Abstract
Vast amounts of information can be obtained by systematic explorations of synergy between phytochemicals and probiotics, which is required for the development of non-dairy probiotic products, globally. Evidence confirms that the same probiotic strain can have different efficiencies depending on the food matrix. One such functional property, viz., antipathogenicity of the probiotic strain against Shigella was investigated in this study. The potential of two fruit based (apple and sea buckthorn) beverage matrices fortified with Lactobacillus rhamnosus GG (ATCC 53103), against outbreak-causing serotypes of Shigella dysenteriae (ATCC 29026) and Shigella flexneri (ATCC 12022) was evaluated. The originality of this study lies in the fact that the functionality assessment was performed with a more realistic approach under storage conditions from 0–14 days at 4 °C. The finding confirms that Lactobacillus rhamnosus GG (LGG) differs in its potential depending on beverage matrices. Principal Component Analysis (PCA) clustered the matrices based on their pathogen clearance. LGG fortified sea buckthorn beverage matrix showed 99% clearance of S. dysenteriae within the first hour compared to 11% in apple beverage matrix. Interestingly, S. flexneri showed more resistance and was cleared (99%) in the LGG fortified sea buckthorn beverage matrix within three hours compared to 5.6% in apple matrix.
1. Introduction
Infectious diarrhea is one of the leading causes of mortality in developing and under developed countries [1]. In 2015, approximately 2.3 billion illnesses and 1.3 million deaths were reported due to diarrheal diseases worldwide, out of which children below five years of age accounted for 40% of diarrheal deaths [2]. The major bacterial entero-pathogens reported as etiological agents for diarrhea associated diseases are Shigella, entero-toxigenic E. coli, Salmonella, Yersenia and Campylobacter [3]. Shigellosis—a severe foodborne illness caused by Shigella—is an invasive infection of the colon, characterized by a series of systemic manifestations, beginning from short-lasting diarrhea to acute inflammatory bowel disease [4]. Shigella sp. is highly communicable at an extremely low infectivity dose, and is generally transmitted person-to-person as a result of poor hygiene [5]. Shigellosis results in about 800,000 fatalities annually throughout the world, predominantly in Sub-Saharan Africa and South Asia [6,7]. The Global Enteric Multicenter Study (GEMS) on the burden and etiology of moderate-to-severe diarrheal illness (MSD) established Shigella as 1 of 4 top pathogens in Sub-Saharan Africa and South-East Asia [8]. Among South-East Asian countries, the occurrence rate of Shigellosis was highest in India (21.7%), followed by Cambodia (19.8%), Philippines (17.9%), and Vietnam (9.0%) [9]. While antimicrobial agents are the mainstay of therapy, the emergence of drug resistance has limited the choice of antibiotics for treating Shigellosis [4,10]. Additionally, the negative influences of antibiotics on gut-microbiota homeostasis have paved the way for alternative treatments of Shigellosis [11].
Probiotics are an efficient alternative therapy for acute infectious gastroenteritis and diarrhea-associated diseases [12]. This is mainly due to immune stimulation and immunomodulatory effects, together with the modulation of the gut microflora [13]. Several studies have reported the beneficial effect of probiotics against entero-pathogenic infections [14,15]. Zhang et al. (2011) reported strong antimicrobial action of four probiotic strains, Lactobacillus paracasei subp. paracasei M5-L, Lactobacillus rhamnosus J10-L, Lactobacillus casei Q8-L and L. rhamnosus GG (LGG) against Shigella sp. [16]. Similarly, Kakisu et al. (2013) explained the antagonistic potential of L. plantarum CIDCA 83114, against Shigella flexneri and Shigella soneii [17].
Evidence from these observations have paved the way for probiotic functional foods [18,19]. Various foods and beverages have been explored as second-generation vehicles for probiotic delivery [20,21,22]. One such class of vehicles are fruits and vegetable matrices [23,24]. However, selection of an appropriate food system as a delivery matrix remains a crucial factor, since the finished products need to have adequate probiotic viability, acceptable sensory attributes and potent bio-efficacies [25,26,27]. There exists a two-way relationship between plant phenolics and gut microflora [28]. Gut microflora, including probiotics, are mostly anaerobes or facultative aerobes; therefore, these phenolic compounds are acting as antioxidants that scavenge and reduce the oxygen, thereby promoting its growth [29]. Furthermore, phenolic compounds enhance the potential of probiotics to produce antimicrobial compounds, thereby upgrading the safety of food and human health [30].
Earlier we have explored two phenolic-rich beverage matrices—apple (APJ) and sea buckthorn (SBT) based matrices—as probiotic delivery vehicles. We demonstrated efficacious pathogen clearance of entero-pathogenic E. coli (ATCC 43887), Salmonella enteritidis (ATCC 13076), Shigella dysenteriae (ATCC 29026) and non-pathogenic E. coli (ATCC 25922) in freshly made beverage matrices [31,32]. However, in order to be effective, any probiotic fortified beverage must retain effectiveness of the probiotic strain in spite of a long shelf life. In this study, we have applied principal component analysis (PCA) to cluster the response of different beverage matrices based on their anti-Shigella potential, when stored for 14 days at 4 °C.
