A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters
Abstract
:1. Introduction
2. Solid State Fermentation for PHA Production
3. Kinetics of PHA Biosynthesis
4. Discontinuous PHA Production Processes
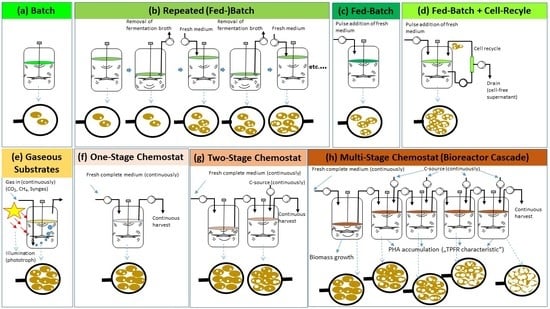
4.1. Batch Systems
4.2. Fed-Batch Systems
4.2.1. General Aspects of Fed-Batch Processes for PHA Production
4.2.2. Fed-Batch Processes with Cell Recycling for Biomass Retention
4.2.3. Repeated Fed-Batch for PHA Production
4.3. “Continuous Fed-Batch” Systems
4.3.1. Use of Liquid Substrates
4.3.2. Use of Gaseous Substrates CH4, CO2 and Syngas
5. Continuous PHA Production Processes Operated as Chemostats
5.1. General
5.2. One-Stage Chemostats
5.2.1. One-Stage Chemostats Based on Pure Cultures
5.2.2. Dual Nutrient Limited Chemostat Cultivation to Utilize “Inefficient” Carbon Sources for PHA Biosynthesis
5.2.3. Non-Sterile Single-Stage Chemostat Processes
5.3. Two-Stage Chemostats
5.3.1. Two-Stage Chemostats under Strict Sterility Precautions
5.3.2. Non-Sterile Two-Stage Chemostat Cultivation for PHA Production
5.4. Multi-Stage Chemostats
6. Conclusions
Conflicts of Interest
References
- Pasteur, L. Mémoire sur la fermentation alcoolique. Ann. Chim. Phys. 1860, 58, 323–426. [Google Scholar]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Kavitha, G.; Rengasamy, R.; Inbakandan, D. Polyhydroxybutyrate production from marine source and its application. Int. J. Biol. Macromol. 2017, 111, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.Y.U.; Castilho, N.A.S.; da Silva, M.A.C.; Miotto, M.C.; de Souza Lima, A.O. Prospecting for marine bacteria for polyhydroxyalkanoate production on low-cost substrates. Bioengineering 2017, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Dubey, S.; Singh, P.; Shrivastava, A.; Mishra, S. Biodegradable polymeric substances produced by a marine bacterium from a surplus stream of the biodiesel industry. Bioengineering 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Svoboda, Z.; Mikulikova, R. Use of controlled exogenous stress for improvement of poly(3-hydroxybutyrate) production in Cupriavidus necator. Folia Microbiol. 2010, 55, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Stankova, M.; Mravcova, L.; Svoboda, Z. Effect of ethanol and hydrogen peroxide on poly (3-hydroxybutyrate) biosynthetic pathway in Cupriavidus necator H16. World J. Microbiol. Biotechnol. 2010, 26, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Samek, O.; Marova, I. Evaluation of 3-hydroxybutyrate as an enzyme-protective agent against heating and oxidative damage and its potential role in stress response of poly(3-hydroxybutyrate) accumulating cells. Appl. Microbiol. Biotechnol. 2016, 100, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Krzyzanek, V.; Nebesarova, J.; Samek, O.; Kucera, D.; Benesova, P.; Hrubanova, K.; Milerova, M.; et al. The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol. 2017, 39, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Setten, L.; Lisi, C.; Maurelis, C.; Mozzicafreddo, M.; Cuccioloni, M.; Angeletti, M.; Ayub, N.D. Hydroxybutyrate prevents protein aggregation in the halotolerant bacterium Pseudomonas sp. CT13 under abiotic stress. Extremophiles 2012, 16, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, A.; Koller, M.; Braunegg, G.; Marqués-Calvo, M.S. Poly[(R)-3-hydroxybutyrate] production under different salinity conditions by a novel Bacillus megaterium strain. New Biotechnol. 2016, 33, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Krzyzanek, V.; Mravec, F.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Marova, I. Accumulation of poly(3-hydroxybutyrate) helps bacterial cells to survive freezing. PLoS ONE 2016, 11, e0157778. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, E.; Sedlacek, P.; Mravec, F.; Mullerova, L.; Samek, O.; Koller, M.; Hesko, O.; Kucera, D.; Marova, I.; Obruca, S. Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl. Microbiol. Biotechnol. 2018, 102, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Poly(hydroxyalkanoates) for food packaging: Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar] [CrossRef]
