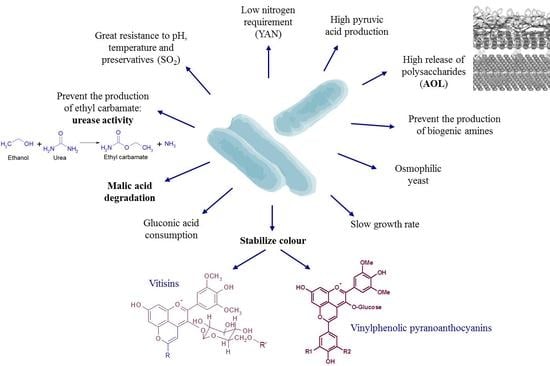
Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition
Abstract
:1. Origin and Features of Schizosaccharomyces pombe
2. Wine Acidity Modulation
3. Influence on Wine Colour
4. Large Release of Polysaccharides During Ageing on Lees
5. Bio-Tool for Ensuring Wine Safety
6. Sparkling Wines and Other Fermented Beverages (Ice Wines, Beers)
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: London, UK, 2010. [Google Scholar]
- Atilgan, E.; Magidson, V.; Khodjakov, A.; Chang, F. Morphogenesis of the fission yeast cell through cell wall expansion. Curr. Biol. 2015, 25, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Vaughan Martini, A. Evaluation of phylogenetic relationships among fission yeast by nDNA/nDNA reassociation and conventional taxonomic criteria. Yeast 1991, 7, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A.; Del Rosario, E.C.; Simanis, V. The role of Schizosaccharomyces pombe dma1 in spore formation during meiosis. J. Cell Sci. 2010, 123, 3284–3293. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int. J. Food Sci. Technol. 2012, 47, 2101–2108. [Google Scholar] [CrossRef]
- Moreno, S.; Klar, A.; Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991, 194, 795–823. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Galvez, L.; Palomero, F.; Calderón, F.; Morata, A.; Palmero, D.; Suárez-Lepe, J.A. Schizosaccharomyces selective differential media. Afr. J. Microbiol. Res. 2013, 7, 3026–3036. [Google Scholar] [CrossRef]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Russell, P. Growth and the environment of Schizosaccharomyces pombe. Cold Spring Harb. Protoc. 2016, 3, 210–226. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Palomero, F.; Benito, S.; Calderón, F.; Morata, A. Oenological versatility of Schizosaccharomyces spp. Eur. Food Res. Technol. 2012, 235, 375–383. [Google Scholar] [CrossRef]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Wine spoilage yeasts: Control strategy. In Yeast-Industrial Applications; InTech: London, UK, 2017; pp. 89–116. [Google Scholar]
- Rankine, B.C. The importance of yeasts in determining the composition and quality of wines. Vitis 1968, 7, 22–49. [Google Scholar]
- Callejo, M.J.; González, C.; Morata, A. Use of non-Saccharomyces yeasts in bottle fermentation of aged beers. In Brewing Technology; InTech: London, UK, 2017; pp. 101–119. [Google Scholar]
- Proenol ProMalic. Biological Deacidification of Musts and Wines. Available online: https://www.proenol.com/web/produtos/leveduras-encapsuladas/promalic-detail (accessed on 22 August 2018).
- Ramon-Portugal, F.; Silva, S.; Taillandier, P.; Strehaiano, P. Inmovilización de Levaduras. Usos enológicos Actuales. Vinidea.net., 2003; pp. 1–8. Available online: https://www.infowine.com/intranet/libretti/libretto922-01-1.pdf (accessed on 22 August 2018).
