1. Introduction
The global trend to develop a more sustainable economy based on renewable resources increases the demand for new innovative processes needed to obtain bio-based chemicals from biomass. The development of biorefineries, where biomass is converted to energy and various biomaterials is gaining ground, leading to an increased need to valorize the generated coproducts [
1,
2].
Wheat and beetroot are widespread crops, which are industrially converted to sugar, food additives, or other components for non-food applications. These processes lead to coproducts, which are generally valorized through animal feed or microbial fermentation for the production of biofuels.
Employing fermentation processes, for the bioconversion of biomass into green building blocks present many advantages, such as using of water as solvent and working at mild temperature. Using agro-industrial coproducts in growth media formulations is a good way to lower manufacturing costs of processes involving microbial systems.
Sugar beet molasses is mainly composed of sugar (~500 g·L
−1, glucose and fructose) and nitrogen compounds, but also contains minerals (e.g., calcium, magnesium, iron, zinc, copper, manganese) and group B vitamins (i.e., thiamin, niacin, riboflavin, and B6), which are necessary nutrients for microbial growth. On the other hand, many components such as organic salts, nitrites and phenolic compounds may induce inhibitory effects on the growth of microorganisms. However, several studies have shown the suitability of incorporating beet molasses in growth media for the production of chemical building blocks by employing different microorganisms, such as
Aspergillus niger to synthesize citric acid [
3],
Bacillus polymyxa to produce polysaccharides [
4], and
Lactobacillus delbrueckii to generate lactic acid [
5,
6].
In this work we investigated the possibility of using combined beet and wheat coproducts as substrates to formulate a growth medium for
L. reuteri DSM17938. This lactic acid bacteria (LAB) strain is of particular interest for its probiotic properties which are used in infant nutrition [
7]. Furthermore,
L. reuteri is capable of naturally converting glycerol into three molecules of industrial interest, 3-hydroxypropionaldehyde (3-HPA), 1,3-propanediol (1,3-PDO), and 3-hydroxypropionic acid (3-HP), which are used as building blocks for the production of superabsorbent polymers and diverse composite materials. In this work, considering the growing interest of this molecule as a building block of particular industrial interest, we will focus on 3-HP as the target molecule [
8].
The bioconversion of glycerol to 3-HP by
L. reuteri occurs in two steps: glycerol is first dehydrated to 3-HPA by a co-enzyme B12-dependent glycerol dehydratase. The synthesized 3-HPA can then either be excreted, reduced to 1,3-PDO via the NADH
2-dependent 1,3-propanediol oxidoreductase, or transformed to 3-HP via an oxidative pathway involving a NAD
+-dependent propionaldehyde dehydrogenase, a phosphotransacetylase and a propionate kinase [
9].
L. reuteri is not able to use glycerol as a carbon source for growth, which limits the coproducts formed during the bioconversion of glycerol by resting cells. The use of resting cells in a restricted medium containing only glycerol is thus interesting as it facilitates the downstream processing [
10,
11].
In this case the glycerol bioconversion process requires a preliminary biomass production step. The growth of lactic acid bacteria is particularly demanding in terms of nutrient availability and environmental parameters (e.g., temperature, pH) and formulating appropriate growth media for their cultivation is challenging. LAB cultivation is generally conducted in MRS (De Man, Rogosa and Sharpe) broth as the reference growth medium. MRS is composed of ten different ingredients among others polypeptones and meat and yeast extracts, which are quite expensive. Pertinent studies have been conducted to develop less expensive media supporting the growth of particular species of lactobacillus, such as
Lactobacillus casei [
12],
Lactobacillus delbrueckii [
5,
6], or
Lactobacillus plantarum [
13] but, to the best of our knowledge, such a study involving
L. reuteri is lacking.
Processes involving microbial production are complex and the culture medium composition must be adapted to the microbial strain as well as to the expected product. Response Surface Methodology (RSM) is an efficient analytical method used to design experiments and optimize a complex set of factors for a specific response, and adapted to growth medium optimization [
14]. The method enables to obtain a large amount of data from a reduced number of experiments and to study the influence of different input variables on a specific response, including the interactions between the studied factors. RSM has been successfully used for the optimization of culture media for different microorganisms including lactobacillus species [
15,
16,
17,
18,
19].
