Chemical Analysis of the Sugar Moiety of Monohexosylceramide Contained in Koji, Japanese Traditional Rice Fermented with Aspergillus
Abstract
:1. Introduction
2. Material and Method
2.1. Materials
2.2. Thin Layer Chromatograph (TLC) Analysis
2.3. Extraction and Purification of Monohexosylceramide
2.4. Acid Hydrolysis of Monohexosylceramide
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
3. Results and Discussion
3.1. Purification of Monohexosylceramide from Koji
3.2. Acid Hydrolysis of Monohexosylceramide




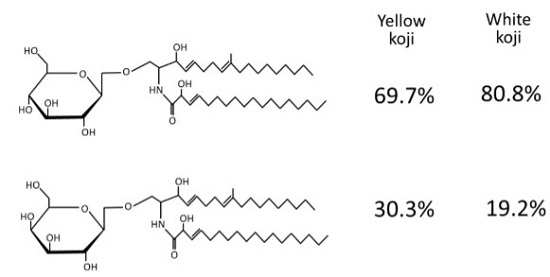
3.3. Quantitation of Sugars which Constitutes Koji Monohexosylceramide


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitagaki, H.; Kitamoto, K. Breeding researches of sake yeasts in Japan: History, recent technological advances, and future perspectives. Annu. Rev. Food. Sci. Technol. 2013, 4, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Yamada, O.; Samson, R.A. Taxonomic re-evaluation of black koji molds. Appl. Microbiol. Biotechnol. 2014, 98, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tadenuma, M.; Sato, S. Studies on the colorants in saké: Presence of ferrichrysin as iron containing colorant in sake. Agric. Biol. Chem. 1967, 31, 1482–1489. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Cereal based functional food of Indian subcontinent: A review. J. Food Sci. Technol. 2012, 49, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Tsuyama, Y.; Hirata, T.; Shiraishi, S.; Sakamoto, K.; Yamada, O.; Akita, O.; Shimoi, H. Identification of a gene involved in the synthesis of a dipeptidyl peptidase IV inhibitor in Aspergillus oryzae. Appl. Environ. Microbiol. 2012, 78, 6996–7002. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.; Challenger, F. The formation of kojic acid (3-hydroxy-6-hydroxymethyl-gamma pyrone) from ethyl alcohol by Aspergillus oryzae. Biochem. J. 1946, 40, lxii. [Google Scholar] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Many ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H.; Cowart, L.A.; Matmati, N.; vaena de Avalos, S.; Novgorodov, S.A.; Zeidan, Y.H.; Bielawski, J.; Obeid, L.M.; Hannun, Y.A. Isc1 regulates sphingolipid metabolism in yeast mitochondria. Biochim. Biophys. Acta 2007, 1768, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Tsuge, K.; Jayakody, L.N.; Urano, Y.; Sawada, K.; Inaba, S.; Nagao, K.; Kitagaki, H. Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by Aspergillus kawachii. J. Agric. Food. Chem. 2012, 60, 11473–11482. [Google Scholar] [CrossRef] [PubMed]
- Fujino, Y.; Ohnishi, M. Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 1976, 486, 161–171. [Google Scholar] [PubMed]
- Tani, Y.; Amaishi, Y.; Funatsu, T.; Ito, M.; Itonori, S.; Hata, Y.; Ashida, H.; Yamamoto, K. Structural analysis of cerebrosides from Aspergillus fungi: The existence of galactosylceramide in A. oryzae. Biotechnol. Lett. 2014, 36, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Uchiyama, R.; Yamauchi, S.; Katayama, T.; Itonori, S.; Sugita, M.; Hada, N.; Yamada-Hada, J.; Takeda, T.; Kumagai, H.; et al. Newly discovered neutral glycosphingolipids in aureobasidin A-resistant zygomycetes: Identification of a novel family of Gala-series glycolipids with core Gal alpha 1–6Gal beta 1–6Gal beta sequences. J. Biol. Chem. 2004, 279, 32028–32034. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Sato, T.; Hamajima, H.; Jayakody, L.N.; Hirata, M.; Yamashiro, M.; Tajima, M.; Mitsutake, S.; Nagao, K.; Tsuge, K.; et al. Glucosylceramide contained in Koji mold-cultured cereal confers membrane and flavor modification and stress tolerance to Saccharomyces cerevisiae during coculture fermentation. Appl. Environ. Microbiol. 2015, 81, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Izumi, K.; Nakahata, E.; Hirata, M.; Sawada, K.; Tsuge, K.; Nagao, K.; Kitagaki, H. Quantitation and structural determination of glucosylceramides contained in sake lees. J. Oleo Sci. 2014, 63, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Inamine, M.; Suzui, M.; Morioka, T.; Kinjo, T.; Kaneshiro, T.; Sugishita, T.; Okada, T.; Yoshimi, N. Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats. Cancer Sci. 2005, 96, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Yazama, H.; Kitatani, K.; Fujiwara, K.; Kato, M.; Hashimoto-Nishimura, M.; Kawamoto, K.; Hasegawa, K.; Kitano, H.; Bielawska, A.; Bielawski, J.; et al. Dietary glucosylceramides suppress tumor growth in a mouse xenograft model of head and neck squamous cell carcinoma by the inhibition of angiogenesis through an increase in ceramide. Int. J. Clin. Oncol. 