- Silva, S.; Ramón-Portugal, F.; Andrade, P.; Abreu, S.; de Fatima Texeira, M.; Strehaiano, P. Malic acid consumption by dry immobilized cells of Schizosaccharomyces pombe. Am. J. Enol. Vitic. 2003, 54, 50–55. [Google Scholar]
- Su, J.; Wang, T.; Wang, Y.; Li, Y.-Y.; Li, H. The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl. Microbiol. Biotechnol. 2014, 98, 2395–2413. [Google Scholar] [CrossRef] [PubMed]
- Rankine, B.C. Decomposition of L-malic acid by wine yeasts. J. Sci. Food Agric. 1966, 17, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Gallander, J.F. Deacidification of eastern table wines with Schizosaccharomyces pombe. Am. J. Enol. Vitic. 1977, 28, 65–68. [Google Scholar]
- Kluyver, A.J. Biochemische Suikerbepalingen. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, January 1914. [Google Scholar]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen–Bloom, M. Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr. Genet. 2003, 43, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Rhee, C.-H.; Park, H.-D. Degradation of malic acid by Issatchenkia orientalis KMBL 5774, an acidophilic yeast strain isolated from Korean grape wine pomace. J. Microbiol. 2007, 45, 521–527. [Google Scholar] [PubMed]
- Hong, S.K.; Lee, H.J.; Park, H.J.; Hong, Y.A.; Rhee, I.K.; Lee, W.H.; Choi, S.W.; Lee, O.S.; Park, H.D. Degradation of malic acid in wine by immobilized Issatchenkia orientalis cells with oriental oak charcoal and alginate. Lett. Appl. Microbiol. 2010, 50, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Zott, K.; Claisse, O.; Lucas, P.; Coulon, J.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Characterization of the yeast ecosystem in grape must and wine using real-time PCR. Food Microbiol. 2010, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Hong, Y.-A.; Park, H.-D. Co-fermentation of grape must by Issatchenkia orientalis and Saccharomyces cerevisiae reduces the malic acid content in wine. Biotechnol. Lett. 2008, 30, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Otaki, A.; Naitoh, A.; Tanaka, H. Controlled simultaneous deacidification and alcohol fermentation of a high-acid grape must using two immobilized yeasts, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1993, 44, 371–377. [Google Scholar]
- Snow, P.G.; Gallander, J.F. Deacidification of white table wines through partial fermentation with Schizosaccharomyces pombe. Am. J. Enol. Vitic. 1979, 30, 45–48. [Google Scholar]
- Morata, A.; González, C.; Tesfaye, W.; Loira, I.; Suárez-Lepe, J.A. Maceration and fermentation. New technologies to increase extraction. In Red Wine Technology; Morata, A., Ed.; Elsevier-Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Suárez-Lepe, J.A. Influence of yeasts in wine colour. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; InTech: London, UK, 2016; pp. 288–289. [Google Scholar]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.C.; Suárez-Lepe, J.A. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int. J. Food Microbiol. 2012, 159, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, P.; Rojas, V.; Genovés, S.; Vallés, S. A preliminary search for anthocyanin-β-d-glucosidase activity in non-Saccharomyces wine yeasts. Int. J. Food Sci. Technol. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lepe, J.A.; Morata, A. Capítulo IV.-El atractivo visual del color del vino tinto: Implicaciones microbiológicas en nuevas formas estables y pérdidas de antocianos durante la fermentación. In Levaduras para vinificación en tinto [Yeasts for red winemaking]; Tecnovino: Bilbao, Spain, 2015. [Google Scholar]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.D.C.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Morata, A.; Loira, I.; Tesfaye, W.; Suarez-Lepe, J.A. Characterization of polymeric pigments and pyranoanthocyanins formed in microfermentations of non-Saccharomyces yeasts. J. Appl. Microbiol. 2016, 121, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Products formed in model wine solutions involving anthocyanins, procyanidin B2, and acetaldehyde. J. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Kraková, L.; Kuchta, T.; Pangallo, D. Assessment of color adsorption by yeast using grape skin agar and impact on red wine color. OENO One 2015, 49, 195–203. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J.A. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Kreger-Van Rij, N.J.W. The Yeasts: A Taxonomic Study; Elsevier: New York, NY, USA, 1984. [Google Scholar]
- Lodder, J. The Yeasts; a Taxonomic Study; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1970. [Google Scholar]
- Manners, D.J.; Meyer, M.T. The molecular structures of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohydr. Res. 1977, 57, 189–203. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S. Cell Wall Chemistry, Morphogenesis, and Taxonomy of Fungi. Annu. Rev. Microbiol. 1968, 22, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Weijman, A.C.M.; Golubev, W.I. Carbohydrate patterns and taxonomy of yeast and yeast-like fungi. In The Expanding Realm of Yeast-Like Fungi; Hoong, G.S., Smith, M.T., Weijman, A.C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 361–371. [Google Scholar]
