Aspergillus niger GH1. Bachelor’s Thesis, Universidad Autónoma de Coahuila, Saltillo, Mexico. September 2020.
- Vauris, A.; Valcauda, S.; Husson, F.; De Coninck, J. A novel method to assess heat transfer and impact of relevant physicochemical parameters for the scaling up of solid state fermentation systems. Biotechnol. Rep. 2022, 36, e00764. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Gharsallaoui, A.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, Q.; Ren, A.; Ge, L.; He, J.; Zhao, M.; He, Q. Roles of water in improved production of mycelial biomass and lignocellulose-degrading enzymes by water-supply solid-state fermentation of Ganoderma lucidum. J. Biosci. Bioeng. 2022, 133, 126–132. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Carboué, Q.; Rébufa, C.; Hamrouni, R.; Roussos, S.; Bombarda, I. Statistical approach to evaluate effect of temperature and moisture content on the production of antioxidant naphtho-gamma-pyrones and hydroxycinnamic acids by Aspergillus tubingensis in solid-state fermentation. Bioprocess. Biosyst. Eng. 2020, 43, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Arely Prado-Barragán, L. Improved extraction of high value-added polyphenols from pomegranate peel by solid-state fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of grapefruit by-products as solid support for solid-state fermentation to produce antioxidant bioactive extracts. Waste Biomass Valorization 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Guía-García, J.L.; López-Romero, J.C.; Torres-Moreno, H.; Moo-Huchin, V.M.; García-Munguía, A.M.; Charles-Rodríguez, A.V. Rhus microphylla leaves extracts obtained by ohmic heating: Physicochemical composition and bioactive properties. Ind. Crops Prod. 2024, 213, 118417. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef]