- De Souza, L.; Shivakumar, S. Polyhydroxyalkanoates (PHAs)-biodegradable polymers for ‘green’ food packaging materials. In Recent Advances in Biotechnology; Delhi Printer & Lamination: New Delhi, India, 2017; p. 149. [Google Scholar]
- Koller, M. Biodegradable and biocompatible polyhydroxyalkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Kowalczuk, M. Biomass-derived polyhydroxyalkanoates: Biomedical applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value, 1st ed.; Popa, V.I., Volf, I., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2018; pp. 271–313. ISBN 978-0-444-63774-1. [Google Scholar]
- Brigham, C.J.; Sinskey, A.J. Applications of polyhydroxyalkanoates in the medical industry. Int. J. Biotechnol. Well. Ind. 2012, 1, 52–60. [Google Scholar] [CrossRef]
- Junyu, Z.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Pierro, L.; Matturro, B.; Rossetti, S.; Sagliaschi, M.; Sucato, S.; Alesi, E.; Bartsch, E.; Arjmand, F.; Papini, M.P. Polyhydroxyalkanoate as a slow-release carbon source for in situ bioremediation of contaminated aquifers: From laboratory investigation to pilot-scale testing in the field. New Biotechnol. 2017, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; Miranda de Sousa Dias, M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, G.; Rocher, M.; Braunegg, G. Effects of low dissolved-oxygen concentrations on poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Alcaligenes eutrophus. Appl. Environ. Microbiol. 1997, 63, 827–833. [Google Scholar] [PubMed]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Fasl, H.; Stelzer, F.; Braunegg, G. Study on the effect of levulinic acid on whey-based biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl. Food Biotechnol. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Koller, M.; Miranda de Sousa Dias, M.; Rodríguez-Contreras, A.; Kunaver, M.; Žagar, E.; Kržan, A.; Braunegg, G. Liquefied wood as inexpensive precursor-feedstock for bio-mediated incorporation of (R)-3-hydroxyvalerate into polyhydroxyalkanoates. Materials 2015, 8, 6543–6557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashby, R.D.; Solaiman, D.K.; Nuñez, A.; Strahan, G.D.; Johnston, D.B. Burkholderia sacchari DSM 17165: A source of compositionally-tunable block-copolymeric short-chain poly(hydroxyalkanoates) from xylose and levulinic acid. Bioresour. Technol. 2017, 253, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, E. Novel precursors for production of 3-hydroxyvalerate-containing poly[(R)-hydroxyalkanoate]s. Biocatal. Biotransform. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Johnston, B.; Jiang, G.; Hill, D.; Adamus, G.; Kwiecień, I.; Zięba, M.; Sikorska, W.; Green, M.; Kowalczuk, M.; Radecka, I. The molecular level characterization of biodegradable polymers originated from polyethylene using non-oxygenated polyethylene wax as a carbon source for polyhydroxyalkanoate production. Bioengineering 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Miranda de Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch synthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.S.; de Almeida, M.C.M.; da Fonseca, M.M.R.; Cesário, M.T. Feeding strategies for tuning poly(3-hydroxybutyrate-co-4-hydroxybutyrate) monomeric composition and productivity using Burkholderia sacchari. Int. J. Biol. Macromol. 2017, 105, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Povolo, S.; Romanelli, M.G.; Basaglia, M.; Ilieva, V.I.; Corti, A.; Morelli, A.; Chiellini, E.; Casella, S. Polyhydroxyalkanoate biosynthesis by Hydrogenophaga pseudoflava DSM 1034 from structurally unrelated carbon sources. New Biotechnol. 2013, 30, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, A.; Yunoue, Y.; Goto, S.; Matsusaki, H. Biosynthesis of polyhydroxyalkanoate from steamed soybean wastewater by a recombinant strain of Pseudomonas sp. 61–63. Bioengineering 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Hajnal, I. The ‘PHAome’. Trends Biotechnol. 2015, 33, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ishii, N.; Sato, S.; Tsuge, T. Thermal properties and crystallization behaviors of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer 2012, 53, 3026–3034. [Google Scholar] [CrossRef]
