Lachancea thermotolerans Applications in Wine Technology
Abstract
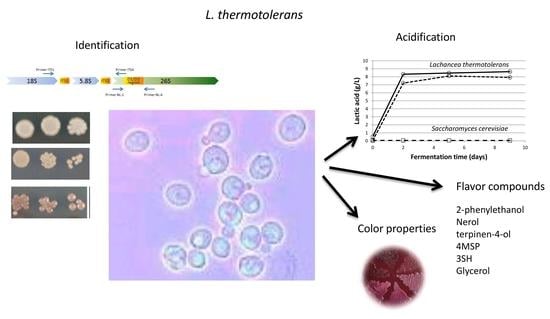
:1. Introduction
2. Isolation and Selection
3. Biotechnological Application: Wine Acidification
4. Metabolic Profile and Influence on Wine Aroma and Flavor
5. Effect on Wine Color
6. Special Wines
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurtzman, C.P. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res. 2003, 4, 233–245. [Google Scholar] [CrossRef]
- Ganter, P.F. Yeast and invertebrate associations. In Biodiversity and Ecophysiology of Yeasts; Rosa, C.A., Gabor, P., Eds.; Springer: Berlin, Germany, 2006; pp. 303–370. [Google Scholar]
- Hranilovic, A.; Bely, M.; Masneuf-Pomarede, I.; Jiranek, V.; Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 2017, 12, e0184652. [Google Scholar] [CrossRef] [PubMed]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Schnierda, T.; Bauer, F.F.; Divol, B.; van Rensburg, E.; Görgens, J.F. Optimization of carbon and nitrogen medium components for biomass production using non-Saccharomyces wine yeasts. Lett. Appl. Microbiol. 2014, 58, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.-A.; Kurtzman, C.P. Chapter 41—Lachancea Kurtzman. In The Yeasts, 5th ed.; Elsevier: New York, NY, USA, 2011; pp. 511–519. ISBN 9780444521491. [Google Scholar]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavor. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Blaiotta, G. Potential role of yeast strains isolated from grapes in the production of Taurasi DOCG. Front. Microbiol. 2016, 7, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues Alda, J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Mills, D.A.; Johannsen, E.A.; Cocolin, L. Yeast diversity and persistence in Botrytis-affected wine fermentations. Appl. Environ. Microbiol. 2002, 68, 4884–4893. [Google Scholar] [CrossRef] [PubMed]
- Holm Hansen, E.; Nissen, P.; Sommer, P.; Nielsen, J.C.; Arneborg, N. The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 2001, 91, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- CHR-Hansen. CONCERTO™. Available online: https://www.chr-hansen.com/en/food-cultures-and-enzymes/wine/cards/product-cards/concerto?countryreset=1 (accessed on 30 June 2018).
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Yeast monitoring of wine mixed or sequential fermentations made by native strains from D.O. “Vinos de Madrid” using Real-Time Quantitative PCR. Front. Microbiol. 2017, 8, 2520. [Google Scholar] [CrossRef] [PubMed]
- Fiori, S.; Urgeghe, P.P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nally, M.C.; Ponsone, M.L.; Pesce, V.M.; Toro, M.E.; Vazquez, F.; Chulze, S. Evaluation of behaviour of Lachancea thermotolerans biocontrol agents on grape fermentations. Lett. Appl. Microbiol. 2018, 67, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Isolation, selection and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Beverage Series; Academic Press-Elsevier: Cambridge, MA, USA, 2018; Volume 19, in press. [Google Scholar]
- Prakitchaiwattana, C.J.; Fleet, G.H.; Heard, G.M. Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res. 2004, 4, 865–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Lopandic, K.; Tiefenbrunner, W.; Gangl, H.; Mandl, K.; Berger, S.; Leitner, G.; Abd-Ellah, G.A.; Querol, A.; Gardner, R.C.; Sterflinger, K.; et al. Molecular profiling of yeasts isolated during spontaneous fermentations of Austrian wines. FEMS Yeast Res. 2008, 8, 1063–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baleiras Couto, M.M.; Reizinho, R.G.; Duarte, F.L. Partial 26S rDNA restriction analysis as a tool to characterise non-Saccharomyces yeasts present during red wine fermentations. Int. J. Food Microbiol. 2005, 102, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.; Ciani, M.; Domizio, P.; Zara, S.; Budroni, M.; Carboni, A.; Mannazzu, I. A culture-independent PCR-based method for the detection of Lachancea thermotolerans in wine. Ann. Microbiol. 2014, 64, 403–406. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.I.; Mulet, A. Growth of yeast species during the fermentation of musts Inoculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1990, 41, 156–159. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Callejo, M.J.; González, C.; Morata, A. Use of non-Saccharomyces yeasts in bottle fermentation of aged beers. In Brewing Technology; Kanauchi, M., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/brewing-technology/use-of-non-saccharomyces-yeasts-in-bottle-fermentation-of-aged-beers (accessed on 30 June 2018).
