Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology
Abstract
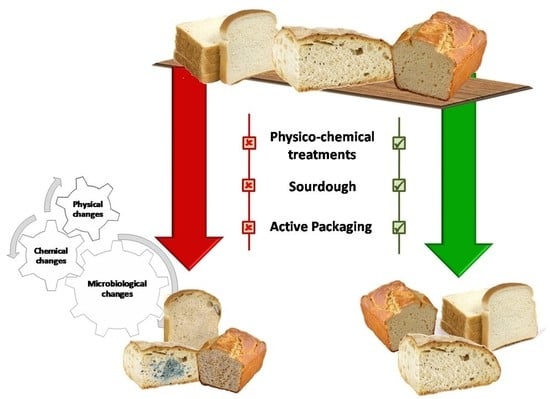
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Including and Excluding Criteria
3. Results
3.1. Factors Affecting Bread Shelf-Life
3.1.1. Bread Staling
3.1.2. Bread Spoilage
3.1.3. Gluten-Free Bread Shelf-Life
3.2. Traditional Methods to Improve Bread Shelf-Life
3.2.1. Physical Treatments
3.2.2. Chemical Treatments
3.2.3. Sourdough
3.3. Novel Strategies to Improve Bread Shelf-Life: Active Packaging
3.3.1. Active Packaging with Oxygen Absorbers
3.3.2. Active Packaging with Releasers: Antimicrobial Releasing Systems
3.3.3. Nanotechnology Application in Active Packaging
3.3.4. Safety Concerns of Active Packaging and Nanotechnology Application in Food Products/Legislation
4. Conclusions and Future Perspectives
Author Contributions
Conflicts of Interest
References
- Association of Plant Bakers. AIBI Aisbl AIBI Bread Report—AIBI. Available online: http://www.aibi.eu/aibi-bread-report/ (accessed on 27 December 2017).
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M.; Webb, C. Food Industry Wastes: Chapter 4. Use of Waste Bread to Produce Fermentation Products; Elsevier Inc.: San Diego, CA, USA, 2013; ISBN 978-0-12-805884-8. [Google Scholar]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Daifas, D.P.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf life and safety concerns of bakery products—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 19–55. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008R1333 (accessed on 27 December 2017).
- Van Long, N.N.; Joly, C.; Dantigny, P. Active packaging with antifungal activities. Int. J. Food Microbiol. 2016, 220, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Cauvain, S.P.; Young, L.S. Technology of Breadmaking; Springer Science & Business Media: New York, NY, USA, 2007; ISBN 978-0-387-38565-5. [Google Scholar]
- Pateras, I.M.C. Bread spoilage and staling. In Technology of Breadmaking; Springer: New York, NY, USA, 1998; pp. 240–261. ISBN 978-1-4613-5922-7. [Google Scholar]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Current and Forward-Looking Approaches to Technological and Nutritional Improvements of Gluten-Free Bread with Legume Flours: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1101–1122. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Cauvain, S. Bread Spoilage and Staling. In Technology of Breadmaking; Springer International Publishing: New York, NY, USA, 2015; pp. 279–302. ISBN 978-3-319-14686-7. [Google Scholar]
- Lavermicocca, P.; Valerio, F.; De Bellis, P.; Sisto, A.; Leguérinel, I. Chapter 16—Sporeforming bacteria associated with bread production: Spoilage and toxigenic potential. In Food Hygiene and Toxicology in Ready-to-Eat Foods; Kotzekidou, P., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 275–293. ISBN 978-0-12-801916-0. [Google Scholar]
- Valerio, F.; De Bellis, P.; Di Biase, M.; Lonigro, S.L.; Giussani, B.; Visconti, A.; Lavermicocca, P.; Sisto, A. Diversity of spore-forming bacteria and identification of Bacillus amyloliquefaciens as a species frequently associated with the ropy spoilage of bread. Int. J. Food Microbiol. 2012, 156, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Gobbetti, M. Physiology and Biochemistry of Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer: Boston, MA, USA, 2013; pp. 183–216. ISBN 978-1-4614-5424-3. [Google Scholar]
- Legan, J.D.; Voysey, P.A. Yeast spoilage of bakery products and ingredients. J. Appl. Bacteriol. 1991, 70, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Deak, T. Handbook of Food Spoilage Yeasts, Second Edition. Available online: https://www.crcpress.com/Handbook-of-Food-Spoilage-Yeasts-Second-Edition/Deak/p/book/9781420044935 (accessed on 6 May 2017).