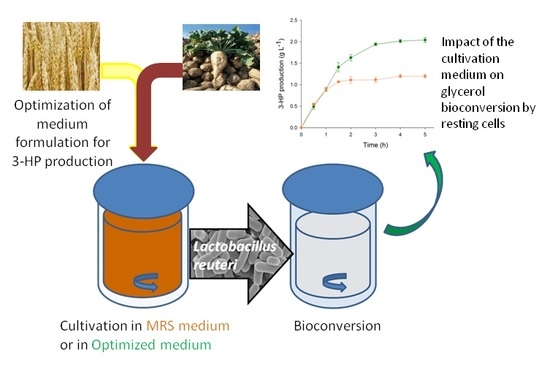
The aim of this study is to elaborate a medium formulation based on agroindustrial coproducts, for the cultivation of L. reuteri and to evaluate the impact of this formulation on the bioconversion of glycerol by resting cells.
2. Materials and Methods
2.1. Microorganism
L. reuteri DSM 17938 was purchased from BioGaia AB (Stockholm, Sweden) and stored on MRS medium with 20% w/v glycerol at −80 °C. Before inoculation the strain was defrosted and grown overnight at 37 °C in MRS medium (Biokar, France).
2.2. Media
Low purity sugar beetroot syrup (LPS), was sampled at Cristal-Union (Pomacle, France) and wheat extract (WE) was supplied by Chamtor (Pomacle, France) and filtered before use to eliminate the suspended solid particles. The total fermentable sugar concentrations (glucose and fructose) were analyzed by HPLC. In LPS, the concentrations of glucose and fructose were identical (290 g·L−1) while WE contained 140 g·L−1 of glucose and 5.8 g·L−1 of fructose. LPS also contained glycerol at a concentration of 17.4 g·L−1. Both LPS and WE were used as sugar sources in the cultivation medium and the total final sugar content was adjusted to 30 g·L−1. The contribution of LPS compared to WE for the sugar concentration was expressed as R. For example, R at 20% means that among the 30 g·L−1 of sugar, 20% come from LPS and 80% from WE.
The growth medium was supplemented with yeast extract (YE, Fisher Scientific, Springfield Township, NJ, USA), Tween 80 (T80, Sigma Aldrich, Saint Louis, MO, USA) and cyanocobalamine (vitamin B12, Fisher Scientific, Springfield, NJ, USA). The initial pH was set to 6.8 by adding HCl 2N (Fisher Scientific, Loughborough, UK).
MRS medium (Biokar, Beauvais, France) was used for the standard cultivations and the precultures. The commercial mixture was supplemented with glucose (Fisher, Loughbourough, UK) to a final concentration of 30 g·L−1.
Glycerol bioconversion was conducted in a 10 g·L−1 glycerol (Fisher, Loughbourough, UK) solution in distilled water.
All media were sterilized for 20 min at 110 °C and cooled down to room temperature before inoculation.
2.3. Cells Cultivation and Glycerol Bioconversion with Resting Cells
Small-volume cultivations were conducted in 15-mL Falcon tubes, with 12 mL medium, at 37 °C in a static incubator, for 8 h. The kinetics of bacterial growth was followed by the variation of the optical density measured at 620 nm (∆OD620nm) with an Agilent (Santa Clara, CA, USA) spectrometer, with the cultivation medium before inoculation as reference.
For the design of experiment as well as for the comparison of carbon sources, the cultivation media were inoculated with the same preculture to a starting ∆OD620nm of 0.1. After 8 h, at the end of the growth phase, the bacteria cells were harvested by centrifugation, 10 min at 5000× g and 10 °C. The supernatant was discarded and the pellet was washed twice in potassium phosphate buffer (pH 6) and re-suspended in 10 g·L−1 glycerol to a final cell concentration of 1×1010 cell·mL−1. Then, the tubes were maintained at 37 °C overnight in an orbital shaker under gentle stirring (110 rpm) for the bioconversion step. At the end of the bioconversion, the bacteria were pelleted by centrifugation, 10 min at 5000× g and 10 °C, and the supernatants were analyzed for the quantification of the glycerol degradation products (3-HP, 3-HPA and 1,3-PDO).