- Li, Z.J.; Qiao, K.; Che, X.M.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the synthesis of the quadripolymer poly(glycolate-co-lactate-co-3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 2017, 44, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Yamada, M.; Matsumoto, K.I.; Tajima, K.; Satoh, Y.; Munekata, M.; Ohno, K.; Kohda, K.; Shimamura, T.; Kambe, H.; et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 17323–17327. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Rehm, B.H. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state fermentation for the production of poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Freire, D.M.G.; Castilho, L.R. Production of poly(3-hydroxybutyrate) by solid-state fermentation with Ralstonia eutropha. Biotechnol. Lett. 2004, 26, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M. Characterization of poly(3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.R.S., Jr. Estudo das Condições de Cultivo para a Produção de PHB por Cupriavidus necator em Fermentação no Estado Sólido. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2005. (In Spanish). [Google Scholar]
- Ramadas, N.V.; Singh, S.K.; Soccol, C.R.; Pandey, A. Polyhydroxybutyrate production using agro-industrial residue as substrate by Bacillus sphaericus NCIM 5149. Braz. Arch. Biol. Technol. 2009, 52, 17–23. [Google Scholar] [CrossRef]
- Braunegg, G.; Lefebvre, G.; Renner, G.; Zeiser, A.; Haage, G.; Loidl-Lanthaler, K. Kinetics as a tool for polyhydroxyalkanoate production optimization. Can. J. Microbiol. 1995, 41, 239–248. [Google Scholar] [CrossRef]
- Braunegg, G.; Korneti, L. Pseudomonas 2 F: Kinetics of growth and accumulation of poly-d(-)-3-hydroxybutyric acid (poly-HB). Biotechnol. Lett. 1984, 6, 825–829. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Koller, M.; Braunegg, M.; Horvat, P. Mathematical modelling as a tool for optimized PHA production. Chem. Biochem. Eng. Q. 2015, 29, 183–220. [Google Scholar] [CrossRef]
- Koller, M.; Vadljia, D.; Braunegg, G.; Atlić, A.; Horvat, P. Formal- and high-structured kinetic process modelling and footprint area analysis of binary imaged cells: Tools to understand and optimize multistage-continuous PHA biosynthesis. EuroBiotech J. 2017, 1, 203–211. [Google Scholar] [CrossRef]
- Cui, B.; Huang, S.; Xu, F.; Zhang, R.; Zhang, Y. Improved productivity of poly(3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl. Microbiol. Biotechnol. 2015, 99, 6009–6019. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.O.; Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Jog, J.P. Production and characterization of a biodegradable poly(hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.S.; Wong, Y.M.; Tsuge, T.; Sudesh, K. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers using jatropha oil as the main carbon source. Proc. Biochem. 2011, 46, 1572–1578. [Google Scholar] [CrossRef]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Gahlawat, G.; Srivastava, A.K. Enhancing the production of polyhydroxyalkanoate biopolymer by Azohydromonas australica using a simple empty and fill bioreactor cultivation strategy. Chem. Biochem. Eng. Q. 2018, 31, 479–485. [Google Scholar] [CrossRef]