2. Materials and Methods
2.1. Raw Materials and Bacterial Strains
Malt extract powder (M) (Imperial Malts Ltd., Gurgaon, Haryana, India), was the major ingredient used to prepare the probiotic fortified apple and sea buckthorn beverages. Probiotic Lactic Acid Bacteria (LAB), Lactobacillus rhamnosus GG (ATCC 53103) was used for the fortification in the fruit matrices. Pathogenic strains of Shigella, namely, Shigella dysenteriae (ATCC 29026), and Shigella flexneri (ATCC 12022) were used as test organisms in this study. All strains were procured from American Type Culture Collection (Microbiologics, MN, USA).
2.2. Development of Juice Matrices
The fruit based probiotic beverages were prepared according to Sireswar et al. (2017) [31]. Briefly, fresh apples and sea buckthorn berries were collected from Himachal Pradesh, India and were subjected to juice extraction, filtration and adjusted to pH 4.5 with tri-sodium citrate (Sigma-Aldrich Co., St Louis, MO, USA). The freshly extracted sea buckthorn and apple juice were separately supplemented with M at a concentration of 5% (w/v) and pasteurized at 95 °C for 5 min. The 2 juice matrices, namely, M-supplemented apple juice (APJ + M) and M-supplemented sea buckthorn juice (SBT + M) were individually fortified with approximately 8 log cfu/mL of L. rhamnosus GG (ATCC 53103) (LR), namely, L. rhamnosus GG fortified, malt-supplemented apple juice (APJ + M + LR) and L. rhamnosus GG fortified, malt-supplemented sea buckthorn juice (SBT + M + LR). All probiotic beverages were tightly sealed and stored at 4 °C for further evaluation.
2.3. Evaluation of Anti-Shigella Potential of the Probiotic Beverages during Shelf Storage
The anti-Shigella potential of the probiotic beverages were evaluated according to Sireswar et al. (2017) with slight modifications [31]. A known volume of each probiotic-fortified beverage (containing approximately 8 log cfu/mL of probiotic), were inoculated with about 6 log cfu/mL of each strain, Shigella dysenteriae and Shigella flexneri, and incubated at 37 °C for 48 h. Aliquots of the sample were taken at 0, 1, 2, 3, 4, 8, 24 and 48 h to enumerate both the pathogens as well as the probiotics. At each time interval, the sample was serially diluted and plated on MacConkey agar for pathogens and incubated at 37 °C for 24 h, and on MRS agar plates for probiotics were incubated at 37 °C for 48 h. The same procedure was repeated at equal intervals up to 14 days of shelf storage at 4 °C. The data are expressed in terms of percentage pathogen clearance:
where A is the initial cfu/mL and B is the final cfu/mL of the pathogen. The initial and final log cfu data has been mentioned in Tables S1 and S2 in the Supplementary File.
log reduction (L) = log10 A − log10 B
percentage pathogen clearance (%) = (1 − 10−L) × 100
2.4. Total Phenolic Content (TPC)
The total phenolic content of the probiotic beverages (APJ + M + LR and SBT + M + LR) were analyzed according to the Folin–Ciocalteu method by Singleton et al. (1999) [33]. Briefly, 20 μL of each juice sample was added to 1.58 mL of water and 100 μL of Folin–Ciocalteu reagent (Sigma-Aldrich co.). The mixture was vortexed for 30 s and allowed to stand for 5 min. To the mixture, 300 μL of saturated sodium carbonate was added and incubated at 20 °C for 2 h and absorbance was determined at 765 nm. The results were expressed as µg/mL of Gallic Acid Equivalent (GAE) using a Gallic acid standard curve of 0, 50, 100, 150, 250 and 500 µg GAE/mL. Unfortified juice matrices were taken as control (APJ + M and SBT + M).
2.5. Statistical Analysis
To test the significance of the antagonistic potential of each beverage against the Shigella strains, Principal Component Analysis (PCA) was applied to the percentage pathogen clearance data set through multivariate exploratory techniques using XLSTAT software (version 2015.6, Addinsoft, SARL, Paris, France). A data matrix was constructed where the samples from each interval, that is 0, 7 and 14 days, were inserted in rows and the antagonistic potential against each strain at each hour of co-incubation were placed in columns and 2D plots were generated to predict the variability among the principal components. All total phenolic content (TPC) data are as an average of triplicate experiments with standard deviation.