- Recent Advances in the Formulation of Gluten-Free Cereal-Based Products—ScienceDirect. Available online: http://www.sciencedirect.com/science/article/pii/S0924224403002590 (accessed on 27 December 2017).
- Zannini, E.; Jones, J.M.; Renzetti, S.; Arendt, E.K. Functional replacements for gluten. Annu. Rev. Food Sci. Technol. 2012, 3, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.; Kunkel, A.; Gormley, T.R.; Arendt, E.K. The effect of dairy and rice powder addition on loaf and crumb characteristics, and on shelf life (intermediate and long-term) of gluten-free breads stored in a modified atmosphere. Eur. Food Res. Technol. 2003, 218, 44–48. [Google Scholar] [CrossRef]
- Moroni, A.V.; Bello, F.D.; Zannini, E.; Arendt, E.K. Impact of sourdough on buckwheat flour, batter and bread: Biochemical, rheological and textural insights. J. Cereal Sci. 2011, 54, 195–202. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Ribotta, P.D.; León, A.E.; Pérez, G.T. Effect of hydrocolloids on gluten-free batter properties and bread quality. Int. J. Food Sci. Technol. 2010, 45, 2306–2312. [Google Scholar] [CrossRef]
- Kadan, R.S.; Robinson, M.G.; Thibodeaux, D.P.; Pepperman, A.B., Jr. Texture and other Physicochemical Properties of Whole Rice Bread. J. Food Sci. 2001, 66, 940–944. [Google Scholar] [CrossRef]
- Hager, A.-S. Cereal Products for Specific Dietary Requirements. Evaluation and Improvement of Technological and Nutritional Properties of Gluten Free Raw Materials and End Products. Ph.D. Thesis, University College Cork, Cork, Ireland, 2013. [Google Scholar]
- Magan, N.; Arroyo, M.; Aldred, D. Mould Prevention in Bread. In Bread Making: Improving Quality; Woodhead Publishing: Boca Raton, FL, USA, 2003; pp. 500–514. ISBN 978-1-85573-553-8. [Google Scholar]
- Levinskaite, L. Susceptibility of food-contaminating Penicillium genus fungi to some preservatives and disinfectants. Ann. Agric. Environ. Med. 2012, 19, 85–89. [Google Scholar] [PubMed]
- Stratford, M.; Nebe-von-Caron, G.; Steels, H.; Novodvorska, M.; Ueckert, J.; Archer, D.B. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. Int. J. Food Microbiol. 2013, 161, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Suhr, K.I.; Nielsen, P.V. Effect of weak acid preservatives on growth of bakery product spoilage fungi at different water activities and pH values. Int. J. Food Microbiol. 2004, 95, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Dantigny, P. Control of food spoilage fungi by ethanol. Food Control 2011, 22, 360–368. [Google Scholar] [CrossRef]
- Katsinis, G.; Rigas, F.; Doulia, D. Synergistic effect of chemical preservatives with ethanol on the microbial shelf life of bread by factorial design. Int. J. Food Sci. Technol. 2008, 43, 208–215. [Google Scholar] [CrossRef]
- Berni, E.; Scaramuzza, N. Effect of ethanol on growth of Chrysonilia sitophila (‘the red bread mould’) and Hyphopichia burtonii (‘the chalky mould’) in sliced bread. Lett. Appl. Microbiol. 2013, 57, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hempel, A.W.; O’Sullivan, M.G.; Papkovsky, D.B.; Kerry, J.P. Use of smart packaging technologies for monitoring and extending the shelf-life quality of modified atmosphere packaged (MAP) bread: Application of intelligent oxygen sensors and active ethanol emitters. Eur. Food Res. Technol. 2013, 237, 117–124. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; De Marco, B.; Balestrieri, F.; Paoletti, F.; Russi, L.; Rossi, J. Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J. Agric. Food Chem. 2000, 48, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.V.; Dal Bello, F.; Arendt, E.K. Sourdough in gluten-free bread-making: An ancient technology to solve a novel issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, L.M.; Von Holy, A. Rope spoilage of bread. S. Afr. J. Sci. 1989, 85, 425–427. [Google Scholar]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Gänzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.A.M.; Dal Bello, F.; Arendt, E.K. The use of sourdough fermented by antifungal LAB to reduce the amount of calcium propionate in bread. Int. J. Food Microbiol. 2008, 125, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Coda, R.; Cassone, A.; Rizzello, C.G.; Nionelli, L.; Cardinali, G.; Gobbetti, M. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: Identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Nigro, F.; De Angelis, M.; Arnault, P.; Gobbetti, M. Long-Term Fungal Inhibitory Activity of Water-Soluble Extracts of Phaseolus vulgaris cv. Pinto and Sourdough Lactic Acid Bacteria during Bread Storage. Appl. Environ. Microbiol. 2008, 74, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L 135, 3–11. [Google Scholar]