- Marang, L.; van Loosdrecht, M.C.; Kleerebezem, R. Enrichment of PHA-producing bacteria under continuous substrate supply. New Biotechnol. 2018, 41, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. M Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Wen, Q.; Guo, Z. Efficient polyhydroxyalkanoate (PHA) accumulation by a new continuous feeding mode in three-stage mixed microbial culture (MMC) PHA production process. J. Biotechnol. 2015, 209, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioproc. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Z.; Wen, Q.; Zhao, L.; Lee, D.J.; Yang, L.; Wang, Y. Insights into Feast-Famine polyhydroxyalkanoate (PHA)-producer selection: Microbial community succession, relationships with system function and underlying driving forces. Water Res. 2018, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Silva, C.E.; Carvalho, G.; Reis, M.A.M. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Carucci, G.; Petrangeli Papinia, M.; Riccardi, C.; Majone, M.; Carrasco, F. Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res. 2005, 39, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Carucci, A.; Dionisi, D.; Majone, M.; Rolle, E.; Smurra, P. Aerobic storage by activated sludge on real wastewater. Water Res. 2001, 35, 3833–3844. [Google Scholar] [CrossRef]
- Carvalho, G.; Pedras, I.; Karst, S.M.; Oliveira, C.S.; Duque, A.F.; Nielsen, P.H.; Reis, M.A.M. Functional redundancy ensures performance robustness in 3-stage PHA-producing mixed cultures under variable feed operation. New Biotechnol. 2018, 40, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rhu, D.H.; Lee, W.H.; Kim, J.Y.; Choi, E. Polyhydroxyalkanoate (PHA) production from waste. Water Sci. Technol. 2003, 48, 221–228. [Google Scholar] [PubMed]
- Burniol-Figols, A.; Varrone, C.; Le, S.B.; Daugaard, A.E.; Skiadas, I.V.; Gavala, H.N. Combined polyhydroxyalkanoates (PHA) and 1, 3-propanediol production from crude glycerol: Selective conversion of volatile fatty acids into PHA by mixed microbial consortia. Water Res. 2018, 136, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wusiman, A.; Liu, X.; Wan, C.; Lee, D.J.; Tay, J. Polyhydroxyalkanoates (PHA) production from phenol in an acclimated consortium: Batch study and impacts of operational conditions. J. Biotechnol. 2018, 267, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl. Environ. Microbiol. 2000, 66, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly(3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol. Lett. 2001, 23, 235–240. [Google Scholar] [CrossRef]
- Da Cruz Pradella, J.G.; Ienczak, J.L.; Delgado, C.R.; Taciro, M.K. Carbon source pulsed feeding to attain high yield and high productivity in poly(3-hydroxybutyrate) (PHB) production from soybean oil using Cupriavidus necator. Biotechnol. Lett. 2012, 34, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwiecień, M.; Adamus, G.; Kowalczuk, M.; Stromeier, K.; Schober, S.; et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Koller, M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-scale production of functionalized mcl-PHA from grape pomace supplemented with fatty acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Quines, L.K.; De Melo, A.A.; Brandellero, M.; Mendes, C.R.; Schmidell, W.; Aragão, G.M.F. High cell density strategy for poly(3-hydroxybutyrate) production by Cupriavidus necator. Braz. J. Chem. Eng. 2011, 28, 585–596. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Penloglou, G.; Vasileiadou, A.; Chatzidoukas, C.; Kiparissides, C. Model-based intensification of a fed-batch microbial process for the maximization of polyhydroxybutyrate (PHB) production rate. Bioproc. Biosyst. Eng. 2017, 40, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of polyhydroxyalkanoates using hydrolyzates of spruce sawdust: Comparison of hydrolyzates detoxification by application of overliming, active carbon, and lignite. Bioengineering 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Ienczak, J.L.; Schmidt, M.; Quines, L.K.; Zanfonato, K.; da Cruz Pradella, J.G.; Schmidell, W.; de Aragao, G.M.F. Poly(3-hydroxybutyrate) production in repeated fed-batch with cell recycle using a medium with low carbon source concentration. Appl. Biochem. Biotechnol. 