Combining Xylose Reductase from Spathaspora arborariae with Xylitol Dehydrogenase from Spathaspora passalidarum to Promote Xylose Consumption and Fermentation into Xylitol by Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media and Growth Conditions
2.2. Molecular Biology Techniques
2.3. Enzyme Assays
2.4. Analytical Methods
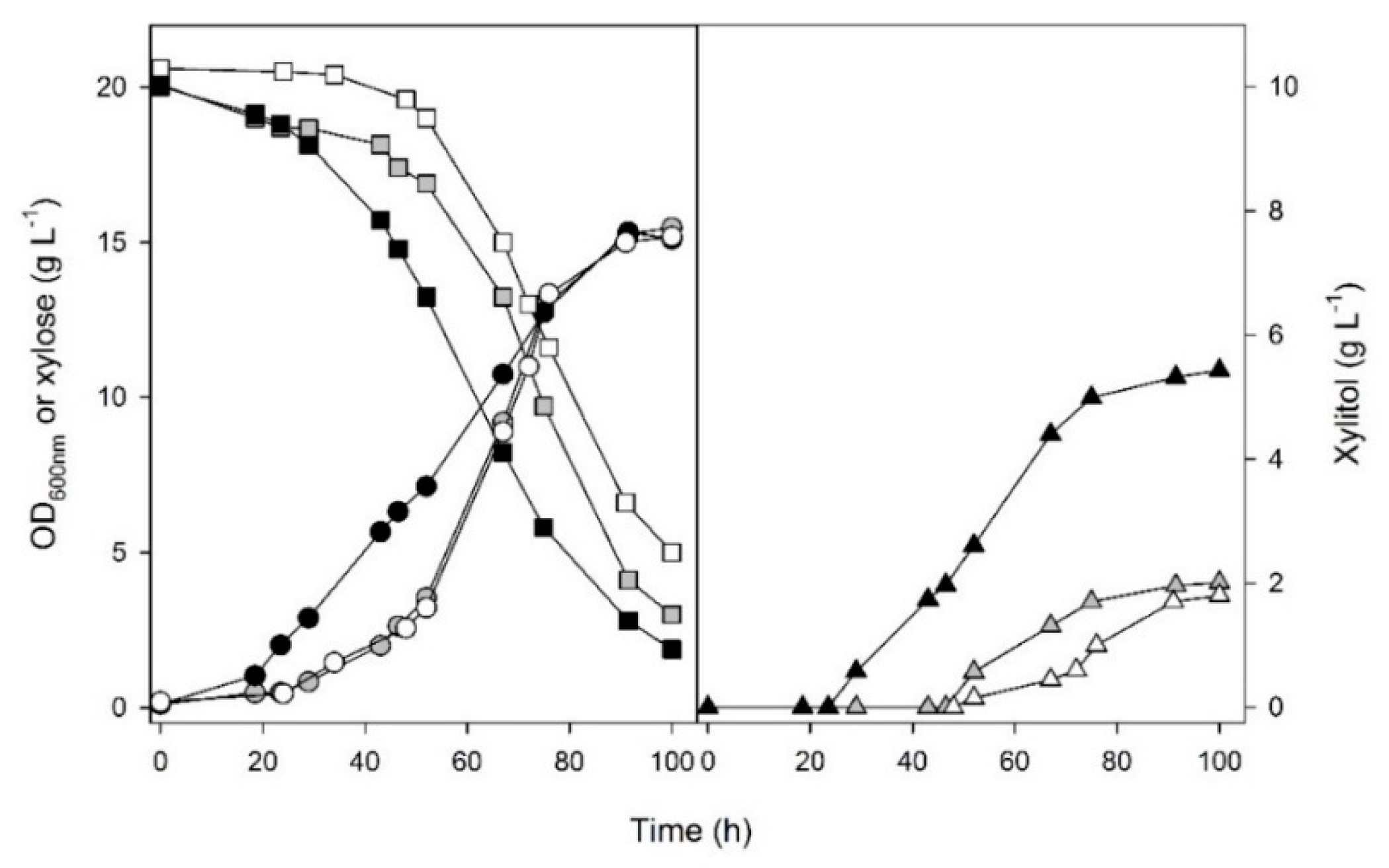