- Ahvenainen, R. Novel Food Packaging Techniques; Woodhead Publishing: Boca Raton, FL, USA, 2003; ISBN 978-1-85573-675-7. [Google Scholar]
- Coles, R.; McDowell, D.; Kirwan, M.J. (Eds.) Food Packaging Technology, 1st ed.; Blackwell: Oxford, UK, 2003; ISBN 978-0-8493-9788-2. [Google Scholar]
- Soares, N.F.F.; Rutishauser, D.M.; Melo, N.; Cruz, R.S.; Andrade, N.J. Inhibition of microbial growth in bread through active packaging. Packag. Technol. Sci. 2002, 15, 129–132. [Google Scholar] [CrossRef]
- Salminen, A.; Latva-Kala, K.; Randell, K.; Hurme, E.; Linko, P.; Ahvenainen, R. The effect of ethanol and oxygen absorption on the shelf-life of packed sliced rye bread. Packag. Technol. Sci. 1996, 9, 29–42. [Google Scholar] [CrossRef]
- Berenzon, S.; Saguy, I.S. Oxygen Absorbers for Extension of Crackers Shelf-life. LWT Food Sci. Technol. 1998, 31, 1–5. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontominas, M.G. Shelf life extension of sliced wheat bread using either an ethanol emitter or an ethanol emitter combined with an oxygen absorber as alternatives to chemical preservatives. J. Cereal Sci. 2010, 52, 457–465. [Google Scholar] [CrossRef]
- Galić, K.; Curić, D.; Gabrić, D. Shelf life of packaged bakery goods—A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 405–426. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance. Off. J. Eur. Union 2011, L 12, 1–89. [Google Scholar]
- Labuza, T.P.; Breene, W.M. Applications of “active Packaging” for Improvement of Shelf-Life and Nutritional Quality of Fresh and Extended Shelf-Life Foods. J. Food Process. Preserv. 1989, 13, 1–69. [Google Scholar] [CrossRef]
- Franke, I.; Wijma, E.; Bouma, K. Shelf life extension of pre-baked buns by an ACTIVE PACKAGING ethanol emitter. Food Addit. Contam. 2002, 19, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Ramaswamy, H.S.; Simpson, B.K. Developments in food packaging technology. Part II. Storage aspects. Trends Food Sci. Technol. 1990, 1, 111–118. [Google Scholar] [CrossRef]
- Koukoutsis, J.; Smith, J.P.; Daifas, D.P.; Yayalan, V.; Cayouette, B.; Ngadi, M.; El-Khoury, W. In vitro studies to control the growth of microorganisms of spoilage and safety concern in high-moisture, high-pH bakery products. J. Food Saf. 2004, 24, 211–230. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.-T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Sekhon, B.S. Food nanotechnology—An overview. Nanotechnol. Sci. Appl. 2010, 3, 1–15. [Google Scholar] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.W.; Fu, Z.H. Nanotechnology and its application in packaging and packaging machinery. Packag. Eng. 2003, 24, 22–24. [Google Scholar]
- Sharma, P.; Oey, I.; Bremer, P.; Everett, D.W. Microbiological and enzymatic activity of bovine whole milk treated by pulsed electric fields. Int. J. Dairy Technol. 2017. [Google Scholar] [CrossRef]
- Azeredo, H. Antimicrobial Activity of Nanomaterials for Food Packaging Applications. In Nano-Antimicrobials; Cioffi, N., Rai, M., Eds.; Springer: Berlin, Germany, 2012; pp. 375–394. ISBN 978-3-642-24427-8. [Google Scholar]