2016, 178, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; El-Najjar, T.; Virgolini, N.; Smerilli, M.; Neureiter, M. High cell-density production of poly(3-hydroxybutyrate) in a membrane bioreactor. New Biotechnol. 2017, 37, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lorantfy, B.; Ruschitzka, P.; Herwig, C. Investigation of physiological limits and conditions for robust bioprocessing of an extreme halophilic archaeon using external cell retention system. Biochem. Eng. J. 2014, 90, 140–148. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Steinbüchel, A. High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl. Environ. Microbiol. 2010, 76, 7890–7895. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Yu, J. Green technology for conversion of food scraps to biodegradable thermoplastic polyhydroxyalkanoates. Environ. Sci. Technol. 2002, 36, 5511–5516. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Mokhtari, Z.B.; Amai, T.; Tanaka, K. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H.; Criddle, C.S.; Lepech, M.D. Cradle-to-gate life cycle assessment for a cradle-to-cradle cycle: Biogas-to-bioplastic (and back). Environ. Sci. Technol. 2012, 46, 9822–9829. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Galega, W.M.; van Nostrand, J.D.; Yuan, T.; Zhou, J.; Criddle, C.S. Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour. Technol. 2015, 198, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cal, A.J.; Sikkema, W.D.; Ponce, M.I.; Franqui-Villanueva, D.; Riiff, T.J.; Orts, W.J.; Pieja, A.J.; Lee, C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016, 87, 302–307. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Arnáiz, E.; Merchán, L.; Lebrero, R.; Muñoz, R. Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: A step further in anaerobic digestion biorefineries. Chem. Eng. J. 2018, 333, 529–536. [Google Scholar] [CrossRef]
- García-Pérez, T.; López, J.C.; Passos, F.; Lebrero, R.; Revah, S.; Muñoz, R. Simultaneous methane abatement and PHB production by Methylocystis hirsuta in a novel gas-recycling bubble column bioreactor. Chem. Eng. J. 2018, 334, 691–697. [Google Scholar] [CrossRef]
- Drosg, B.; Fritz, I.; Gattermayr, F.; Silvestrini, L. Photo-autotrophic production of poly (hydroxyalkanoates) in cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Koller, M.; Maršalek, L. Cyanobacterial polyhydroxyalkanoate production: Status quo and Quo vadis? Curr. Biotechnol. 2015, 4, 464–480. [Google Scholar] [CrossRef]
- Ghysels, S.; Mozumder, M.S.I.; De Wever, H.; Volcke, E.I.; Garcia-Gonzalez, L. Targeted poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic production from carbon dioxide. Bioresour. Technol. 2018, 249, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M. Screening and Selection of a Cyanobacteria for Production of poly-β-hydroxybutyrate in a Closed Photobioreactor. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2009. [Google Scholar]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria biorefinery—Production of poly (3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB) synthesis by Spirulina sp. LEB 18 using biopolymer extraction waste. Appl. Biochem. Biotechnol. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015, 370164. [Google Scholar] [CrossRef]
- Chaudhari, S.T.; Dalai, A.K.; Bakhshi, N.N. Production of hydrogen and/or syngas (H2 + CO) via steam gasification of biomass-derived chars. Energy Fuel. 2003, 17, 1062–1067. [Google Scholar] [CrossRef]
- Revelles, O.; Calvillo, I.; Prieto, A.; Prieto, M.A. Syngas Fermentation for Polyhydroxyalkanoate Production in Rhodospirillum rubrum. In Hydrocarbon and Lipid Microbiology Protocols, 1st ed.; McGenity, T., Timmis, K., Nogales, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–119. ISBN 978-3-662-491317. [Google Scholar]
