Application of a Pyruvate-Producing Escherichia coli Strain LAFCPCPt-accBC-aceE: A Case Study for d-Lactate Production
Abstract
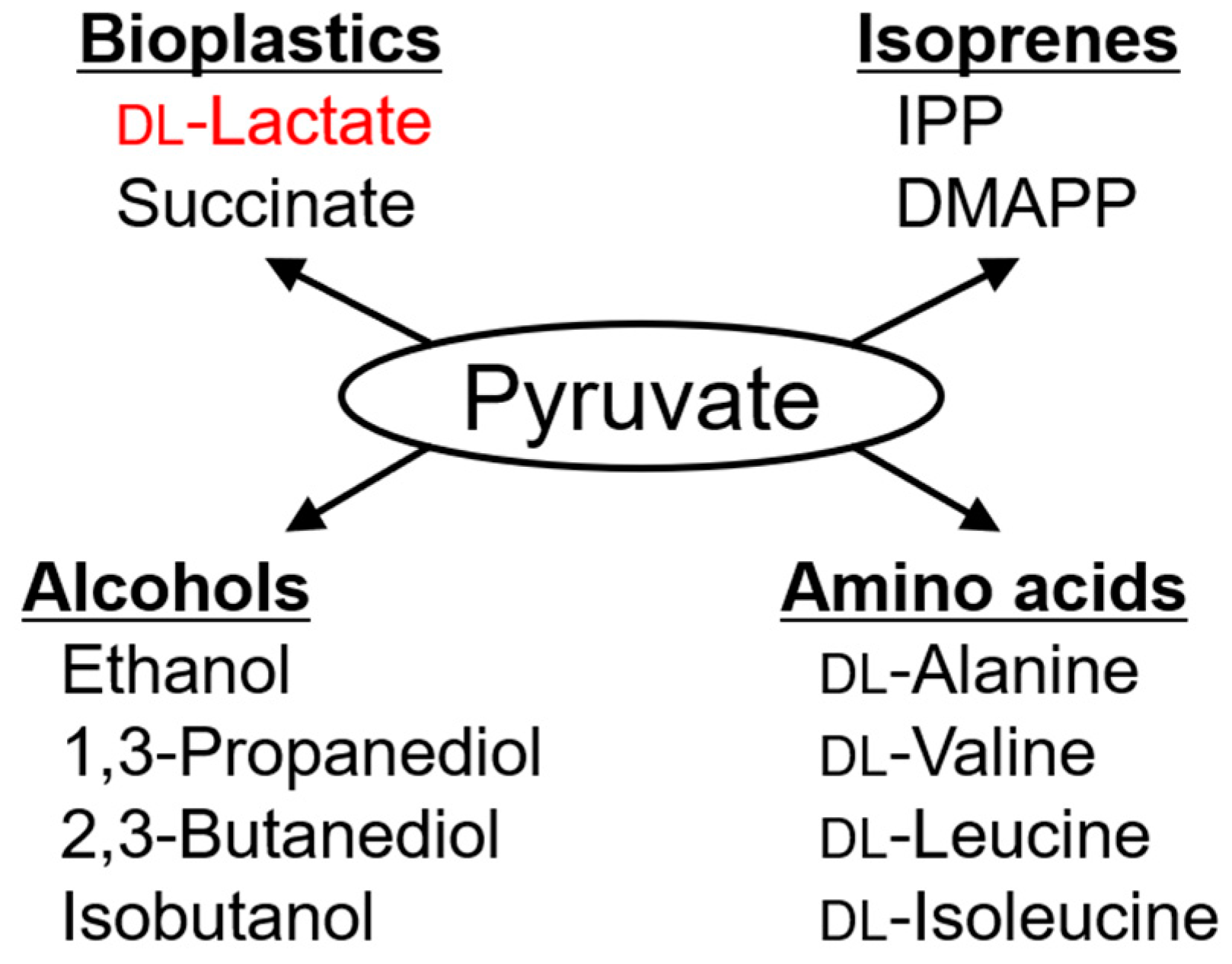
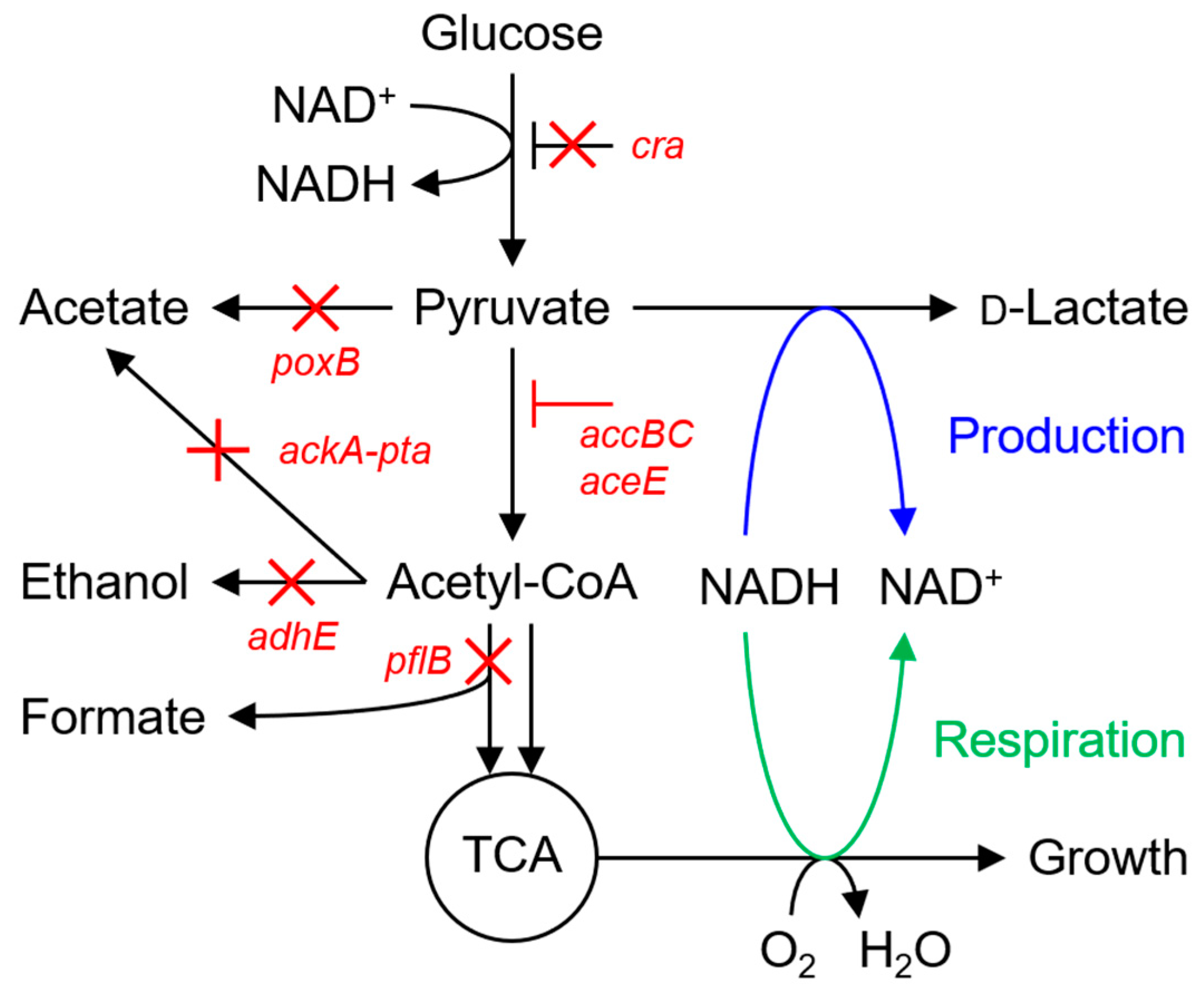
:1. Introduction
2. Materials and Methods
2.1. Construction of Bacterial Strains and Plasmids
2.1.1. Plasmids
2.1.2. Bacterial Strains
2.2. Culture Conditions for d-Lactate Production
2.3. Quantification of Metabolites
2.3.1. Extracellular Metabolites
2.3.2. Intracellular NAD+ and NADH
2.4. Enzyme Assays of DLDH
3. Results
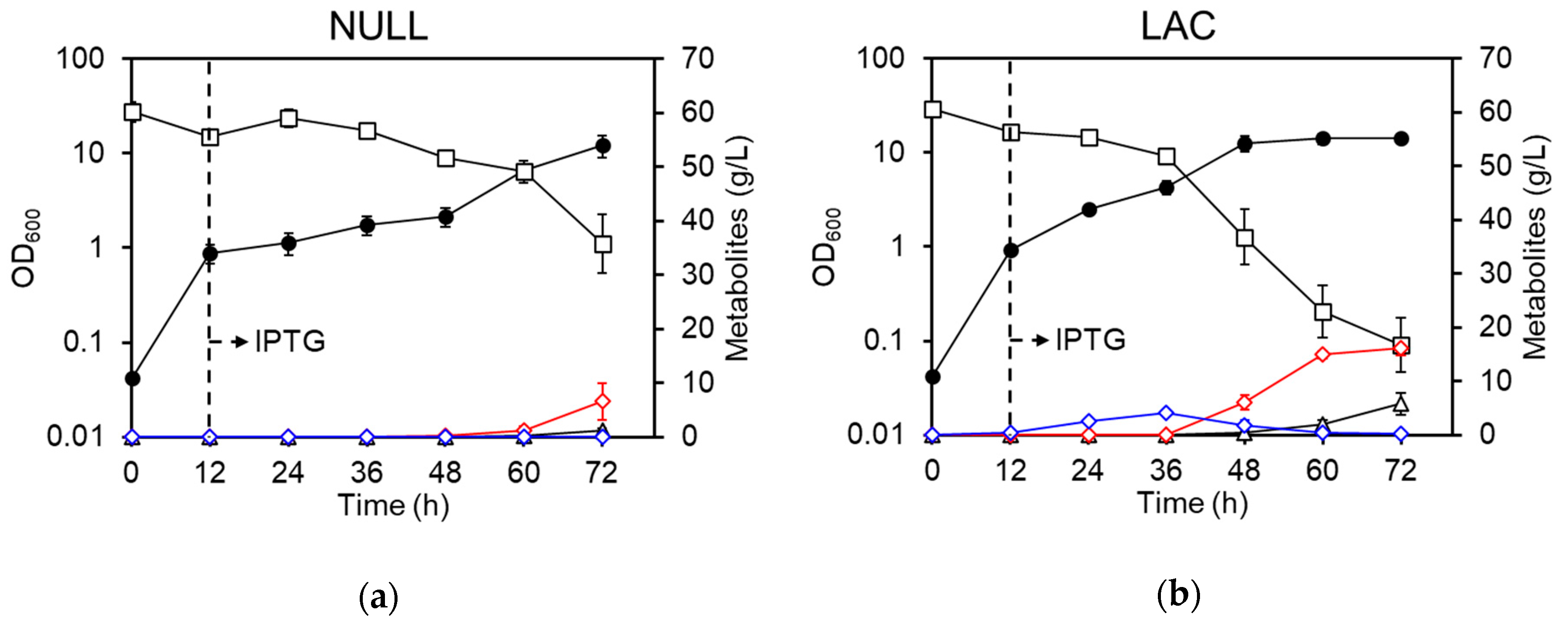
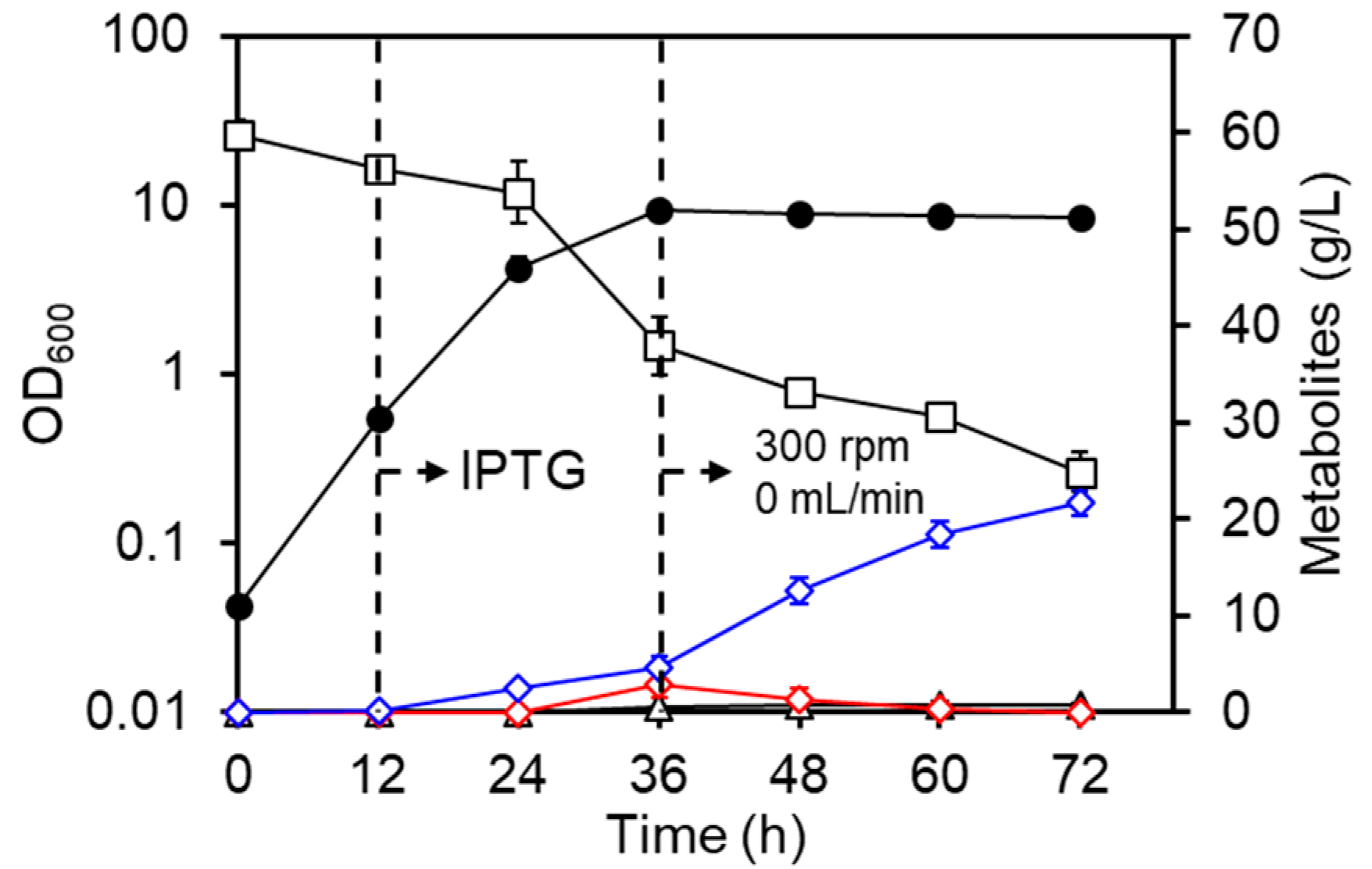
3.1. Culture Profiles of the LAC Strain under Basal Conditions
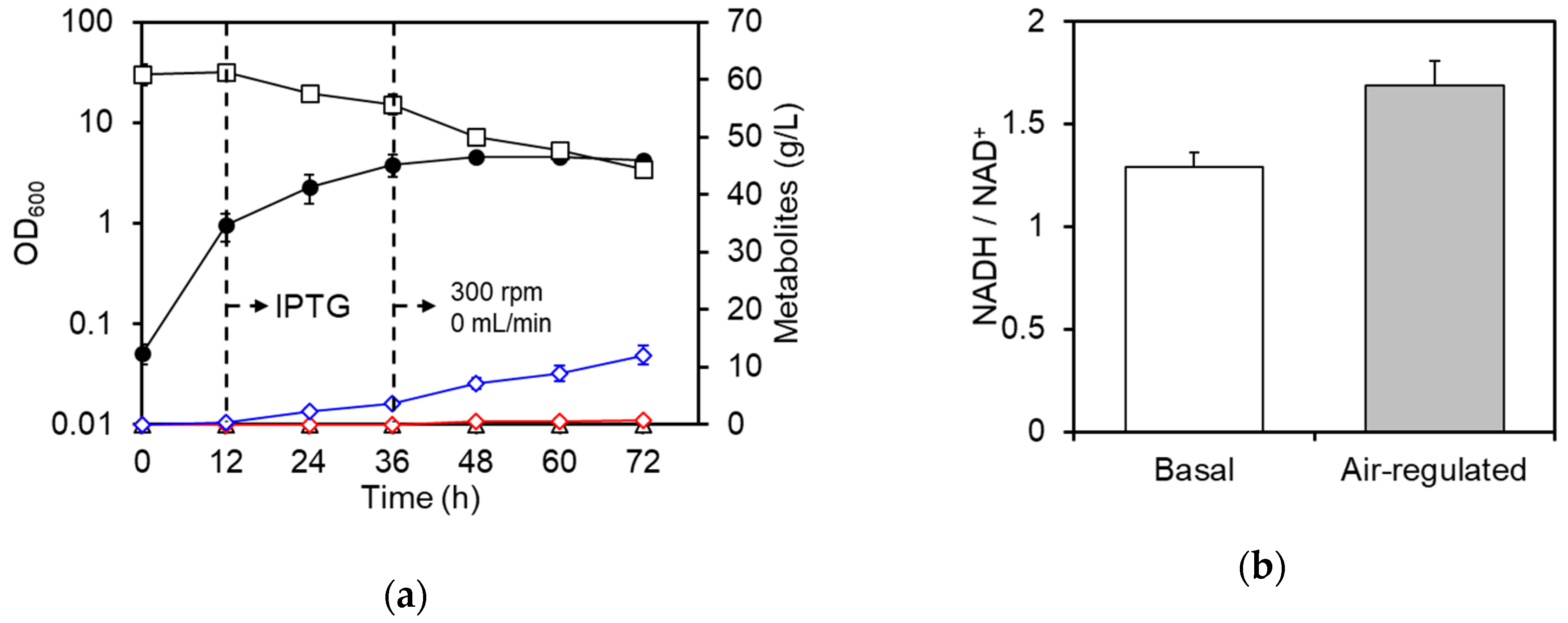
3.2. Regulation of Aeration Improves d-Lactate Production
3.3. Improvement of d-Lactate Production and Cell Density by Pre-Induction of DLDH
3.4. Summary of Culture Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maleki, N.; Eiteman, M.A. Recent progress in the microbial production of pyruvic acid. Fermentation 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, J.; Lun, S.Y. Biotechnological production of pyruvic acid. Appl. Microb. Biotechnol. 2001, 57, 451–459. [Google Scholar] [CrossRef]