3. Results and Discussion
Percentage Pathogen Clearance and Principal Component Analysis (PCA)
It is well known that plant phenolic compounds have a significant impact on the functionality of probiotics [34,35,36]. A few reports specifically on the potential of sea buckthorn and apple juice against Shigella sp. also exist [37,38]. The complex matrix environment and its physicochemical attributes, especially of beverage matrices for probiotic delivery, alter the potential of probiotic strains by modifying their efficacy and functionality during storage [39]. There is sufficient evidence of change in functionality of probiotics without any alteration in the level of viable cells during storage [40]. Hence, it is necessary to know the alterations that may occur in the functionality of probiotics in a food matrix during storage, even though the viable count may not have changed. Earlier we have reported the symbiosis of apple and sea buckthorn phenolics, with probiotic action against entero-pathogens [31]. Those observations formed the premise for the current evaluation, where we apply PCA to evaluate the efficiency of the influence of beverage matrices fortified with L. rhamnosus GG for elimination of Shigella dysenteriae and Shigella flexneri during a storage period.
Table 1 and Table 2 represent the percentage pathogen clearance of the unfortified matrices, APJ + M, SBT + M, and probiotic fortified beverages, APJ + M + LR and SBT + M + LR during shelf storage at regular intervals (Day 0, Day 7 and Day 14) against S. dysenteriae and S. flexneri, respectively. Results indicated that the highest pathogen clearance was demonstrated in SBT + M + LR against both Shigella species. A pathogen clearance of 99.99% was observed within 1 h of co-incubation in SBT + M + LR at day 0 against S. dysenteriae. Similar pathogen clearance (99.99%) was observed only after 4 h in APJ + M + LR. For SBT + M + LR, when co-incubated for 1 h with S. flexneri, only 8.4% clearance was shown, and for APJ + M + LR clearance was as low as 5.09%. However, S. flexneri was finally eliminated after 3 h of incubation in the case of SBT + M + LR. Interestingly, SBT + M + LR, when stored for 7 days showed nearly complete pathogen clearance after a lag of 2 h, and APJ + M + LR after lag of 4 h, indicating that storage may influence the functionality of the probiotic. Similar functional evaluations of stored probiotic beverages fortified with LGG have not been carried out and hence are not available for comparison. However, a somewhat similar assessment of elimination of P. aeruginosa, E. coli, S. aureus and S. enteritidis by L. casei and B. animalis-fortified whey cheese matrix was performed by Madureira et al. (2011) [40]. The authors reported elimination of E. coli, S. aureus and S. enteritidis at the much lower contaminant inoculum level of 103–104 cfu/g after a much longer co-incubation period. No elimination was observed with respect to P. aeruginosa in L. casei-fortified matrix after 14 days of storage [40].

Table 1.
Percentage pathogen clearance potential of L. rhamnosus GG fortified sea buckthorn (SBT) and apple juice (APJ) against Shigella dysteneriae during 14 days of shelf storage.

Table 2.
Percentage pathogen clearance potential of L. rhamnosus GG fortified sea buckthorn (SBT) and apple juice (APJ) against Shigella flexneri during 14 days of shelf storage.
Another important observation of the study was that fortification of probiotic strain L. rhamnosus GG significantly enhanced the anti-Shigella potential of both SBT and APJ matrices. Unfortified juice matrices (SBT + M) showed 76.1% pathogen clearance for S. dysenteriae and 56% pathogen clearance for S. flexneri after 8 h of co-incubation. While comparing the two pathogens, S. dysenteriae was more easily susceptible to inhibition than S. flexneri. This may be because of the higher stress resistance and acid tolerance of S. flexneri. In and colleagues in 2013 reported the sensitivity of S. dysenteriae to organic acids in comparison to other Shigella serotypes, namely, S. soneii, S. boydii and S. flexneri [41]. Our results corroborate the previous results of Bagamboula et al. (2002), which established the survival of S. flexneri in highly acidic apple juice for 14 days [42]. The lag in pathogen inhibition could also be due to the hfq protein in S. flexneri, acting as a key factor in maximal adaptation to environmental stress—especially low pH conditions—thereby regulating acid stress tolerance within the matrix environment [43].