- Do, Y.S.; Smeenk, J.; Broer, K.M.; Kisting, C.J.; Brown, R.; Heindel, T.J.; Bobik, T.A.; DiSpririto, A.A. Growth of Rhodospirillum rubrum on synthesis gas: Conversion of CO to H2 and poly-β-hydroxyalkanoate. Biotechnol. Bioeng. 2007, 97, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; West, T.P.; Gibbons, W.R. Rhodospirillum rubrum: Utilization of condensed corn solubles for poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production. J. Appl. Microbiol. 2008, 104, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Revelles, O.; Beneroso, D.; Menendez, J.A.; Arenillas, A.; García, J.L.; Prieto, M.A. Syngas obtained by microwave pyrolysis of household wastes as feedstock for polyhydroxyalkanoate production in Rhodospirillum rubrum. Microb. Biotechnol. 2017, 10, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 2014, 160, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Karmann, S.; Follonier, S.; Egger, D.; Hebel, D.; Panke, S.; Zinn, M. Tailor-made PAT platform for safe syngas fermentations in batch, fed-batch and chemostat mode with Rhodospirillum rubrum. Microb. Biotechnol. 2017, 10, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Klask, C.; Raberg, M.; Heinrich, D.; Steinbüchel, A. Heterologous expression of various PHA synthase genes in Rhodospirillum rubrum. Chem. Biochem. Eng. Q. 2015, 29, 75–85. [Google Scholar] [CrossRef]
- Heinrich, D.; Raberg, M.; Fricke, P.; Kenny, S.T.; Morales-Gamez, L.; Babu, R.P.; O’Connor, K.E.; Steinbüchel, A. Synthesis gas (Syngas)-derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum rubrum. Appl. Environ. Microbiol. 2016, 82, 6132–6140. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A. Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem. Biochem. Eng. Q. 2014, 28, 65–77. [Google Scholar]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Senior, P.J.; Beech, G.A.; Ritchie, G.A.F.; Dawes, E.A. The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem. J. 1972, 128, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, B.A.; Lomaliza, K.; Chavarie, C.; Dube, B.; Bataille, P.; Ramsay, J.A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl. Environ. Microbiol. 1990, 56, 2093–2098. [Google Scholar] [PubMed]
- Zinn, M.; Witholt, B.; Egli, T. Dual nutrient limited growth: Models, experimental observations, and applications. J. Biotechnol. 2004, 113, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Hazenberg, W.; Prieto, M.; Witholt, B. Two-stage continuous process development for the production of medium-chain-length poly(3-hydroxyalkanoates). Biotechnol. Bioeng. 2001, 72, 19–24. [Google Scholar] [CrossRef]
- Durner, R.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth with octanoate in continuous culture at different dilution rates. Appl. Environ. Mmicrobiol. 2000, 66, 3408–3414. [Google Scholar] [CrossRef]
- Durner, R.; Zinn, M.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources. Biotechnol. Bioeng. 2001, 72, 278–288. [Google Scholar] [CrossRef]
- Zinn, M.; Weilenmann, H.U.; Hany, R.; Schmid, M.; Egli, T.H. Tailored synthesis of poly([R]-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/HV) in Ralstonia eutropha DSM 428. Eng. Life Sci. 2003, 23, 309–316. [Google Scholar] [CrossRef]
- Hartmann, R.; Hany, R.; Geiger, T.; Egli, T.; Witholt, B.; Zinn, M. Tailored biosynthesis of olefinic medium-chain-length Poly[(R)-3-hydroxyalkanoates] in Pseudomonas putida GPo1 with improved thermal properties. Macromolecules 2004, 37, 6780–6785. [Google Scholar] [CrossRef]
- Hany, R.; Brinkmann, M.; Ferri, D.; Hartmann, R.; Pletscher, E.; Rentsch, D.; Zinn, M. Crystallization of an aromatic biopolyester. Macromolecules 2009, 42, 6322–6326. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.C.; Wu, Q.; Chen, G.Q. Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 2015, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of culture conditions on poly(β-hydroxybutyric acid) production by Haloferax mediterranei. Appl. Environ. Microb. 1990, 56, 2517–2521. [Google Scholar]