2015, 20, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Kitatani, K.; Fukushima, K.; Yazama, H.; Umehara, H.; Kikuchi, M.; Igarashi, Y.; Kitano, H.; Okazaki, T. Inhibitory effects of dietary glucosylceramides on squamous cell carcinoma of the head and neck in NOD/SCID mice. Int. J. Clin. Oncol. 2011, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.; Hougeir, F.; Bikowski, J. Atopic dermatitis, and the role for a ceramide-dominant, physiologic lipid-based barrier repair emulsion. J. Drugs Dermatol. 2013, 12, 1024–1027. [Google Scholar] [PubMed]
- Duan, J.; Sugawara, T.; Hirose, M.; Aida, K.; Sakai, S.; Fujii, A.; Hirata, T. Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp. Dermatol. 2012, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Mitsutake, S.; Ishikawa, J.; Takagi, Y.; Akiyama, M.; Shimizu, H.; Tomiyama, T.; Igarashi, Y. Dietary glucosylceramide improves skin barrier function in hairless mice. J. Dermatol. Sci. 2006, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Takatori, R.; le Vu, P.; Iwamoto, T.; Satsu, H.; Totsuka, M.; Chida, K.; Shimizu, M. Effects of oral administration of glucosylceramide on gene expression changes in hairless mouse skin: Comparison of whole skin, epidermis, and dermis. Biosci. Biotechnol. Biochem. 2013, 77, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Fujita, J.; Shimizu, R.; Hiyoshi, A.; Fukuda, H.; Kizaki, Y.; Wakabayashi, S. Production of cellulose- and xylan-degrading enzymes by a koji mold, Aspergillus oryzae, and their contribution to the maceration of rice endosperm cell wall. J. Biosci. Bioeng. 2002, 93, 9–14. [Google Scholar] [CrossRef]
- Miranda, R.U.; Gómez-Quiroz, L.E.; Mendoza, M.; Pérez-Sánchez, A.; Fierro, F.; Barrios-González, J. Reactive oxygen species regulate lovastatin biosynthesis in Aspergillus terreus during submerged and solid-state fermentations. Fungal Biol. 2014, 118, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Yao, L.Y.; Jiao, R.H.; Lu, Y.H.; Tan, R.X. Enhanced production of Fumigaclavine C in liquid culture of Aspergillus fumigatus under a two-stage process. Bioresour Technol. 2014, 152, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloante Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Japan Federation of Miso Manufacturers’ Cooperatives. Available online: http://www.zenmi.jp/miso_toukei.html (accessed on 18 September 2015).
- Yunoki, K.; Ogawa, T.; Ono, J.; Miyashita, R.; Aida, K.; Oda, Y.; Ohnishi, M. Analysis of sphingolipid classes and their contents in meals. Biosci Biotechnol Biochem. 2008, 72, 222–225. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamajima, H.; Fujikawa, A.; Yamashiro, M.; Ogami, T.; Kitamura, S.; Tsubata, M.; Tan, S.; Matsunaga, H.; Sawada, K.; Kumagai, S.; et al. Chemical Analysis of the Sugar Moiety of Monohexosylceramide Contained in Koji, Japanese Traditional Rice Fermented with Aspergillus. Fermentation 2016, 2, 2. https://doi.org/10.3390/fermentation2010002
Hamajima H, Fujikawa A, Yamashiro M, Ogami T, Kitamura S, Tsubata M, Tan S, Matsunaga H, Sawada K, Kumagai S, et al. Chemical Analysis of the Sugar Moiety of Monohexosylceramide Contained in Koji, Japanese Traditional Rice Fermented with Aspergillus. Fermentation. 2016; 2(1):2. https://doi.org/10.3390/fermentation2010002
Chicago/Turabian StyleHamajima, Hiroshi, Ayami Fujikawa, Mikako Yamashiro, Takatoshi Ogami, Seiichi Kitamura, Masahito Tsubata, Sei Tan, Haruka Matsunaga, Kazutaka Sawada, Satoshi Kumagai, and et al. 2016. "Chemical Analysis of the Sugar Moiety of Monohexosylceramide Contained in Koji, Japanese Traditional Rice Fermented with Aspergillus" Fermentation 2, no. 1: 2. https://doi.org/10.3390/fermentation2010002
APA StyleHamajima, H., Fujikawa, A., Yamashiro, M., Ogami, T., Kitamura, S., Tsubata, M., Tan, S., Matsunaga, H., Sawada, K., Kumagai, S., Hayashi, N., Nagao, K., Yanagita, T., Oka, T., Mitsutake, S., & Kitagaki, H. (2016). Chemical Analysis of the Sugar Moiety of Monohexosylceramide Contained in Koji, Japanese Traditional Rice Fermented with Aspergillus. Fermentation, 2(1), 2. https://doi.org/10.3390/fermentation2010002