Cultivations in 2 L were conducted in Biostat B bioreactor (Sartorius, Goettingen, Germany). Cultivations in the optimized medium (WE-LPS) and MRS were done in parallel, under gentle stirring (100 rpm). The temperature was maintained at 37 °C with water circulation. For the bioconversion kinetics experiments, the bacterial cultivation was conducted overnight with a starting ∆OD620nm of 0.001. The bacterial biomass was then harvested after 15 h as described above, resuspended in 10 g·L−1 glycerol to a concentration of 1 × 1010 cell·mL−1 and the bioconversion was followed for 5 h by sampling the bioconversion medium every 30 min.
2.4. Substrate and Products Quantification
Glycerol, 3-HP, 3-HPA and 1,3-PDO were analyzed by HPLC on an Aminex 87H column (300 mm × 7.8 mm, Bio-Rad, Richmond, VA, USA) equipped with a cation H+ Micro-Guard column (30 mm × 4.6 mm, Bio-Rad) thermostated to 50 °C. H2SO4 (Fisher, Loughbourough, UK) 4 mM was used as mobile phase. The elution flow rate was set at 0.6 mL·min−1. 3-HP was detected with a UV detector (Dionex, Sunnyvale, CA, USA) 210 nm; retention time: 13.1 min), residual glycerol, 3-HPA, and 1,3-PDO were detected with a refractometer (RI-101, Shodex, Japan) (RT: 13.3, 15.0 and 17.5 min respectively). Citric acid (0.5 g·L−1) was added as an internal standard and samples were filtered on 0.22 µm syringe-filter.
Quantification was performed using five-point standard curves obtained under the same conditions of analysis from standard samples prepared from pure products. Glycerol was purchased from Fisher Chemicals (Loughbourough, UK), 3-HP from TCI (Tokyo, Japan) and 1,3-PDO from Sigma-Aldrich (Saint Louis, MO, USA). 3-HPA was synthesized according to Burgé et al. [
20].
2.5. Experimental Design and Statistical Analysis
Response surface methodology based on four-factor-multi level D-optimal design was used to optimize the growth medium composition. The different formulations were compared according to two responses, the first one being the growth of
L. reuteri assessed as the ∆OD
620nm of the broth at the end of the cultivation phase, and the second one being the concentration of 3-HP in the supernatant at the end of the bioconversion phase. MODDE 8 software (Umetrics, AB, Umeå, Sweden) was used for statistical analysis of the experimental design [
21]. The factors tested were the LPS/WE ratio (R) (from 10% to 90%), and the concentrations of YE (from 0 to 35 g·L
−1), B12 (from 0 to 200 µg·L
−1), and T80 (from 0 to 5 mL·L
−1).
Growth on MRS medium supplemented with glucose (30 g·L−1) was used as a standard to compare the results obtained with the tested media.
The three independent variables levels used for the experimental design are presented in
Table 1. The runs set by the D-optimal design and the respective experimental responses obtained for ∆OD
620nm (Y1) and 3-HP production (Y2) are also presented in
Table 1. Thirty experiments with three replications at the center point were designed for the above mentioned variables. The experiments were made in two different blocks, e.g., two series made by two different operators at two different dates, to eliminate a potential influence of the operator on the results. The data were fitted using multiple linear regressions (MLR). The significant parameters in the model were determined by analysis of variance (ANOVA) for each response. The model validation was based on R
2, Q
2, and lack of fit test. R
2 (0.991 for ∆OD
620nm and 0.977 for 3-HP) expresses the percentage of the variation of the response explained by the model, whereas Q
2 (0.982 for ∆OD
620nm and 0.940 for 3-HP) expresses the percentage of the variation predicted by the model according to cross validation. The probability for lack of fit were, respectively, 0.079 for ∆OD
620nm and 0.102 for 3-HP and the model validity was evaluated to 0.354 for ∆OD
620nm and 0.495 for 3-HP, a minimal value of 0.25 being requested to consider the model as valid.