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. A novel bacteria-free method for sour beer production. Food Microbiol. 2018, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P. Use of Lachancea thermotolerans to Improve pH in Red Wines. Effect of the Coinoculation with Oenococcus oeni. Master’s Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2018. [Google Scholar]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic Fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of non-Saccharomyces yeasts using different methodologies and evaluation of their compatibility with malolactic fermentation. S. Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Carlina, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R.; Maifreni, M. Glycerol and other fermentation products of apiculate wine yeasts. J. Appl. Microbiol. 1997, 82, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing oxygen pulses to modulate Lachancea thermotolerans–Saccharomyces cerevisiae Chardonnay fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Nieuwoudt, H.H.; Prior, B.A.; Pretorius, I.S.; Bauer, F.F. Glycerol in South African table wines: An assessment of its relationship to wine quality. S. Afr. J. Enol. Vitic. 2002, 23, 22–30. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine fermentation microbiome: A landscape from different Portuguese wine appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of Yeasts in Wine Colour. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016; pp. 285–305. Available online: https://www.intechopen.com/books/grape-and-wine-biotechnology/influence-of-yeasts-in-wine-colour (accessed on 30 June 2018).
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Escott, C.; Morata, A.; Loira, I.; Tesfaye, W.; Suarez-Lepe, J.A. Characterization of polymeric pigments and pyranoanthocyanins formed in microfermentations of non-Saccharomyces yeasts. J. Appl. Microbiol. 2016, 121, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef] [PubMed]





| Biotechnology | LT Fermentation Time | Initial pH | Final pH | Comments |
|---|---|---|---|---|
| Sequential fermentation LT → S. Cerevisiae LT → S. pombe | 0–4 days Most of the acidification is performed in the 3 first days | 3.9–4.2 | 3.5–3.7 depending on LT strains and implantation success | Population inoculated of LT must be >log6 CFU/mL |
| Mixed fermentation LT + S. Cerevisiae LT + S. pombe | 0–6 days | 3.9–4.2 | 3.5–3.7 depending on LT strains and implantation success | Population inoculated of LT must be >log6 CFU/mL Ratio between LT + S. cerevisiae (or S. pombe) must be log6/log2 including wild Sacch. |
| Coinoculation LT + LAB (O. oeni) and subsequent inoculation of S. cerevisiae | 0–6 days | 3.9–4.2 | 3.3–3.5 depending on LT strains, implantation success and lactic acid production by LAB | Strong pH reduction. Light alcohol degree reduction |
| Acidification pH or Lactic Acid (g/L) | Fermentative Power (Ethanol % v/v) | Aroma, Flavor, Polysaccharides and Color | Molecules | Reference |
|---|---|---|---|---|
| 3.5 → 3.2; 5.1 g/L lactic acid | 9 | Acidity | Lactic acid | [16] |
| 3.2 → 2.9 in coinoculation | 4–8 | [12] | ||
| 3.53 → 3.33 sequential 48 h 0.1 units reduction sequential 24 h | 10.5 | [13] | ||
| 1–16.6 g/L | [28] | |||
| 1.2–2.6 g/ | 9.5–10.4 | [14] | ||
| Esters | 2-phenylethanol, phenethyl propionate, ethyl salicylate, methyl salicylate, 3-methylthio-1-propanol | [50] | ||
| Enhanced formation of terpenes & Thiols | Nerol, terpinen-4-ol 4MSP & 3SH | [48] | ||
| β-d-glucosidase Carbon-sulfur lyase | Free terpenes and thiols | [47,48] | ||
| 7.7 | Polysaccharides/mannoproteins | N-acetyl hexosamines | [51] | |
| Polyalcohols | Glycerol | [12,16] | ||
| Polymeric pigments | malvidin-3-glucoside-ethyl-catechin dimer | [40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. https://doi.org/10.3390/fermentation4030053
Morata A, Loira I, Tesfaye W, Bañuelos MA, González C, Suárez Lepe JA. Lachancea thermotolerans Applications in Wine Technology. Fermentation. 2018; 4(3):53. https://doi.org/10.3390/fermentation4030053
Chicago/Turabian StyleMorata, Antonio, Iris Loira, Wendu Tesfaye, María Antonia Bañuelos, Carmen González, and José Antonio Suárez Lepe. 2018. "Lachancea thermotolerans Applications in Wine Technology" Fermentation 4, no. 3: 53. https://doi.org/10.3390/fermentation4030053
APA StyleMorata, A., Loira, I., Tesfaye, W., Bañuelos, M. A., González, C., & Suárez Lepe, J. A. (2018). Lachancea thermotolerans Applications in Wine Technology. Fermentation, 4(3), 53. https://doi.org/10.3390/fermentation4030053