- Peciulyte, A.; Pisano, M.; de Vries, R.P.; Olsson, L. Hydrolytic potential of five fungal supernatants to enhance a commercial enzyme cocktail. Biotechnol. Lett. 2017, 39, 1403–1411. [Google Scholar] [CrossRef]
- Kango, N.; Jana, U.K.; Choukade, R. Fungal enzymes: Sources and biotechnological applications. In Advancing Frontiers in Mycology & Mycotechnology; Springer: Singapore, 2019; pp. 515–538. [Google Scholar]
- Namnuch, N.; Thammasittirong, A.; Thammasittirong, S.N.R. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology 2021, 12, 119–127. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Gutierrez-Sánchez, G.; Prado-Barragán, L.A.; Rodríguez-Herrera, R.; Aguilar-Zárate, P.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Tafolla-Arellano, J.C.; Aguilar, C.N. Influence of culture conditions on ellagitannase expression and fungal ellagitannin degradation. Bioresour. Technol. 2021, 337, 125462. [Google Scholar] [CrossRef]
- Godoy, M.G.; Amorim, G.M.; Barreto, M.S.; Freire, D.M. Agricultural residues as animal feed: Protein enrichment and detoxification using solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–256. [Google Scholar]
- García, J.E.; Gómez, L.; Mendoza-de-Gives, P.; Rivera-Corona, J.L.; Millán-Orozco, J.; Ascacio, J.A.; Mellado, M. Anthelmintic efficacy of hydro-methanolic extracts of Larrea tridentata against larvae of Haemonchus contortus. Trop. Anim. Health Prod. 2018, 50, 1099–1105. