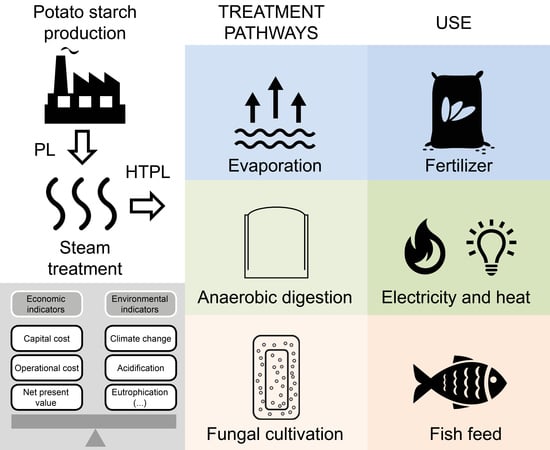
1. Introduction
Potato is one of the most important food crops in the world, and it accounts for 13.3% of the starch produced in the European Union (EU). The processing of potato to produce starch results in two major byproducts: potato pulp (PP) and potato liquor (PL). PP contains the insoluble polysaccharides cellulose, hemicellulose, pectin and residual starch. Proteins, minerals and trace elements in high concentrations, are the major ingredients of PL [
1]. Each metric ton of processed potato yields approximately 200 kg of starch and generates ca 700 kg PL [
2] containing 30–41% protein per total solid (TS) [
3]. The proteins present in the PL are of good quality, similar to those of whole eggs [
3]. Different methods to recover these proteins have been reported. They include thermal coagulation, acid precipitation, salting out, isoelectric precipitation, complexing with carboxymethylcellulose or bentonite, ultrafiltration, expanded bed adsorption and dry separation [
3,
4,
5,
6]. However, the only method that is presently being used for industrial recovery of protein from PL is heat coagulation [
3,
4,
5,
6,
7]. In this method, steam is injected into the liquor at a pH of 5.5 to increase the temperature to 99 °C. This coagulates the proteins, which are then precipitated and collected [
8]. However, the proteins obtained by this method lose their functional properties and are mostly used as an additive to cattle feed. Moreover, they have a salty, bitter taste, which prevents them from being used as food additives [
3,
7,
9]. After the proteins have been removed from PL, the residual liquid—called HTPL—is further evaporated at 140 °C to produce potato protein liquor (PPL), containing 40% (
w/
w) TS [
1].
The most common method to manage PPL is to use it as fertilizer. However, most potato processing occurs during the winter. The reduced biological activity in the soil during this period prevents the uptake of the liquor, which forces the potato starch facilities to store large volumes of PPL for several months [
9]. Additionally, the use of PPL as fertilizer causes contamination of groundwater and emits a bad odor that can disturb citizens living in neighboring areas [
10]. However, as mentioned above, despite the high content of residual proteins in the PPL, they are of poor quality, hence preventing them from being used as a supplement for cattle feed [
1].
A few alternative management strategies for PPL have been presented in the literature. The biodegradable nature of the byproduct has stimulated its use in bioprocesses to produce yeast biomass [
11], acetate and ethanol [
12], enzymes [
10] and fungal protein [
13]. Additionally, the filamentous zygomycete fungus
Rhizopus oryzae has been used to reduce the chemical oxygen demand (COD) of the PPL and produce a protein-rich biomass with a potential application as fish feed [
1].
One of the reasons for the growing interest in producing supplements for fish feed is that aquaculture has been growing at about 8% per year since the late 1970s, which is higher than the rate of human population growth. It has been suggested that the reason for this large growth is the widespread knowledge of the importance of fish for a healthy lifestyle, mainly because fish contains ω-3 polyunsaturated fatty acids. Fish feed is a major cost in intensive fish farming [
14], and zygomycete fungi can be used as a substitute for fish feed [
15]. These are filamentous fungi that contain large amounts of polyunsaturated fatty acids and protein, resulting in an increased content of these components in the fish feed [
16]. Ferreira et al. (2016) [
17] reported that ascomycete biomass (which has protein and fatty acid compositions comparable to zygomycete) is a good substitute for soybean-based feeds in the diet of animals like poultry, cattle, chicken and fish.
Among the zygomycete strains that have been investigated for fish feed,
R. oryzae is a promising microorganism.
R. oryzae has been used for many centuries in Asian cuisine to prepare fermented food, such as tempeh. Therefore, it is Generally Regarded As Safe (GRAS), which is a very favorable property when investigating its potential use as animal feed [
18]. Due to these arguments, the possibility of using HTPL to cultivate
R. oryzae is studied in this work. Alternatively, HTPL can be used in anaerobic digestion (AD) to produce biogas. AD is considered to be a sustainable form of treating industrial waste while simultaneously producing energy in the form of biogas [
2]. Production of biogas from waste can have several benefits, including reduction in the costs of waste treatment, contribution to global energy needs using relatively cheap feedstock, and lower environmental impact than conventional types of energy [
19].