- Yue, H.; Ling, C.; Yang, T.; Chen, X.; Chen, Y.; Deng, H.; Wu, Q.; Chen, J.; Chen, G.Q. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 2014, 7, 108–119. [Google Scholar] [CrossRef]
- Du, G.; Chen, J.; Yu, J.; Lun, S. Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-stage culture system. J. Biotechnol. 2001, 88, 59–65. [Google Scholar] [CrossRef]
- Mothes, G.; Ackermann, J.U. Synthesis of poly(3-hydroxybutyrate-co-4-hydrobutyrate) with a target mole fraction of 4-hydroxybutyric acid units by two-stage continuous cultivation of Delftia acidovorans P4a. Eng. Life Sci. 2005, 5, 58–62. [Google Scholar] [CrossRef]
- Hartmann, R.; Hany, R.; Witholt, B.; Zinn, M. Simultaneous biosynthesis of two copolymers in Pseudomonas putida GPo1 using a two-stage continuous culture system. Biomacromolecules 2010, 11, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Pederson, E.N.; McChalicher, C.W.; Srienc, F. Bacterial synthesis of PHA block copolymers. Biomacromolecules 2006, 7, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Xue, Y.S.; Aibaidula, G.; Chen, G.Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol. 2011, 102, 8130–8136. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wu, Q.; Chen, J.C.; Chen, G.Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metab. Eng. 2014, 26, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Horvat, P.; Vrana Špoljarić, I.; Lopar, M.; Atlić, A.; Koller, M.; Braunegg, G. Mathematical modelling and process optimization of a continuous 5-stage bioreactor cascade for production of poly[-(R)-3-hydroxybutyrate] by Cupriavidus necator. Bioproc. Biosyst. Eng. 2013, 36, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Lopar, M.; Vrana Špoljarić, I.; Atlić, A.; Koller, M.; Braunegg, G.; Horvat, P. Five-step continuous production of PHB analyzed by elementary flux, modes, yield space analysis and high structured metabolic model. Biochem. Eng. J. 2013, 79, 57–70. [Google Scholar] [CrossRef]
- Vadlja, D.; Koller, M.; Novak, M.; Braunegg, G.; Horvat, P. Footprint area analysis of binary imaged Cupriavidus necator cells to study PHB production at balanced, transient, and limited growth conditions in a cascade process. Appl. Microbiol. Biotechnol. 2016, 100, 10065–10080. [Google Scholar] [CrossRef] [PubMed]


| Production Strain | Raw Material | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|
| C. necator | Soya cake supplemented with molasses | PHB | 4.9 g PHB per kg solid | [49] |
| C. necator | Solid biodiesel waste supplemented with molasses | PHB | 2.1 g PHB per kg solid | [50] |
| B. sphaericus NII 0838 | Jack fruit seed hydrolysate on PU foam supports | PHB | 170 g PHB per kg PU support | [51] |
| Production Strain | Process Regime | Substrate | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|---|
| Chelatococcus daeguensis TAD1 | Batch | Glycerol | PHB | 0.81 g/g PHA in CDM, 0.01 g/(L h) | [59] |
| Halomonas campisalis | Batch | Maltose | PHB | 0.81 g/g PHA in CDM, 0.03 g/(L h) | [60] |
| Cupriavidus necator H16 | Batch | Jatropha oil | PHB | 0.9 g/g PHA in CDM, 0.17 g/(L h) | [61] |
| Bacillus firmus NI 0830 | Batch | Rice straw hydrolyzate | PHB | 0.89 g/g PHA in CDM, 0.02 g/(L h) | [62] |
| Azohydromonas australica DSM 1124 | Repeated batch | Sucrose | PHB | 0.82 g/g PHA in CDM, 0.31 g/(L h) | [63] |
| Chelatococcus sp. MW10 | Repeated batch (“cyclic batch”) | Glucose | PHB | 0.32 g/g PHA in CDM, 0.02 g/(L h) | [96] |
| C. necator DSM 545 | Fed-batch | Soybean oil | PHB | 0.81 g/g PHA in CDM, 2.5 g/(L h) | [83] |
| Burkholderia sacchari | Fed-batch | Sucrose | PHB | 0.72 g/g PHA in CDM, 1.87 g/(L h) | [34] |
| Hfx. mediterranei | Fed-batch | Crude glycerol phase | PHBHV | 0.76 g/g PHA in CDM, 0.12 g/(L h) | [84] |
| Pseudomonas citronellolis | Fed-batch | Low-quality biodiesel | mcl-PHA | 0.27 g/g PHA in CDM, 0.055 g/(L h) | [85] |
