Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Dairy Substrates
2.3. Cultivations
2.4. Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Effect of pH on Fat Degradation by A. oryzae
3.2. Fatty Acids Released by A. oryzae Fat Degradation
3.3. Lactic Acid and Glycerol Consumption by A. oryzae
3.4. Cultivation of A. oryzae in Different Fractions of Cream
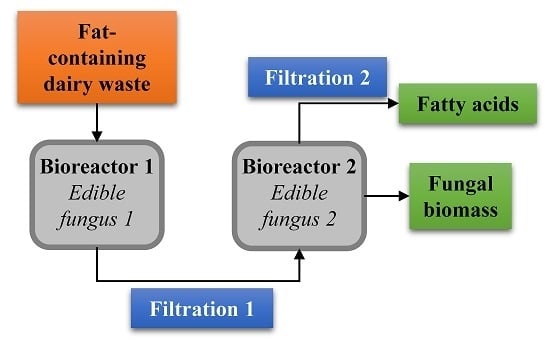
3.5. Proposed Integrated Bioconversion Unit of Dairy Substrates to Various Value-Added Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreira, J.A.; Lennartsson, P.R.; Edebo, L.; Taherzadeh, M.J. Zygomycetes-based biorefinery: Present status and future prospects. Bioresour. Technol. 2013, 135, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2017, 59, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Barbesgaard, P.; Heldt-Hansen, H.P.; Diderichsen, B. On the safety of Aspergillus oryzae: A review. Appl. Microbiol. Biotechnol. 1992, 36, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.A. Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Lock, A. Milk fatty acid composition: Challenges and opportunities related to human health. In Proceedings of the XXVI World Buiatrics Congress, Santiago, Chile, 14–18 November 2010; Cornell University: New York, NY, USA, 2010; pp. 278–289. [Google Scholar]
- Nettleton, J. Introduction to Fatty Acids. In Omega-3 Fatty Acids and Health; Springer: Berlin, Germany, 1995; 63p. [Google Scholar]
- Yilmaz-Ersan, L. Fatty acid composition of cream fermented by probiotic bacteria. Mljekarstvo 2013, 63, 132–139. [Google Scholar]
- Meunier-Goddik, L. Sour Cream and Creme Fraiche. In Handbook of Food and Beverage Fermentation Technology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Sues, A.; Millati, R.; Edebo, L.; Taherzadeh, M.J. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 2005, 5, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.L.; Huang, B.; Nie, Z.Y.; Wang, W. Production and characterization of alkaline extracellular lipase from newly isolated strain Aspergillus awamori HB-03. J. Cent. South Univ. Technol. 2011, 18, 1425–1433. [Google Scholar] [CrossRef]
- Toida, J.; Kondoh, K.; Fukuzawa, M.; Ohnishi, K.; Sekiguchi, J. Purification and characterization of a lipase from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1995, 59, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Colla, L.M.; Ficanha, A.M.M.; Rizzardi, J.; Bertolin, T.E.; Reinehr, C.O.; Costa, J.A.V. Production and characterization of lipases by two new isolates of Aspergillus through solid-state and submerged fermentation. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Yoshida, Y.; Toita, J.; Sekiguchi, J. Purification and characterization of a novel lipolytic enzyme from Aspergillus oryzae. J. Ferment. Bioeng. 1994, 78, 413–419. [Google Scholar] [CrossRef]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Dairy Chemistry and Biochemistry; Springer: Berlin, Germany, 1998. [Google Scholar]
- Gerez, C.L.; Torres, M.J.; Font de Valdez, G.; Rollán, G. Control of spoilage fungi by lactic acid bacteria. Biol. Control 2013, 64, 231–237. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z. Screening of Lactic Acid Bacteria for Antifungal Activity against Aspergillus oryzae. Am. J. Appl. Sci. 2011, 8, 447–451. [Google Scholar] [CrossRef]
- Torres, A.; Li, S.M.; Roussos, S.; Vert, M. Screening of microorganisms for biodegradation of poly (lactic-acid) and lactic acid-containing polymers. Appl. Environ. Microbiol. 1996, 62, 2393–2397. [Google Scholar] [PubMed]
- Torres, A.; Li, S.M.; Roussos, S.; Vert, M. Degradation of L- and DL-lactic acid oligomers in the presence ofFusarium moniliforme andPseudomonas putida. J. Environ. Polym. Degrad. 1996, 4, 213–223. [Google Scholar] [CrossRef]
- De Vrese, M.; Laue, C.; Offick, B.; Soeth, E.; Repenning, F.; Thoß, A.; Schrezenmeir, J. A combination of acid lactase from Aspergillus oryzae and yogurt bacteria improves lactose digestion in lactose maldigesters synergistically: A randomized, controlled, double-blind cross-over trial. Clin. Nutr. 2014, 34, 394–399. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates. Fermentation 2017, 3, 48. https://doi.org/10.3390/fermentation3040048
Mahboubi A, Ferreira JA, Taherzadeh MJ, Lennartsson PR. Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates. Fermentation. 2017; 3(4):48. https://doi.org/10.3390/fermentation3040048
Chicago/Turabian StyleMahboubi, Amir, Jorge A. Ferreira, Mohammad J. Taherzadeh, and Patrik R. Lennartsson. 2017. "Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates" Fermentation 3, no. 4: 48. https://doi.org/10.3390/fermentation3040048
APA StyleMahboubi, A., Ferreira, J. A., Taherzadeh, M. J., & Lennartsson, P. R. (2017). Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates. Fermentation, 3(4), 48. https://doi.org/10.3390/fermentation3040048