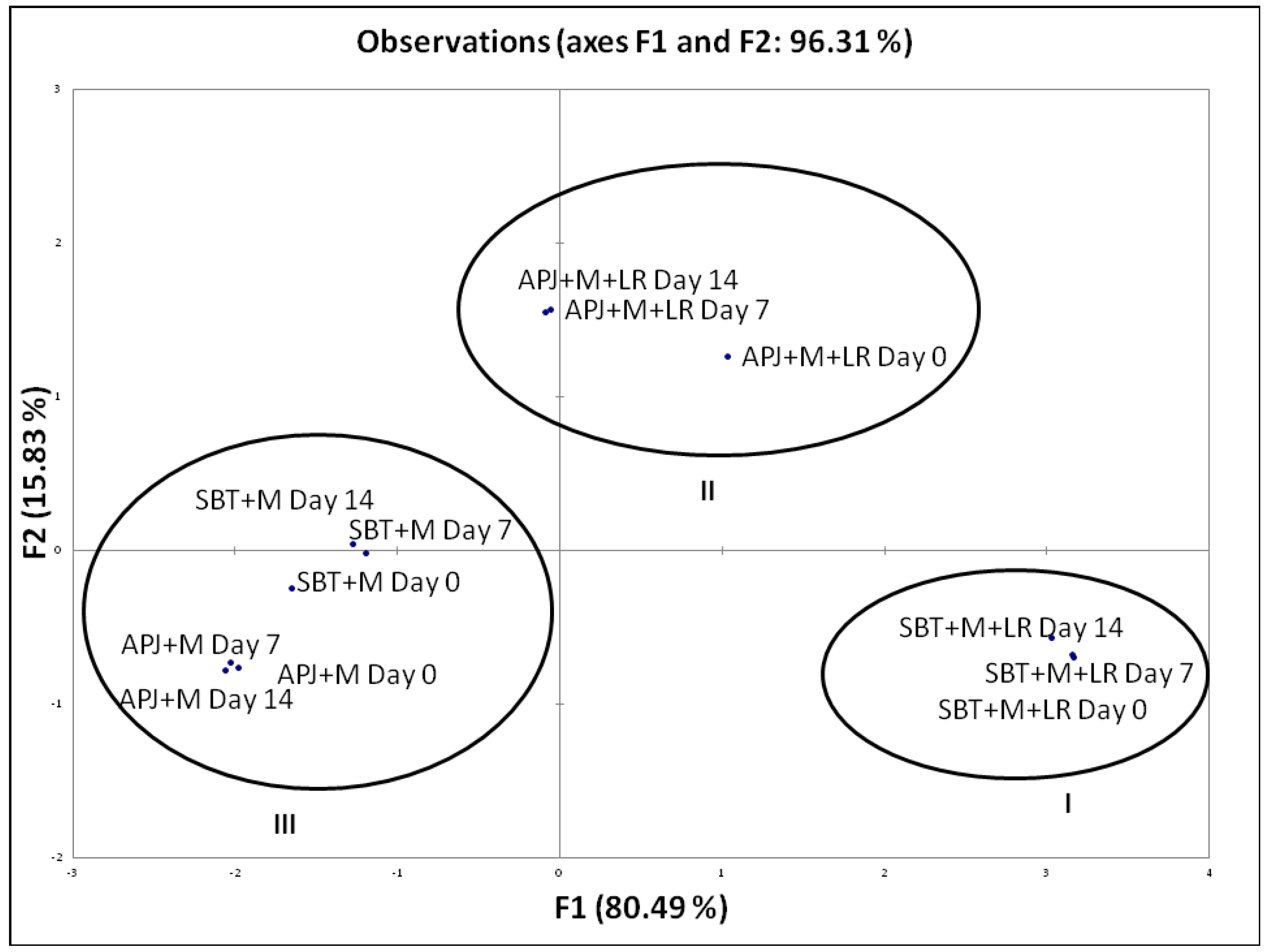
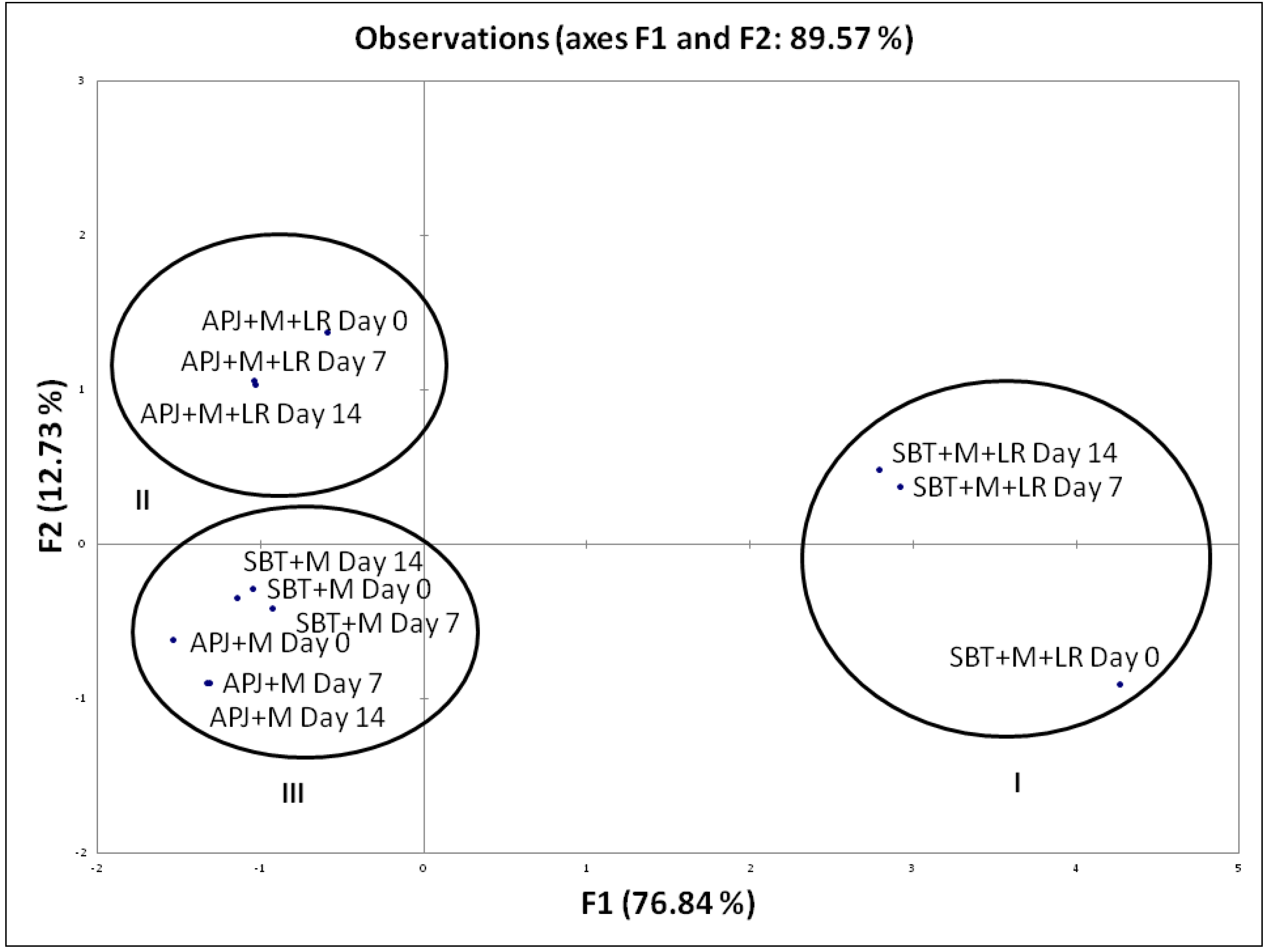
To distinctively discriminate the pathogen clearance ability of the probiotic fortified matrices, we carried out a Principal Component Analysis (PCA) based on their anti-Shigella potential during shelf storage. This analysis is of importance as it provides an indication of the matrix-specific pathogen clearance potential. The biplot (Figure 1) explains 96.31% of total variation, with factor 1 on x-axis depicting 80.49% of the data and factor 2 on the y-axis explaining 15.83%. Similarly, in Figure 2, the biplot explains 89.57% of total variation, with factor 1 on x-axis depicting 76.84% of the data and factor 2 on the y-axis explaining 12.73%. Factor 1 accounts for the highest percentage pathogen clearance against S. dysenteriae and S. flexneri respectively. Factor 2 was characterized by the lag in time with respect to pathogen clearance.
Figure 1.
PCA biplot of the percentage pathogen clearance of unfortified matrices and L. rhamnosus GG fortified beverages against S. dysenteriae. The anti-S. dysenteriae activity of the LGG fortified beverages were performed at regular