- Kopecká, M.; Fleet, G.H.; Phaff, H.J. Ultrastructure of the Cell Wall of Schizosaccharomyces pombe Following Treatment with Various Glucanases. J. Struct. Biol. 1995, 114, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Maclean, N. Electron microscopy of a fission yeast, Schizosaccharomyces pombe. J. Bacteriol. 1964, 88, 1459–1466. [Google Scholar] [PubMed]
- Vivas, N.; Vivas de Gaulejac, N.; Nonier, M.F.; Nedjma, M. Les phénomènes colloïdaux et l’interêt des lies dans l’élevage des vins rouges: Une nouvelle approche technologique et méthodologique. 2° partie-Méthodes destinés aux élevages en cuves de grande capacité. Revue Française D’oenologie 2001, 190, 32–35. [Google Scholar]
- Vivas, N.; Vivas de Gaulejac, N.; Nonier, M.F.; Nedjma, M. Les phénomènes colloïdaux et l’interêt des lies dans l’élevage des vins rouges: Une nouvelle approche technologique et méthodologique. 1° partie-Methodes traditionnelles d’élevage sur lie destinés aux vins en fûts. Revue Française D’oenologie 2001, 189, 33–38. [Google Scholar]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Leger, B.; Charpentier, C.; Feuillat, M. Effet colloide-protecteur d’extraits de parois de levures sur la stabilité tartrique d’une solution hydro-alcoolique modele. J. Int. des Sci. la Vigne du Vin 1993, 27, 13–22. [Google Scholar] [CrossRef]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine-Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Moine-Ledoux, V.; Dubourdieu, D. An invertase fragment responsible for improving the protein stability of dry white wines. J. Sci. Food Agric. 1999, 79, 537–543. [Google Scholar] [CrossRef]
- Waters, E.J.; Pellerin, P.; Brillouet, J.-M. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Frankel, E.N.; German, J.B.; Kinsella, J.; Parks, E.; Kanner, J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Salmon, J.M.; Fornairon-Bonnefond, C.; Mazauric, J.P. Interactions between Wine Lees and Polyphenols: Influence on Oxygen Consumption Capacity during Simulation of Wine Aging. J. Food Sci. 2002, 67, 1604–1609. [Google Scholar] [CrossRef]
- Mazauric, J.-P.; Salmon, J.-M. Interactions between Yeast Lees and Wine Polyphenols during Simulation of Wine Aging: I. Analysis of Remnant Polyphenolic Compounds in the Resulting Wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.-P.; Salmon, J.-M. Interactions between Yeast Lees and Wine Polyphenols during Simulation of Wine Aging: II. Analysis of Desorbed Polyphenol Compounds from Yeast Lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicas: Quantification and characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Lencioni, L.; Gobbi, M.; Mannazzu, I.; Ciani, M.; Domizio, P. Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking. Fermentation 2018, 4, 14. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- De Fatima, M.; Centeno, F.; Palacios, A. Desacidificación biológica de mosto a través de la inoculación de levadura Schizosaccharomyces pombe encapsulada como alternativa a la no producción de aminas biógenas. In Proceedings of the International Symposium of Microbiology and Food Safety in Wine “Microsafetywine”, Vilafranca del Penedès, Spain, 20–21 November 2007. [Google Scholar]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Ortega, J.M.; Medina, M.; Mauricio, J.C. Gluconic acid consumption in wines by Schizosaccharomyces pombe and its effect on the concentrations of major volatile compounds and polyols. J. Agric. Food Chem. 2004, 52, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C.; Medina, M.; Moreno, J.J. Effect of Schizosaccharomyces pombe on aromatic compounds in dry sherry wines containing high levels of gluconic acid. J. Agric. Food Chem. 2004, 52, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, M.W.; Rodriguez, S.B.; Honey, N.K.; Thornton, R.J. Purification and characterization of urease from Schizosaccharomyces pombe. Can. J. Microbiol. 1996, 42, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Uthurry, C.A.; Suárez-Lepe, J.A.; Lombardero, J.; García Del Hierro, J.R. Ethyl carbamate production by selected yeasts and lactic acid bacteria in red wine. Food Chem. 2006, 94, 262–270. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT-Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Li, J.; Ma, T.; Han, S.; Morata, A.; Suárez Lepe, J.A. Biotechnology of ice wine production. In Advances in Biotechnology for Food Industry; Elsevier: New York, NY, USA, 2018; pp. 267–300. [Google Scholar]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation 2018, 4, 70. https://doi.org/10.3390/fermentation4030070
Loira I, Morata A, Palomero F, González C, Suárez-Lepe JA. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation. 2018; 4(3):70. https://doi.org/10.3390/fermentation4030070
Chicago/Turabian StyleLoira, Iris, Antonio Morata, Felipe Palomero, Carmen González, and José Antonio Suárez-Lepe. 2018. "Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition" Fermentation 4, no. 3: 70. https://doi.org/10.3390/fermentation4030070
APA StyleLoira, I., Morata, A., Palomero, F., González, C., & Suárez-Lepe, J. A. (2018). Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation, 4(3), 70. https://doi.org/10.3390/fermentation4030070