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Deng, Y.T.; Liang, G.; Shi, Y.; Li, H.L.; Zhang, J.; Mao, X.M.; Fu, Q.R.; Peng, W.X.; Chen, Q.X.; Shen, D.Y. Condensed tannins from Ficus altissima leaves: Structural, antioxidant, and antityrosinase properties. Process Biochem. 2016, 51, 1092–1099. [Google Scholar] [CrossRef]

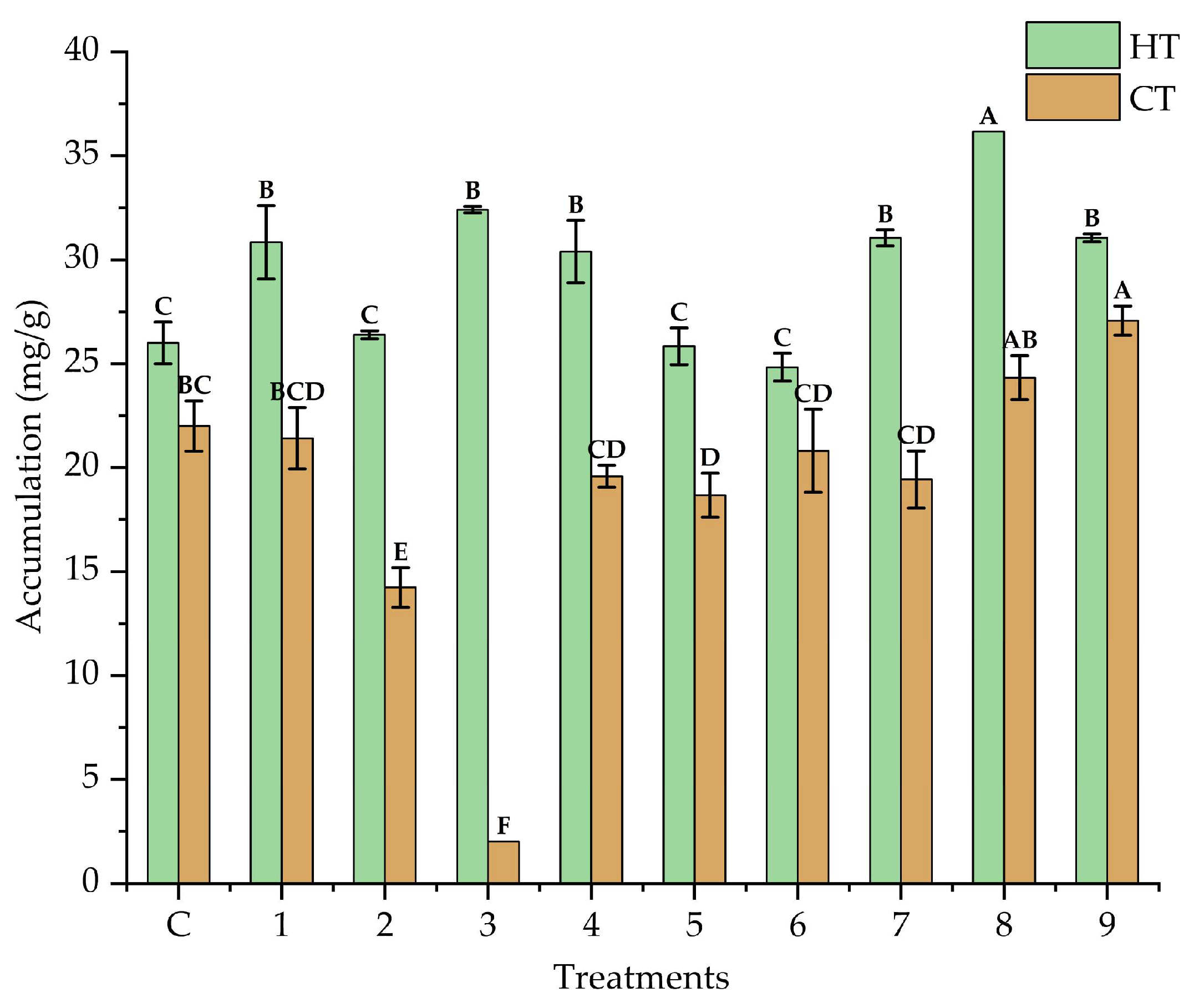

| Treatment | pH | MgSO4 | |
|---|---|---|---|
| 1 | −1 | −1 | |
| 2 | −1 | 0 | |
| 3 | −1 | 1 | |
| 4 | 0 | −1 | |
| 5 | 0 | 0 | |
| 6 | 0 | 1 | |
| 7 | 1 | −1 | |
| 8 | 1 | 0 | |
| 9 | 1 | 1 | |
| Factor | Levels | ||
| −1 | 0 | 1 | |
| pH | 4.5 | 5 | 5.5 |
| MgSO4 (g L−1) | 0.26 | 0.76 | 1.26 |
| Parameters | Results |
|---|---|
| Moisture (%) | 5.00 ± 0.00 |
| Solids (%) | 95.00 ± 0.00 |
| Water absorption capacity (g of gel/g of dry weight) | 3.62 ± 0.02 |
| Maximum moisture of the support/substrate (%) | 73.76 ± 0.01 |
| Fat content (%) | 5.11 ± 0.08 |
| Fiber content (%) | 16.82 ± 3.47 |
| Protein content (%) | 1.28 ± 0.01 |
| Total sugar content (%) | 37.06 ± 0.09 |
| Ash content (%) | 9.41 ± 0.87 |
| Retention time (min) | Mass [M-H]− | Compound | Family |
|---|---|---|---|
| 22.451 | 304.9 | (+)-Gallocatechin | Catechins |
| 25.562 | 283.9 | Methylgalangin | Methoxyflavonols |
| 27.071 | 288.9 | (+)-Catechin | Catechins |
| 29.740 | 754.7 | Quercetin 3-O-rhamnosyl-rhamnosyl-glucoside | Flavonols |
| 31.425 | 305.0 | (−)-Epigallocatechin | Catechins |
| 33.215 | 608.9 | Quercetin 3-O-rutinoside | Flavonols |
| 35.462 | 596.9 | Delphinidin 3-O-sambubioside | Anthocyanins |