intervals (Day 0, Day 7 and Day 14) during the storage period. S. dysenteriae was co-incubated in different beverage matrices at 37 °C and evaluated for viable counts. The pathogen clearance percentage was calculated as per the formula provided in Section 2.3.
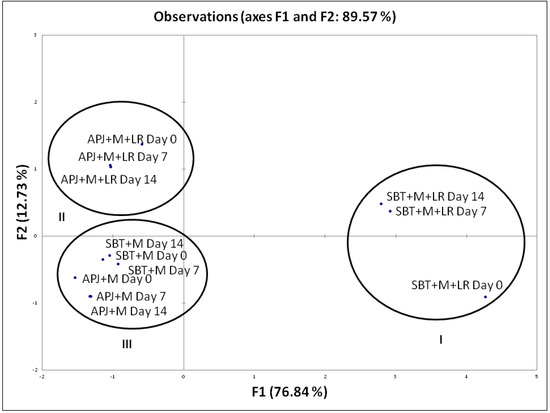
Figure 2.
PCA biplot of the percentage pathogen clearance of unfortified matrices and L. rhamnosus GG fortified beverages against S. flexneri. The anti-S. flexneri activity of the LGG fortified beverages were performed at regular intervals (Day 0, Day 7 and Day 14) during the storage period. S. dysenteriae was co-incubated in different beverage matrices at 37 °C and evaluated for viable counts. The pathogen clearance percentage was calculated as per the formula provided in Section 2.3.
The PCA biplot (Figure 1 and Figure 2) illustrated specific functionality differences between matrices and clustered the beverages according to their composition; Cluster I—SBT + M + LR, Cluster II—APJ + M + LR, Cluster III—APJ + M and SBT + M. As mentioned earlier, the absence of probiotics had a negative impact on the pathogen clearance ability of the juice matrices.