- Soma, Y.; Tsuruno, K.; Wada, M.; Yokota, A.; Hanai, T. Metabolic flux redirection from a central metabolic pathway toward a synthetic pathway using a metabolic toggle switch. Metab. Eng. 2014, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.S.; Jang, W.D.; Jang, Y.S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. [Google Scholar] [CrossRef]
- Dayem, L.C.; Carney, J.R.; Santi, D.V.; Pfeifer, B.A.; Khosla, C.; Kealey, J.T. Metabolic engineering of a methylmalonyl-CoA mutase-epimerase pathway for complex polyketide biosynthesis in Escherichia coli. Biochemistry 2002, 41, 5193–5201. [Google Scholar] [CrossRef]
- Capa-Robles, W.; Paniagua-Michel, J.; Soto, J.O. The biosynthesis and accumulation of β-carotene in Dunaliella salina proceed via the glyceraldehyde 3-phosphate/pyruvate pathway. Nat. Prod. Res. 2009, 23, 1021–1028. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, F.; Du, M.; Ma, C.; Qiu, J.; Gu, L.; He, X.; Xu, P. An efficient method for N-acetyl-d-neuraminic acid production using coupled bacterial cells with a safe temperature-induced system. Appl. Microbiol. Biotechnol. 2010, 86, 481–489. [Google Scholar] [CrossRef]
- Causey, T.B.; Shanmugam, K.T.; Yomano, L.P.; Ingram, L.O. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. USA 2004, 101, 2235–2240. [Google Scholar] [CrossRef] [Green Version]