| Pseudomonas chlororaphis | Fed-batch | Low-quality biodiesel | mcl-PHA | 0.29 g/g PHA in CDM, 0.138 g/(L h) | [86] |
| Chelatococcus sp. MW10 | Fed-batch | Glucose | PHB | 0.51 g/g PHA in CDM, 0.05 g/(L h) | [96] |
| Pseudomonas putida KT2440 | Growth phase: Batch; Accumulation phase: Fed-batch | Growth phase: Grape pomace; accumulation phase: octanoic acid & 10-undecenoic acid | tailored mcl-PHA | 0.41 g/g PHA in CDM, 0.10 g/(L h) | [87] |
| Rec. Escherichia coli | Fed-batch; pH-stat | Whey powder | PHB | 0.81 g/g PHA in CDM, 2.57 g/(L h) | [81] |
| Rec. Escherichia coli | Fed-batch with cell recycle | Whey powder | PHB | 0.87 g/g PHA in CDM, 4.6 g/(L h) | [82] |
| C. necator DSM 545 | Fed-batch with cell recycle | Glucose & fructose | PHB | 0.69 g/g PHA in CDM, 1.0 g/(L h) | [93] |
| C. necator DSM 545 | Fed-batch with cell recycle | Glucose | PHB | 0.76 g/g PHA in CDM, 3.1 g/(L h) | [94] |
| Chelatococcus sp. MW10 | Repeated fed-batch (“cyclic fed-batch”) | Glucose | PHB | 0.12 g/g PHA in CDM, 0.07 g/(L h) | [96] |
| C. necator ATCC 17699 | “Continuous fed-batch” in airlift reactor | Organic acid cocktail | PHB & PHBHV | 0.60 g/g PHB in CDM; 0.73 g/g PHBHV in CDM | [97] |
| Methylocystis hirsuta | “Continuous fed-batch” in bubble column | Biogas with and without VFAs | PHB & PHBHV | 0.45 g/g PHB; 0.48-0.54 g/g PHBHV | [102] |
| Anabaena solitaria | “Continuous fed-batch” in flat panel bubble column PBR | CO2 | PHB | 0.03 g/g PHB in CDM; 0.191 g/(L d) | [107] |
| Synechocystis salina CCALA 192 | “Continuous fed-batch” in 200 L pilot plant tubular glass PBR | CO2 from industrial effluent gas | PHB | 0.09 g/g PHB in CDM | [108] |
| Rhodospirillum rubrum | “Continuous fed-batch” in bubbled and stirred laboratory reactor | Syngas from corn seed gasification | PHBHV | 0.09 g/g PHB in CDM; 0,0002 g/(L h) | [115] |
| Production Strain | Process Regime | Substrate | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|---|
| Azotobacter beijerinkii NCIB 9067 | One-stage chemostat | Glucose | PHB | 0.44 g/g PHA in CDM, g/(L h) | [124] |
| C. necator DSM 545 | One-stage chemostat | Glucose & propionic acid | PHBHV | 0.33 g/g PHA in CDM, 0.3 g/(L h) | [125] |
| P. putida GPo1 | One-stage chemostat; DNL | Octanoate | mcl-PHA | Up to 0.56 g/g PHA in CDM | [128] |
| C. necator DSM 428 | One-stage chemostat; DNL | Butyrate & valerate | PHBHV | n.r. | [130] |
| P. putida GPo1 | One-stage chemostat; DNL | 5-phenylpentanoate, octanoate, & 10-undecenoate | mcl-PHA | Up to 0.4 g/g PHA in CDM | [131] |
| Hfx. mediterranei | One stage chemostat; non-sterile | Glucose | PHB | 0.42 g/g PHA in CDM, 0.03 g/(L h) | [134] |
| Halomonas campaniensis LS21 | One stage chemostat; non-sterile | Mixed substrates | PHB | 0.7 g/g PHA in CDM, g/(L h) | [135] |
| Azohydromonas lata DSM 1124 | Two stage chemostat | Sucrose & propionic acid | PHBHV | 0.55 g/g PHA in CDM, 0. g/(L h) | [121] |
| C. necator WSH3 | Two stage chemostat | Crude glycerol phase | PHBHV | 0.72 g/g PHA in CDM, 1.24 g/(L h) | [136] |
| Delftia acidovorans | Two stage chemostat | Acetate & GBL | Poly(3HB-co-4HB) | 0.63 g/g PHA in CDM, 1.06 g/(L h) | [137] |
| Halomonas TD01 | Two stage chemostat; non-sterile | Glucose | PHB | 0.7 g/g PHA in CDM, g/(L h) | [140] |
| C. necator DSM 545 | Five stage chemostat | Glucose | PHB | 0.77 g/g PHA in CDM, 2.31 g/(L h) | [142] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. https://doi.org/10.3390/fermentation4020030
Koller M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation. 2018; 4(2):30. https://doi.org/10.3390/fermentation4020030
Chicago/Turabian StyleKoller, Martin. 2018. "A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters" Fermentation 4, no. 2: 30. https://doi.org/10.3390/fermentation4020030
APA StyleKoller, M. (2018). A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation, 4(2), 30. https://doi.org/10.3390/fermentation4020030