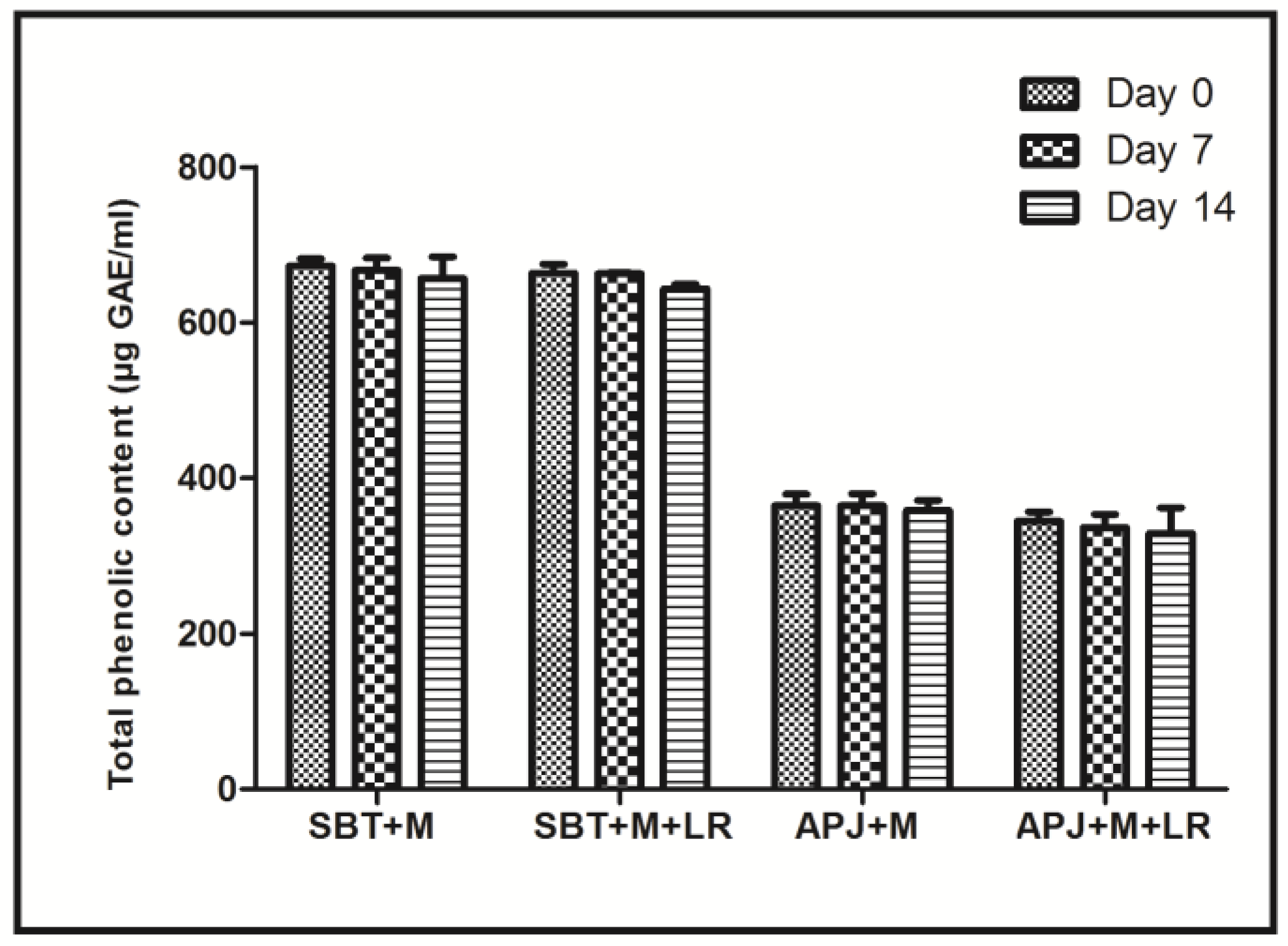
Another important conclusion that may be drawn from the PCA is that among probiotic fortified beverages, the total phenolic content of the matrices also influenced the efficiency. A synergistic interaction of probiotics along with phenolics is indicated. Reports of the selective stimulation of probiotic strains Lactobacillus bulgaricus and Bifidibacterium bifidis in pasteurized blueberry juice along with the inhibition of several foodborne pathogens indicated that irrespective of the presence of a probiotic strain, the phenolic content synergistically played a role in the elimination of both the Shigella stains [44]. To confirm the synergistic role, the total phenolic content of the beverage matrices were estimated (Figure 3). The SBT + M + LR had nearly two-fold more phenolic content (663.18 µgGAE/mL) as compared to APJ + M + LR (342.87 µgGAE/mL).
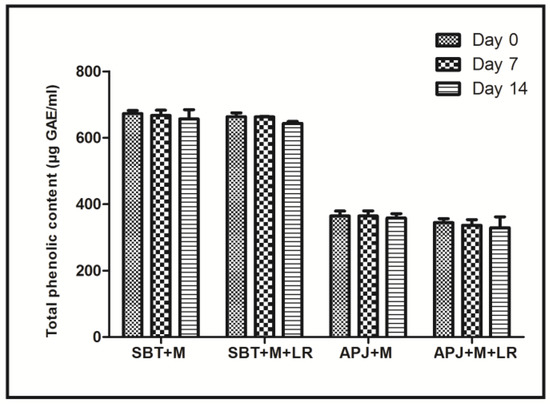
Figure 3.