- Kawata, Y.; Nishimura, T.; Matsuhita, I.; Tsubota, J. Efficient production and secretion of pyruvate from Halomonas sp. KM-1 under aerobic conditions. AMB Express 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Enhanced pyruvate production in Candida glabrata by engineering ATP futile cycle system. ACS Synth. Biol. 2019, 8, 787–795. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Fang, Y.; Tan, T. Cofactor engineering fore more efficient production of chemicals and biofuels. Biotevhnol. Adv. 2017, 35, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Du, C.; Liu, M.; Cao, Z. Inactivation of aldehyde dehydrogenase: A key factor for engineering 1,3-propanediol production by Klebsiella pneumoniae. Metab. Eng. 2006, 8, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Uematsu, K.; Natsuma, Y.; Suda, M.; Hiraga, K.; Jojima, T.; Inui, M.; Yukawa, H. Improvement of the redox balance increase l-valine production by Corynebacterium glutamicum under oxygen deprivation conditions. Appl. Environ. Microbiol. 2012, 78, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akita, H.; Nakashima, N.; Hoshino, T. Pyruvate production using engineered Escherichia coli. AMB Express 2016, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, N.; Ohno, S.; Yoshikawa, K.; Shimizu, H.; Tamura, T. A vector library for silencing central carbon metabolism genes with antisense RNAs in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, N.; Tamura, T. Gene silencing in Escherichia coli using antisense RNAs expressed from doxycycline-inducible vectors. Lett. Appl. Microbiol. 2013, 56, 436–442. [Google Scholar] [CrossRef]