The experimental data were further analyzed using multiple regressions and a second order polynomial model fitted for predicting optimal levels was expressed as follows:
Yi is the predicted response,
β0 is the intercept coefficient of the
y axis,
βi is a linear coefficient,
βij and
βii are the quadratic coefficients of the model, and
Xi and
Xj are the independent variables and ε the residual error.
3. Results and Discussion
3.1. Influence of Medium Composition on Bacterial Growth and 3-HP Production
A growth medium based on wheat (WE) and sugar beet (LPS) coproducts was formulated for the cultivation of L. reuteri DSM17938 with the perspective of using the resting cells for the bioconversion of glycerol into 3-HP.
A design of experiment was set up as a screening using a four-factor three-level factorial experiment design with three replications at the central point. Twenty-eight different growth medium formulations were tested, varying in the proportion of LPS versus WE to reach the total sugar concentration of 30 g·L−1, the concentration of yeast extract, Tween 80, and vitamin B12. The performance of the tested media was assessed according to two responses (i) the maximal optical density (∆OD620nm) obtained after the cultivation phase and (ii) the 3-HP production at the end of the bioconversion phase.
The maximum yield of biomass production was obtained from the culture condition of run number 4 (∆OD
620nm 12.8) while the maximal production of 3-HP (2.3 g·L
−1) was obtained for run number 13 (
Table 1).
In parallel, three control fermentations were conducted in MRS under the same conditions. The average ∆OD obtained was 6.7 ± 0.8 and the 3-HP production at the end of the bioconversion phase was 1.3 ± 0.1 g·L−1.
The contributions of each parameter on both responses were different (
Figure 1). The highest contribution to both responses was due to the concentration of yeast extract, which impacts positively both the final ∆OD and the 3-HP production.
The 3-HP production was also positively impacted by the addition of vitamin B12 to the culture broth during the preliminary cultivation phase (
Figure 1b), although no significant impact was noticeable on ∆OD (
Figure 1a). This positive influence of vitamin B12 on the 3-HP production is surprising, considering that
L. reuteri is able to synthesize de novo vitamin B12 from glutamate or glycine [
22]. Furthermore, this characteristic has been shown to be directly associated with the ability of the strain to produce 3-HPA [
23].
On the other hand, the LPS/WE ratio (R) had a negative impact on the 3-HP production, which means that a lower proportion of LPS compared to WE as carbon source led to a higher 3-HP synthesis, while ∆OD was not significantly influenced by this parameter. Besides, the squared effects had a negative impact on the results, which suggests that the range of tested values included the optimal value for the parameters (
Figure 1a,b).
Interestingly, when compared to the control data obtained after bacterial cultivation in MRS medium, thirteen formulations tested led to a higher production of 3-HP, but were not necessarily associated with higher bacterial growth (
Figure 2). Conversely, three formulations that allowed higher biomass production led to lower levels of 3-HP when compared to the control cultivation.
These results highlight that the ability of L. reuteri to convert glycerol into 3-HP highly depends on the cultivation phase, in particular on the composition of the cultivation medium. In addition, a high bacterial biomass production is not sufficient to ensure a good production of 3-HP.
3.2. Growth Medium Optimization
Response Surface Methodology (RSM) was used to determine the optimized growth medium formulation with the goal to maximise the 3-HP production. Thus, the software prediction tool MODDE8 determined the optimal composition of the medium (WE-LPS medium) as follows (g for 100 g medium): WE: 10.3; LPS: 2.6 yeast extract, 1.5; Tween 80, 0.5; and vitamin B12, 0.01. According to the model, a maximal 3-HP titre of 2.3 g·L−1 can be expected when using WE-LPS as cultivation medium for the production of bacterial biomass.
3.3. Interest of Using Both LPS and WE as Carbon Sources in the Medium Formulation
Control fermentations were carried out in order to assess the influence of LPS and WE and the relevance of utilizing both ingredients in the medium formulation employed for the synthesis of 3-HP. Four media were prepared using LPS, WE or commercial glucose as the carbon source. In every formulation the final total sugar concentration was adjusted to 30 g·L
−1. The other ingredients were added at the same concentration in the four formulations (
Table 2). The performance of the media in terms of final ΔOD were compared to the optimized medium (WE-LPS) and to the MRS medium, in 15 mL Falcon tubes containing 12 mL. All experiments were done in triplicate.