- Völker, C.; Oetken, M.; Oehlmann, J. The biological effects and possible modes of action of nanosilver. Rev. Environ. Contam. Toxicol. 2013, 223, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Glińska, S.; Gapińska, M.; Ruman, T.; Nowak, A.; Aydin, E.; Gutarowska, B. Silver nanoparticles: A mechanism of action on moulds. Metallomics 2016, 8, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Shankar, S.; Wang, L.; Sothornvit, R.; Rhim, J. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, L.N.; Alvarez, V.A.; Vazquez, A. Processing and microstructure of PCL/clay nanocomposites. Mater. Sci. Eng. A 2007, 460–461, 121–129. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.; Chandra, T.S. Effect of electrospun montmorillonite-Nylon 6 nanofibrous membrane coated packaging on potato chips and bread. Innov. Food Sci. Emerg. Technol. 2014. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Peter, A.; Mihaly Cozmuta, L.; Nicula, C.; Crisan, L.; Baia, L.; Turila, A. Active Packaging System Based on Ag/TiO2 Nanocomposite Used for Extending the Shelf Life of Bread. Chemical and Microbiological Investigations. Packag. Technol. Sci. 2015, 28, 271–284. [Google Scholar] [CrossRef]
- Peter, A.; Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Nicula, C.; Ziemkowska, W.; Basiak, D.; Danciu, V.; Vulpoi, A.; Baia, L.; Falup, A.; et al. Changes in the microbiological and chemical characteristics of white bread during storage in paper packages modified with Ag/TiO2-SiO2, Ag/N-TiO2 or Au/TiO2. Food Chem. 2016, 197, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Ko, S. Nano-food packaging: An overview of market, migration research, and safety regulations. J. Food Sci. 2015, 80, R910–923. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food Antimicrobials Nanocarriers. Available online: https://www.hindawi.com/journals/tswj/2014/837215/ (accessed on 27 December 2017).
- Barbosa-Canovas, G.V.; Mortimer, A.; Lineback, D.; Spiess, W.; Buckle, K.; Colonna, P. Global Issues in Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; ISBN 978-0-08-092081-8. [Google Scholar]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A.; de Fátima Ferreira Soares, N. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Batlle, R.; Andújar, S.; Sánchez, C.; Nerín, C. Evaluation of Antimicrobial Active Packaging to Increase Shelf Life of Gluten-Free Sliced Bread. Packag. Technol. Sci. 2011, 24, 485–494. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- European Parliament and the Council. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, L 338, 4-17.004. [Google Scholar]
- Linsinger, T.P.J.; Chaudhry, Q.; Dehalu, V.; Delahaut, P.; Dudkiewicz, A.; Grombe, R.; von der Kammer, F.; Larsen, E.H.; Legros, S.; Loeschner, K.; et al. Validation of methods for the detection and quantification of engineered nanoparticles in food. Food Chem. 2013, 138, 1959–1966. [Google Scholar] [CrossRef] [PubMed]

| Spoilage Agents | Properties of Colony | |
|---|---|---|
| Moulds | Penicillium spp. | Blue/green, flat, spread rather slowly |
| Aspergillus niger | Black, fluffy, spreading with sporeheads often clearly visible | |
| Aspergillus flavus | Olive green | |
| Aspergillus candidus | Cream | |
| Aspergillus glaucus | Pale green | |
| Cladosporium spp. | Dark olive green, flat, spread slowly | |
| Neurospora stophila | Salmon pink, fluffy and fast spreading | |
| Rhizopus nigricans | Grey/black, very fluffy and fast spreading | |
| Mucor spp. | Grey | |
| Bacteria | Bacillus subtilis or Bacillus licheniformis | Irregular shape, white and dull colour |
| Yeasts | Hyphopichia burtonii | Slow growth on bread surface, low, white, spreading colonies |
| Pichia anomala | ||
| Scopsisfi buligera | ||
| Pichia burtonii | Very fast growth on bread | |
| Zygosaccharomyces bailii | Smooth, round, convex and white to cream coloured | |
| Torulaspora delbrueckii | ||
| Pichia membranifaciens | ||
| Candida parapsilosis | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, V.; Melini, F. Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation 2018, 4, 9. https://doi.org/10.3390/fermentation4010009
Melini V, Melini F. Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation. 2018; 4(1):9. https://doi.org/10.3390/fermentation4010009
Chicago/Turabian StyleMelini, Valentina, and Francesca Melini. 2018. "Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology" Fermentation 4, no. 1: 9. https://doi.org/10.3390/fermentation4010009
APA StyleMelini, V., & Melini, F. (2018). Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation, 4(1), 9. https://doi.org/10.3390/fermentation4010009