In the present study, techno-economic and life cycle assessments of the treatment and use of HTPL are performed for three scenarios. The current strategy of PPL production is compared with two alternative scenarios where the HTPL is used for (i) the cultivation of filamentous fungus R. oryzae biomass or (ii) the production of biogas. The data used in the analyses are obtained from experiment, the literature and industrial potato starch production plants.
3. Results and Discussion
Production of potato starch generates a protein-rich side stream which is exploited by the industry to produce protein isolate. The residual wastewater is given (without charge) to the farmers to be used as fertilizer. This scenario was compared to other scenarios in which the wastewater is used to produce fungal biomass for use as fish feed or to produce biogas.
3.1. Technical Analysis
In the evaporation scenario, 19,641 kg/h of HTPL are pumped to a boiler operating at 2 bar to concentrate the HTPL to PPL, which contains approximately 40% (w/w) solids. The boiler produces 18,126 kg/h of steam which is used to preheat the HTPL before it enters the boiler. Approximately 267 MW·h/day of energy are consumed in this process.
The fungus scenario, involving the production of
R. oryzae biomass to be used as fish feed, was evaluated for the same flow rate used for the evaporation scenario (19,641 kg/h of HTPL during six months a year). The cultivation of
R. oryzae yielded a biomass production of 2475 kg/day (445 metric tons for an operational period of six months) containing 46.6% crude protein. The energy consumption in this process is 24.5 MW·h/day, which is primarily due to the aeration of the bioreactor (95% of the energy consumed in the scenario is for aeration). A daily volume of 473.8 m
3 of wastewater is discarded by the plant. An airlift bioreactor was chosen because of the improved agitation achieved in this design without the use of internal parts (e.g., impellers and baffles) in which the fungus can grow around interfering in the mass transfer [
40].
In the biogas scenario, 279.1 Nm3/h of biogas containing 58.8% (v/v) of methane is produced. At this concentration, the biogas contains a low heat value of 5.47 kW·h/Nm3. The biogas production is equivalent to 36.6 MW·h/day, while the energy consumption is 11.9 MW·h/day, i.e., less than half of the energy needed for the fungus scenario. This is because the anaerobic digester uses mechanical agitation to create homogeneous conditions inside the reactor, as opposed to the airlift bioreactor used in the fungus scenario, which uses aeration as the source of agitation. This decreases the energy demand in the biogas scenario. 474.0 m3 of wastewater is generated each day. Compared to the evaporation scenario, which is presently the most common alternative in potato starch plants, both bioprocess scenarios reduce the energy consumption.
3.2. Economic Analysis
Treatment of HTPL to PPL in the evaporation scenario requires an operating cost of €1.7 million per operating period (6 months). No income when using the PPL as fertilizer was accounted for in this scenario, since PPL is given without charge to the farmers for use as fertilizer. The excess steam produced and not used to preheat the HTPL represents an income of €671,414. The capital cost for this scenario is approximately €16.5 million. The evaporation of HTPL, containing as little as 3.3% (
w/
w) of TS, which is required to obtain the highly-concentrated PPL, demands the highest amount of heat when compared to the other scenarios. A standard vertical vessel was used to estimate the cost of the boiler for direct steam injection. Also, a shell and tube heat exchanger was designed to preheat the HTPL. The equipment, size and construction material used in the simulation, as well as the individual prices, are presented in
Table 6.
Production of fungal biomass (fungus scenario) demands much less energy. Only 9.2% of the energy presently used in the evaporation scenario would be required to produce the fungal biomass from HTPL. Cultivating the fungus on the potato starch wastewater has a capital cost of about €7.5 million. The cost of the airlift bioreactor was considered to be the same as the cost of a jacketed vertical tank. A rotary drum filter was used to collect the biomass after fermentation. Drying the biomass was achieved using a direct contact rotary dryer. The compressor used to provide air to the bioreactor was designed to provide an aeration of 0.04 vvm, and was calculated using Equation (4). The costs of the equipment are presented in
Table 6. The operational cost of the plant was estimated to be €1.84 million per operating period, and the fungal biomass obtained during this period was sold as fish feed supplement for €282,150.
The biogas scenario has the lowest energy consumption (11.9 MW·h/day). The capital investment for this scenario is about €14.2 million. Operational costs are €1.4 million/period (including sending wastewater to a municipal treatment plant), and €216,785/period would be obtained from selling the biogas. Compared to the fungus scenario, the biogas scenario demands 89% more capital investment and the operational cost is 24% lower. The capital cost, operating cost and product sales for the proposed scenarios are presented in
Figure 3. The digestate from the AD still contains nutrients which can be recovered in the form of fertilizer. However, the low concentration of such components in the digestate would require processes that have high energy demands, e.g., evaporation or centrifugation, or the transportation of large volumes of liquid. This would increase the costs associated with biogas scenario. Therefore, the wastewater produced in fungus and biogas scenarios is sent to the municipal wastewater treatment plant.