Furthermore, after the bioconversion phase, bioconversion broths were analyzed for residual glycerol and the products of glycerol bioconversion, 3-HP, 3-HPA, and 1,3-PDO (
Table 2).
As many aldehydes, 3-HPA is known to display antimicrobial properties and is suspected to cause a rapid loss of glycerol conversion activity during the 3-HP production process [
24]. Thus, the limitation of the 3-HPA titre in the conversion broth is of utmost importance for the 3-HP production process. Therefore, the performance of the tested media were evaluated according to three parameters i.e., (i) the total consumed glycerol (Y
gly/gly); (ii) the glycerol conversion yield to 3-HP (Y
3-HP/gly); and (iii) the glycerol conversion yield to 3-HPA (Y
3-HPA/gly). Regarding these criteria, bacteria growth on LPS medium exhibited the best performances. However, 19% of the glycerol was consumed (
Table 2) which therefore led to the lowest 3-HP concentration recorded across all tested media. When considering the total products of glycerol bioconversion (3-HP, 3-HPA and 1,3-PDO), the WE-LPS medium distinguished itself from the others, with a lower production of 3-HPA, whereas the synthesis of 3-HP and 1,3-PDO was up to 70% higher compared to standard medium MRS.
Concerning the total glycerol consumption, glycerol was better converted by bacteria cultivated in MRS and WE medium, but 3-HPA was the main final product, with 0.68 and 0.5 g 3-HPA per g of consumed glycerol, respectively (
Table 2). Interestingly, after cultivation in WE-LPS medium, the total glycerol consumption was 45% lower compared to the control (MRS), but the bacteria grown in this medium displayed the highest Y
3-HP/gly (0.40 g·g
−1) and the lowest Y
3-HPA/gly (0.17 g·g
−1). Furthermore, the highest final 3-HP titre was obtained after growth in WE-LPS medium
The comparison of the five cultivation conditions highlighted the influence of the cultivation medium on both glycerol uptake by the cells and the conversion of 3-HPA to 3-HP and 1,3-PDO. While the biomass cultivated on WE medium was the most efficient in terms of glycerol conversion yield, the consumed glycerol was mainly converted into 3-HPA. Finally, the combination of WE and LPS provided the best balance in terms of glycerol uptake and 3-HPA conversion to 3-HP and 1,3-PDO. Thus, the combination of LPS and WE provides a real added value to the 3-HP production process.
This work clearly shows that the metabolism of glycerol in resting cells is highly influenced by the growth medium composition.
The complementation of beet molasses with other industrial crop products to formulate bacterial growth media was reported in a few studies. Beet molasses as a sole fermentation medium generally leads to low biomass growth, due to its high salt concentration and pH, and the presence of betaine as the main nitrogen source [
4]. In combination with wheat stillage as nitrogen sources, however, adding beet molasses led to higher bacterial biomass [
13]. The authors attributed the increase of LAB biomass to the rise of sugar concentration. In our case, the total sugar concentration was kept constant across the different formulations, but LPS and WE provided fructose to the medium which in addition to glucose has been shown to enhance the growth performance of
L. reuteri ATCC 55730 by allowing a better redox balance [
25]. However, the presence of fructose in the growth medium does not explain the high biomass density (ΔOD) obtained after cultivation in media containing WE (WE-LPS and WE,
Table 2), as the biomass density obtained after growth on LPS alone was four times as low as the one obtained in medium containing WE (
Table 2).
The influence of the bacteria cultivation medium on the bioconversion step using resting cells is difficult to explain at this stage. The presence of glycerol in LPS may provide an advantage for strain growth and its adaptation to the bioconversion medium [
26,
27]. Additionally, the process used here for the bioconversion of glycerol involves a succession of steps, which represent environmental changes likely to weaken the cells. The microorganisms cultivated in a complex medium derived from agro-industrial coproducts containing potential inhibitory molecules may develop adaptation mechanisms, providing the cells with a technological advantage for the bioconversion step [
28,
29].