- Akita, H.; Nakashima, N.; Hoshino, T. Production of d-lactate using a pyruvate-producing Escherichia coli strain. Biosci. Biotechnol. Biochem. 2017, 81, 1452–1455. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.; Toya, Y.; Banno, S.; Yoshikawa, K.; Matsuda, F.; Shimizu, H. 13C-metabolic flux analysis for mevalonate-producing strain of Escherichia coli. J. Biosci. Bioeng. 2017, 123, 177–182. [Google Scholar] [CrossRef]
- Tegal, H.; Ottosson, J.; Hober, S. Enhancing the protein production levels in Escherichia coli with a strong promoter. FEBS J. 2011, 278, 729–739. [Google Scholar] [CrossRef]
- Liu, H.; Kang, J.; Qi, Q.; Chen, G. Production of lactate in Escherichia coli by redox regulation genetically and physiologically. Appl. Biochem. Biotechnol. 2011, 164, 165–169. [Google Scholar] [CrossRef]
- Chang, D.E.; Jung, H.C.; Rhee, J.S.; Pan, J.G. Homofermentative production of d- or l-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 1999, 65, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabar, T.B.; Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Ingram, L.O. Methlglyoxal bypass identified as source of chiral contamination in l(+) and d(−)-lactate fermentations by recombinant Escherichia coli. Biotechnol. Lett. 2006, 28, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, X.; Yu, X.; Cui, Z.; Wang, Z.; Chen, T.; Zhao, X. Evolutionary engineering of Escherichia coli for improved anaerobic growth in minimal medium accelerated lactate production. Appl. Microbiol. Biotechnol. 2019, 103, 2155–2170. [Google Scholar] [CrossRef]
- Clark, D.P. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 1989, 5, 223–234. [Google Scholar] [CrossRef]
- Förster, A.H.; Gescher, J. Metabolic engineering of Escherichia coli for production of mixed-acid fermentation end products. Front Bioeng. Biotechnol. 2014, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Causey, T.B.; Hasona, A.; Shanmugam, K.T.; Ingram, L.O. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 2003, 69, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zuo, Z.R.; Chen, X.Z.; Niu, D.D.; Tian, K.M.; Prior, B.A.; Shen, W.; Shi, G.Y.; Singh, S.; Wang, Z.X. Evaluation of genetic manipulation strategies on d-lactate production by Escherichia coli. Curr. Microbiol. 2011, 62, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Terasawa, Y.; Takaoka, N.; Shimizu, H.; Tomita, F. Pyruvic acid production by an F1-ATPase-defective mutant of Escherichia coli W1485lip2. Biosci. Biotechnol. Biochem. 1994, 58, 2164–2167. [Google Scholar] [CrossRef] [Green Version]