Total phenolic content of unfortified and L. rhamnosus GG fortified sea buckthorn and apple beverages during 14 days of cold storage.
Importantly, under storage conditions, the levels of phenolics in the beverage matrices did not change significantly during the 14 days period. Thus, it may be concluded that the functionality difference and the clustering shown by the beverage matrices is due to the two differentiators, viz., the presence of probiotics and presence of phenolics. In future we will be reporting on the phenolic profile of the matrices.
Some articles have been dedicated to the evaluation of antipathogenic potential of dairy probiotic products [45,46,47]. This is probably a rare study where the probiotic functionality has been evaluated in fruit beverage matrix with respect to its antipathogenic activity in more realistic conditions where it has been stored for 7–14 days at 4 °C.
4. Conclusions
We were able to successfully establish that together, phenolic and probiotic strain, L. rhamnosus GG can form an effective barrier against two potent diarrhea-causing Shigella strains. Such rational designing of fruit based probiotic beverages could be of great help to second-generation probiotic product developers who could utilize competitive LAB strains and juice phenolics for creating effective barriers against Shigella during storage.
Supplementary Materials
The following are available online at http://www.mdpi.com/2311-5637/4/2/34/s1, Table S1: Antagonistic activity of L. rhamnosus GG fortified sea buckthorn (SBT) and apple juice (APJ) against Shigella dysteneriae during 14 days storage, Table S2: Antagonistic activity of L. rhamnosus GG fortified sea buckthorn (SBT) and apple juice (APJ) against Shigella flexneri during 14 days storage.
Author Contributions
G.D. conceived and designed the experiments; S.S. performed the experiments and analyzed the data; Department of Science and Technology (DST) provided reagents/materials/analysis tools; S.S. and G.D. wrote the paper. D.M. provided his expert comments during the manuscript preparation.
Acknowledgments
The work was funded under the project No.: SEED/TSP/CODER/008/2012.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- GBD. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 acute and chronic diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar]
- Kotloff, K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. N. Am. 2017, 64, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.K. Shigellosis. J. Microbiol. 2005, 43, 133–143. [Google Scholar] [PubMed]
- Sur, D.; Ramamurthy, T.; Deen, J.; Bhattacharya, S.K. Shigellosis: Challenges & management issues. Indian J. Med. Res. 2004, 20, 454–462. [Google Scholar]
- Greenhill, C. Diarrhoea: Tackling the problem of moderate-to-severe diarrhoea in developing countries. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Rabaa, M.A.; Thanh, D.P.; De Lappe, N.; Cormican, M.; Valcanis, M.; Howden, B.P.; Wangchuk, S.; Bodhidatta, L.; Mason, C.J.; Nguyen, T.N.; et al. South Asia as a reservoir for the global spread of ciprofloxacin-resistant Shigella sonnei: A cross-sectional study. PLoS Med. 2016, 13, e1002055. [Google Scholar] [CrossRef]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Youn, S.K.; Lee, S.; Choi, Y.H. Epidemiological Characteristics of Imported Shigellosis in Korea, 2010–2011. Osong Public Health Res. Perspect. 2013, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kosek, M.; Yori, P.P.; Olortegui, M.P. Shigellosis update: Advancing antibiotic resistance, investment empowered vaccine development, and green bananas. Curr. Opin. Infect. Dis. 2017, 23, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.S.; Jalgaonkar, S.; Shahani, S.; Kulkarni, V.N. Probiotics: Current trends in the treatment of diarrhoea. Hong Kong Med. J. 2010, 16, 213–218. [Google Scholar] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Davoodabadi, A.; Dallal, M.M.; Foroushani, A.R.; Douraghi, M.; Harati, F.A. Antibacterial activity of Lactobacillus spp. isolated from the feces of healthy infants against enteropathogenic bacteria. Anaerobe 2015, 34, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Du, M.; Yi, H.; Guo, C.; Tuo, Y.; Han, X.; Li, J.; Zhang, L.; Yang, L. Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol. Res. 2011, 167, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kakisu, E.; Bolla, P.; Abraham, A.G.; De Urraza, P.; de Antoni, G.L. Lactobacillus plantarum isolated from kefir: Protection of cultured Hep-2 cells against Shigella invasion. Int. Dairy J. 2013, 33, 22–26. [Google Scholar] [CrossRef]