3.4. Impact of Growth Medium on the Glycerol Bioconversion Pathway
To validate the model and its predictions, L. reuteri cultivation in WE-LPSP medium and glycerol bioconversion were successively conducted in 2 L bioreactors using the same conditions as during the previous small-scale setup. The kinetics of glycerol bioconversion was followed by sampling the supernatant every thirty minutes for the first two hours and then every hour. Glycerol and its products were then quantified by HPLC. As for the previous small-scale experiments, bacterial growth in MRS medium, followed by glycerol bioconversion, was performed as a control. Experiments were done in triplicate.
As in small scale experiments, the concentration of 3-HP at the end of the bioconversion in the medium was 70% higher as after a growth phase in MRS (
Figure 3b). The 3-HPA and the 1,3-PDO concentrations were also impacted; the production of 1,3-PDO increasing by 60% (
Figure 3b) while the maximum 3-HPA concentration was greatly reduced from 4.9 to 1.0 g·L
−1 (
Figure 3a) compared to the results obtained after growth on MRS medium.
Interestingly, the 3-HPA production was greatly impacted by the growth condition (
Figure 3a). After growth on MRS, 3-HPA accumulated quickly in the bioconversion medium, with a production rate of 70.7 ± 3.8 mmol·L
−1·h
−1, while after growth on WE-LPS medium, the production rate at the beginning of the bioconversion phase was ten times lower (7.5 ± 0.9 mmol·L
−1·h
−1). On the other hand, the 3-HP and 1,3-PDO production rates measured at the onset of the bioconversion process were the same for both growth media (11.0 mmol·L
−1·h
−1 for 3-HP and 17 mmol·L
−1·h
−1 for 1,3-PDO). However, after growth in MRS medium, the production rates decreased quickly and the glycerol bioconversion stopped after only 90 min, while the bioconversion activity was maintained for 180 min after growth on WE-LPS medium.
The metabolic fluxes through the different pathways, presented in
Figure 4, were calculated at the onset of the bioconversion phase and are representative of the glycerol dehydratase (GDH) activity of the bacterial population [
27].
The main impact of the growth medium composition on the glycerol bioconversion was the limitation of the 3-HPA synthesis flux from glycerol (ν1). After growth on MRS medium, ν1, was, respectively, 9 and 6 times faster than the subsequent oxidative (ν2) and reductive (ν3) pathways leading to the synthesis of 3-HP and 1,3-PDO. These results are consistent with the ones reported by Dishisha [
10,
11] using
L. reuteri DSM 20016. Besides, in the same study, the limitation of the glycerol consumption rate using a fed-batch approach allowed maintaining a low concentration of 3-HPA while increasing the 3-HP and 1,3-PDO production rates. The antimicrobial activity of 3-HPA has been demonstrated for several micro-organisms [
30,
31]. Indeed, the in situ complexation of 3-HPA with bisulfites increases the productivity and the lifetime of microorganisms [
32]. Although less widely studied, 3-HP can also display a toxic activity towards a range of microorganisms [
33]. In the case of growth in MRS medium, the drop in bioconversion activity can be attributed to a decrease in cell viability due to the rapid accumulation of 3-HPA as observed by Burgé [
24]. Here, the limitation of the 3-HPA production rate, allowed maintaining the cell activity and increased the glycerol conversion yield to 3-HP and 1,3-PDO from 0.1 g·g
−1 in the control condition to 0.4 g·g
−1 after growth in WE-LPS medium. The reason for this limitation after growth on WE-LPS medium is still to clarify. Considering the glycerol consumption flux, the restriction of glycerol intake through the cell membrane could be a factor. On the other hand, the addition of vitamin B12 to the growth medium has a positive influence on the 3-HP production, as observed in the first part of the study. According to the link between the synthesis of both vitamin B12 and 3-HPA by
L. reuteri, the presence of an exogenous source of vitamin B12 might moderate the expression of the glycerol dehydratase, reducing the 3-HPA production flux [
23].