| 38.108 | 333.0 | Gallic acid 4-O-glucoside | Hydroxybenzoic acids |
| 39.529 | 386.9 | Medioresinol | Lignans |
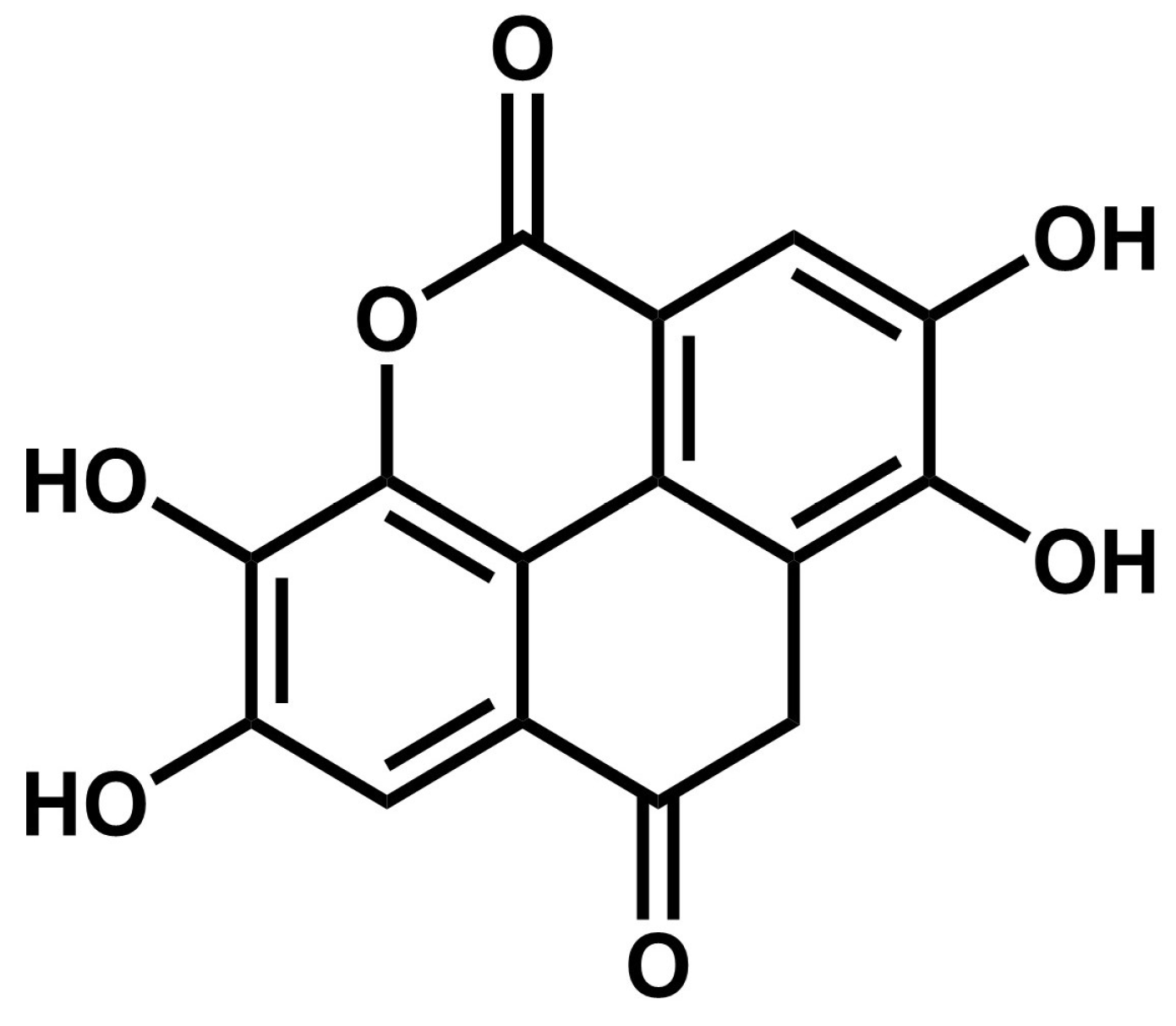
| 43.635 | 300.9 | Ellagic acid (EA) | Hydroxybenzoic acids |
| 47.439 | 330 | 3,7-Dimethylquercetin | Methoxyflavonols |
| 49.317 | 298.9 | 4-Hydroxybenzoic acid 4-O-glucoside | Hydroxybenzoic acids |
| 51.841 | 347.0 | 5-Heptadecylresorcinol | Alkylphenols |
| 54.047 | 301.1 | Nordihydroguaiaretic acid (NDGA) | Lignans |
| 56.551 | 313.0 | Cirsimaritin | Methoxyflavones |
| 58.322 | 630.7 | Pelargonidin 3,5-O-diglucoside | Anthocyanins |
| Antioxidant Assay | Inhibition (%) |
|---|---|
| DPPH | 55.69 ± 1.51 |
| ABTS | 84.84 ± 1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascacio-Valdés, A.; Barrera-Martínez, C.L.; Ascacio-Valdés, J.A.; Sepúlveda, L. Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1. Fermentation 2025, 11, 229. https://doi.org/10.3390/fermentation11040229
Ascacio-Valdés A, Barrera-Martínez CL, Ascacio-Valdés JA, Sepúlveda L. Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1. Fermentation. 2025; 11(4):229. https://doi.org/10.3390/fermentation11040229
Chicago/Turabian StyleAscacio-Valdés, Alonso, Cynthia L. Barrera-Martínez, Juan A. Ascacio-Valdés, and Leonardo Sepúlveda. 2025. "Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1" Fermentation 11, no. 4: 229. https://doi.org/10.3390/fermentation11040229
APA StyleAscacio-Valdés, A., Barrera-Martínez, C. L., Ascacio-Valdés, J. A., & Sepúlveda, L. (2025). Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1. Fermentation, 11(4), 229. https://doi.org/10.3390/fermentation11040229