- Partnoy, V.A.; Herrgård, M.J.; Palsson, B.Ø. Aerobic fermentation of d-glucose by an evolved cytochrome oxidase-deficient Escherichia coli strain. Appl. Environ. Microbiol. 2008, 74, 7561–7569. [Google Scholar] [CrossRef] [Green Version]
- Kihira, C.; Hayashi, Y.; Azuma, N.; Noda, S.; Maeda, S.; Fukiya, S.; Wada, M.; Masushita, K.; Yokota, A. Alterations of glucose metabolism in Escherichia coli mutants defective in respiratory-chain enzymes. J. Biotechnol. 2012, 158, 215–223. [Google Scholar] [CrossRef]
| Strains | Relevant Descriptions 1 | References |
|---|---|---|
| PYR | Derived of the MG1655; ΔldhA ΔadhE ΔpflB Δ(ackA-pta) ΔpoxB Δcra PaccBC::Ptet-accBC PaceEF::Ptet-aceE | [14] |
| old-LAC | Derived of the PYR strain; [pMAL-c5X-DLDH] | [17] |
| PYR(DE3) | Derived of the PYR strain; λ(DE3) | This study |
| NULL | Derived of the PYR(DE3) strain; [pETIK] 1 | This study |
| LAC | Derived of the PYR(DE3) strain; [pETIK-DLDH] | This study |
| Strains | Conditions | Consumed Glucose (g·L−1) | Produced d-Lactate (g·L−1) | Produced Pyruvate (g·L−1) | Yields 1 (g·g−1) | References |
|---|---|---|---|---|---|---|
| PYR | Basal | 48.0 ± 4.7 | N/A 2 | 26.1 ± 0.9 | 54 ± 2 | [14] |
| old-LAC | Basal 3 | 4.0 ± 1.9 | 1.1 ± 0.0 | N/A | 33 ± 12 | [17] |
| LAC | Basal | 43.8 ± 4.7 | 0.2 ± 0.1 | 16.1 ± 1.4 | 1 ± 0 | This study |