- Carlson, J.; Slavin, J. Health benefits of fibre, prebiotics and probiotics: A review of intestinal health and related health claims. Qual. Assur. Saf. Crop 2016, 8, 539–554. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Galgano, F.; Condelli, N.; Caruso, M.C.; Colangelo, M.A.; Favati, F. Probiotics and prebiotics in fruits and vegetables: Technological and sensory aspects. In Beneficial Microbes in Fermented and Functional Foods; CRC Press-Taylor & Francis Group: Abingdon, UK, 2014; pp. 189–206. [Google Scholar]
- Güler-Akın, M.B.; Ferliarslan, I.; Akın, M.S. Apricot probiotic drinking yoghurt supplied with inulin and oat fiber. Adv. Microbiol. 2016, 6, 999–1009. [Google Scholar] [CrossRef]
- Homayoni Rad, A.; Vaghef Mehrabany, E.; Alipoor, B.; Vaghef Mehrabany, L. The comparison of food and supplement as probiotic delivery vehicles. Crit. Rev. Food Sci. Nutr. 2016, 56, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Alwis, A.D.P.S.; Perera, O.D.A.N.; Weerahewa, H.L.D. Development of a Novel Carrot-based Synbiotic Beverage using Lactobacillus casei 431®. J. Agric. Sci. 2016, 11, 178–185. [Google Scholar] [CrossRef][Green Version]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.N.; Messaoud, G.B.; Desobry, S.; Costa, J.M.C.; Rodrigues, S. Effect of drying technique and feed flow rate on bacterial survival and physicochemical properties of a non-dairy fermented probiotic juice powder. J. Food Eng. 2016, 189, 45–54. [Google Scholar] [CrossRef]
- Dharmasena, M.; Barron, F.; Fraser, A.; Jiang, X. Refrigerated Shelf Life of a Coconut Water-Oatmeal Mix and the Viability of Lactobacillus Plantarum Lp 115-400B. Foods 2015, 4, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl. Microbiol. Biotechnol. 2012, 95, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wishon, L.M.; Song, D.; Ibrahim, S. Effect of metals on growth and functionality of Lactobacillus and Bifidobacteria. Milchwissenschaft 2010, 65, 369–372. [Google Scholar]
- Sireswar, S.; Dey, G.; Sreesoundarya, T.K.; Sarkar, D. Design of probiotic-fortified food matrices influence their antipathogenic potential. Food Biosci. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Sireswar, S.; Dey, G.; Dey, K.; Kundu, A. Evaluation of Probiotic L. rhamnosus GG as a Protective Culture in Sea Buckthorn-Based Beverage. Beverages 2017, 3, 48. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 11, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Gunenc, A.; Khoury, C.; Legault, C.; Mirrashed, H.; Rijke, J.; Hosseinian, F. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT-Food Sci. Technol. 2016, 66, 490–495. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT-Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Arora, R.; Mundra, S.; Yadav, A.; Srivastava, R.B.; Stobdan, T. Antimicrobial activity of seed, pomace and leaf extracts of sea buckthorn (Hippophae rhamnoides L.) against foodborne and food spoilage pathogens. Afr. J. Biotechnol. 2012, 11, 10424–10430. [Google Scholar] [CrossRef]
- Van Opstal, I.; Bagamboula, C.F.; Theys, T.; Vanmuysen, S.C.M.; Michiels, C.W. Inactivation of Escherichia coli and Shigella in acidic fruit and vegetable juices by peroxidase systems. J. Appl Microbiol. 2006, 101, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Binetti, A.; Burns, P.; Reinheimer, J. Cell viability and functionality of probiotic bacteria in dairy products. Front. Microbiol. 2011, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Amorim, M.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res. Int. 2011, 44, 465–470. [Google Scholar] [CrossRef]
- In, Y.W.; Kim, J.J.; Kim, H.J.; Oh, S.W. Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J. Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Acid tolerance of Shigella sonnei and Shigella flexneri. J. Appl. Microbiol. 2002, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, L.; Wang, Y.; Li, P.; Zhu, J.; Qiu, S.; Hao, R.; Wu, Z.; Li, W.; Song, H. hfq regulates acid tolerance and virulence by responding to acid stress in Shigella flexneri. Res. Microbiol. 2015, 166, 166–476. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Wu, V.C.; White, J.; Tadepalli, S.; Andre, E.E. The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiol. 2012, 30, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez, E.; Calzada, J.; Arqués, J.L.; Rodrıguez, J.M.; Nunez, M.; Medina, M. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157: H7 in cheese. Int. Dairy J. 2005, 15, 51–57. [Google Scholar] [CrossRef]
- McAuliffe, O.; Hill, C.; Ross, R.P. Inhibition of Listeria monocytogenes in cottage cheese manufactured with a lacticin 3147-producing starter culture. J. Appl. Microbiol. 1999, 86, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Arqués, J.L.; Rodríguez, E.; Gaya, P.; Medina, M.; Guamis, B.; Nunez, M. Inactivation of Staphylococcus aureus in raw milk cheese by combinations of high-pressure treatments and bacteriocin-producing lactic acid bacteria. J. Appl. Microbiol. 2005, 98, 254–260. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).