The NPV diagram after 10, 15 and 20 years is presented in
Figure 4. No scenario returns the investment made. Fungal cultivation (fungus scenario) results in a NPV that is less negative than AD (biogas scenario). After 15 years, the NPV of the biogas scenario becomes similar to the NPV of the evaporation scenario and, at the end of the lifetime of the plant, evaporation and fungus scenarios have comparable NPV. This is caused by the large capital cost and low operational cost of the evaporation scenario, opposed to the low capital cost and large operational cost of the fungus scenario. The contrasting characteristics of the scenarios lead to a shift in the NPV during the last five years of the plant’s lifetime. Rajendran et al. [
20] estimated that the capital cost for a municipal solid waste (MSW) AD plant is about 40 million USD with operating costs of about 3 million USD per year. The plant was designed to treat 55,000 m
3 of MSW and to produce compressed biogas (CBG) for the transport sector. The MSW, which has a high TS content, yields 64 Nm
3 of raw biogas per m
3 of MSW versus 14.2 Nm
3 of raw biogas per m
3 of HTPL. This creates a situation where a plant can make a profit from waste treatment. In the case of the HTPL, the dilute nature of the waste stream requires larger equipment and higher energy consumption, and returns lower quantities of biogas, hence making it difficult to operate the treatment processes with a positive economic balance.
3.3. Life Cycle Assessment
Figure 5 shows the environmental impacts of the three scenarios for HTPL treatment and use. The results show that the evaporation scenario has the largest impact in all of the impact categories except freshwater ecotoxicity. This is primarily due to the impacts related to the large amount of heat required for the evaporation of HTPL to PPL, which is part of the process emissions seen in the figure. Due to this heat requirement, the evaporation scenario has a large environmental impact despite the abatement from the avoided production of mineral fertilizer (seen as nutrients recovery in
Figure 5).
The fungus scenario has lower impacts than the evaporation scenario in all of the impact categories. It also has a lower impact than the biogas scenario in all of the impact categories except climate change. The lower impact of the biogas scenario on climate change is mainly due to the avoided marginal energy production, which is a result from the biogas firing in a CHP plant. It can also be noted that biogas scenario has lower impacts than the evaporation scenario in all impact categories except freshwater ecotoxicity.
The trends seen in the acidification, terrestrial eutrophication, marine eutrophication and freshwater eutrophication impact categories are the same, with the fungus scenario having the lowest impact and the evaporation scenario the highest. The impact of fungus scenario on freshwater eutrophication is 77% and 55% lower compared to the evaporation and biogas scenarios, respectively (
Figure 5). The results show that for the freshwater ecotoxicity category, the fungus scenario has the best performance with impacts that are 48% and 51% lower compared to the evaporation and biogas scenarios, respectively (
Figure 5).
3.4. Comparison of the Different Scenarios
The fungus scenario has the lowest impact in five out of the six environmental impact categories that were analyzed. This may indicate that it is the preferred option. However, it must be emphasized that this scenario has a larger impact than the biogas scenario in the climate change category. Since this impact category is considered by the United Nations “the single biggest threat to development” [
41], this result may have a central role when selecting the preferred scenario.
The fungus scenario is also the preferred scenario according to the economic analysis, since it has the lowest capital cost and the best NPV during the first fifteen years of operation. In contrast, at the end of the plant’s lifetime, the evaporation scenario becomes economically more viable. However, the difference between the two scenarios is only 1% of the evaporation scenario’s NPV.
The evaporation scenario has the largest impact in five out of the six environmental impact categories, in addition to having the largest capital investment of all scenarios.
Since none of the scenarios was best in all of the analyzed parameters, it is not possible to draw a simple conclusion regarding the preferred scenario. A decision would ultimately be made according to the political, environmental or economic agenda of the decision-makers and identifying the crucial factors can be difficult [
42]. The most important contribution of this study is to highlight the trade-offs inherently involved in the decision process.
4. Conclusions
Technical, economic and environmental analyses were performed to determine potential benefits of two proposed scenarios to a plant discarding 19.64 ton/h of HTPL. The two proposed scenarios are to use the HTPL (i) to cultivate filamentous fungus R. oryzae to produce a protein-rich biomass (fungus scenario) and (ii) to produce biogas via AD (biogas scenario). These two scenarios are compared to the most commonly used treatment method, which is concentrating the HTPL before using it as a fertilizer. Both proposed scenarios reduce the capital cost and the energy consumption of the wastewater treatment. Moreover, the current study highlights the environmental benefits of cultivating fungi in the HTPL (fungus scenario), since it has the lowest impact in acidification, freshwater ecotoxicity as well as the terrestrial, freshwater, and marine eutrophication categories. In contrast, the greenhouse gas emissions were higher from fungus scenario compared to biogas scenario, where the residue was anaerobically digested. The results show that the substituted products in the system expansion, such as mineral fertilizers, electricity and heat, substantially reduce the environmental footprints of fungus and biogas Scenarios. This study presents the techno-economic and environmental trade-offs that are necessary to take into account when selecting one of the scenarios in preference to the others.