| LAC | Air-regulated | 16.4 ± 2.6 | 12.1 ± 1.6 | 0.7 ± 0.6 | 74 ± 8 | This study |
| LAC | Combined | 35.0 ± 0.8 | 21.7 ± 1.4 | 0.1 ± 0.0 | 62 ± 3 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, K.; Fujii, T.; Inoue, H.; Akita, H.; Morita, T.; Matsushika, A. Application of a Pyruvate-Producing Escherichia coli Strain LAFCPCPt-accBC-aceE: A Case Study for d-Lactate Production. Fermentation 2020, 6, 70. https://doi.org/10.3390/fermentation6030070
Wada K, Fujii T, Inoue H, Akita H, Morita T, Matsushika A. Application of a Pyruvate-Producing Escherichia coli Strain LAFCPCPt-accBC-aceE: A Case Study for d-Lactate Production. Fermentation. 2020; 6(3):70. https://doi.org/10.3390/fermentation6030070
Chicago/Turabian StyleWada, Keisuke, Tatsuya Fujii, Hiroyuki Inoue, Hironaga Akita, Tomotake Morita, and Akinori Matsushika. 2020. "Application of a Pyruvate-Producing Escherichia coli Strain LAFCPCPt-accBC-aceE: A Case Study for d-Lactate Production" Fermentation 6, no. 3: 70. https://doi.org/10.3390/fermentation6030070
APA StyleWada, K., Fujii, T., Inoue, H., Akita, H., Morita, T., & Matsushika, A. (2020). Application of a Pyruvate-Producing Escherichia coli Strain LAFCPCPt-accBC-aceE: A Case Study for d-Lactate Production. Fermentation, 6(3), 70. https://doi.org/10.3390/fermentation6030070