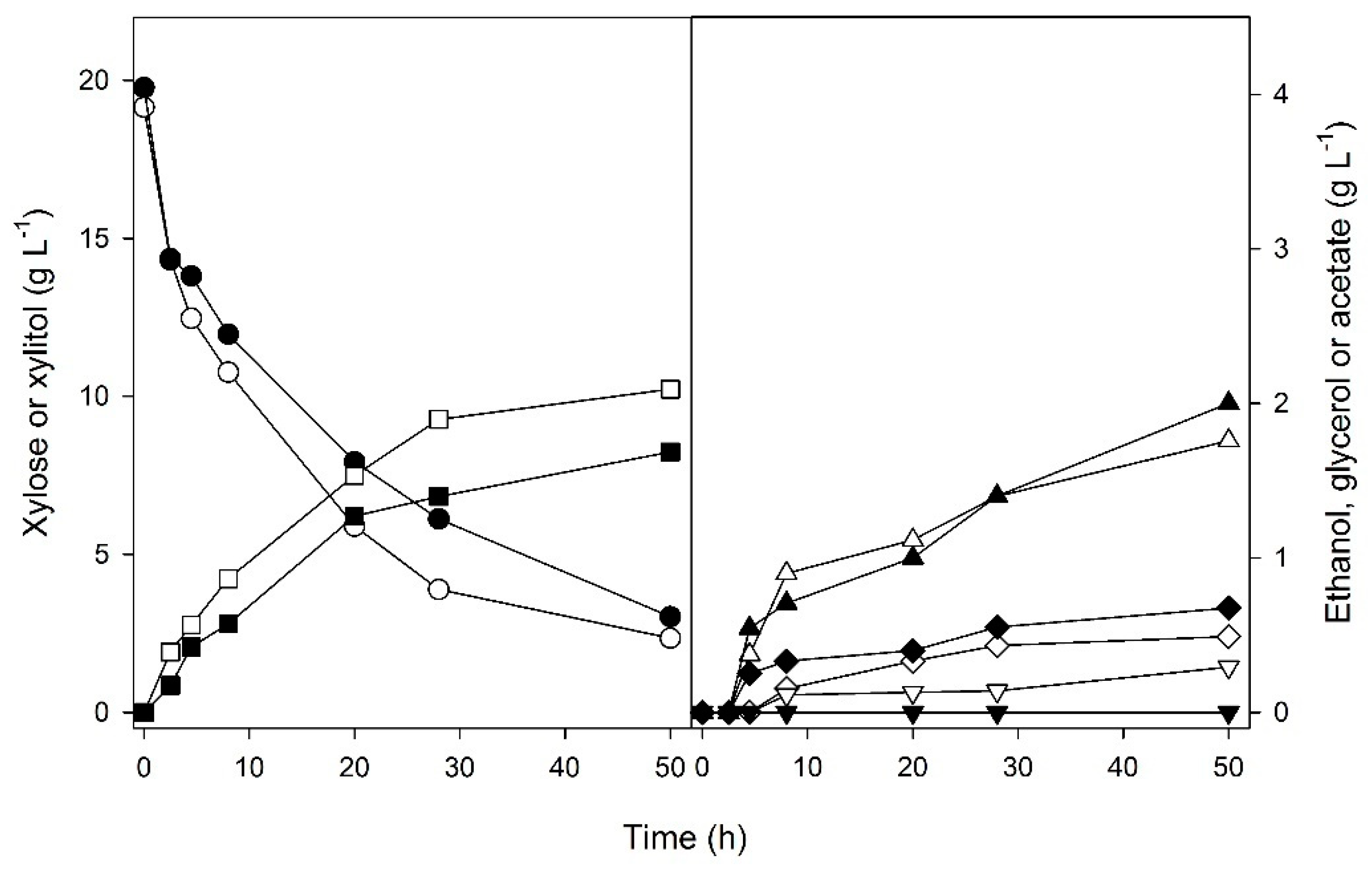
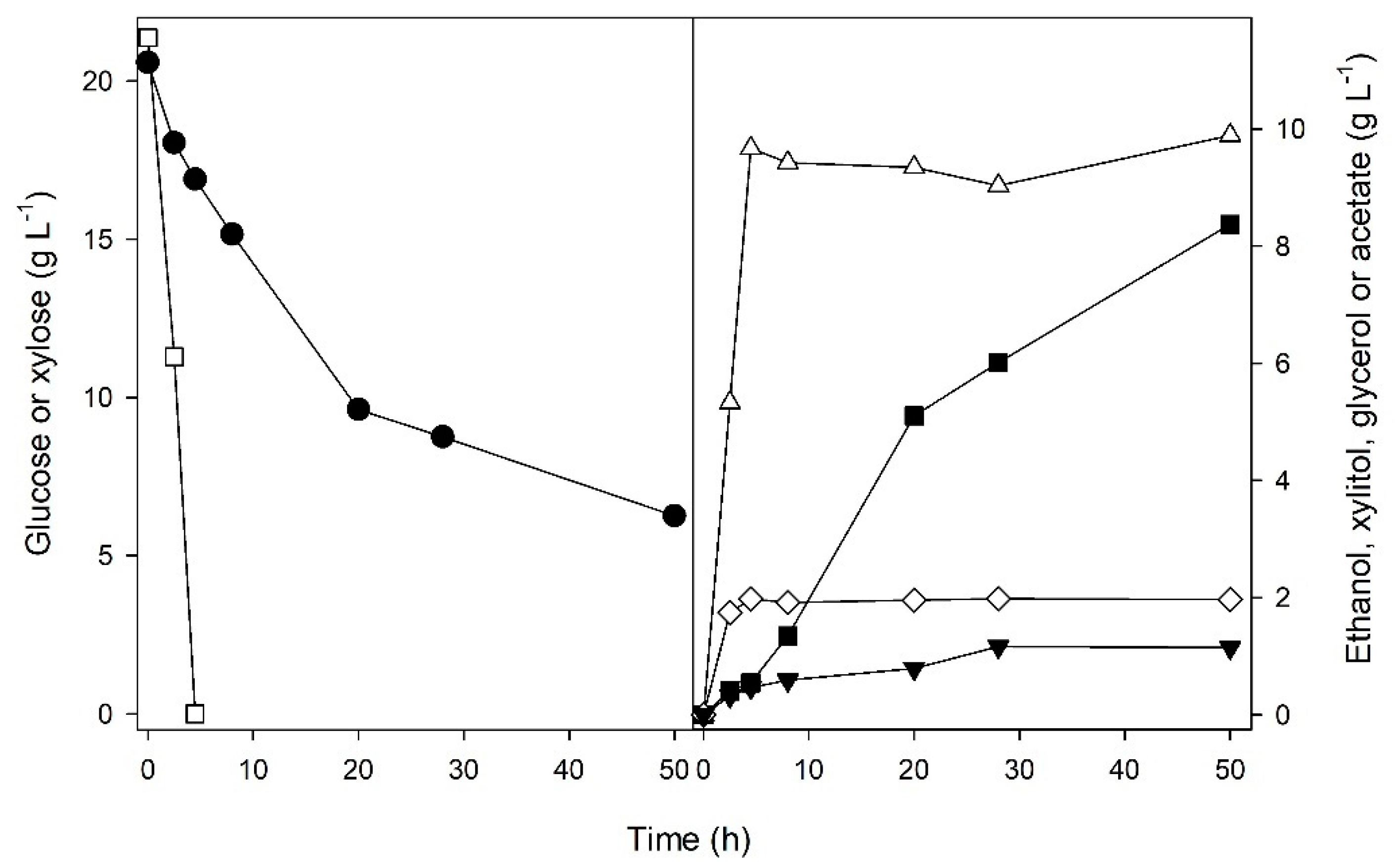
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hermann, B.G.; Blok, K.; Patel, M.K. Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 2007, 41, 7915–7921. [Google Scholar] [CrossRef]
- Uihlein, A.; Schebek, L. Environmental impacts of a lignocellulose feedstock biorefinery system: An assessment. Biomass Bioenergy 2009, 33, 793–802. [Google Scholar] [CrossRef]
- Stambuk, B.U.; Eleutherio, E.C.A.; Florez-Pardo, L.M.; Souto-Maior, A.M.; Bon, E.P.S. Brazilian potential for biomass ethanol: Challenge of using hexose and pentose cofermenting yeast strains. J. Sci. Ind. Res. 2008, 67, 918–926. [Google Scholar]
- Zhang, G.C.; Liu, J.J.; Kong, I.I.; Kwak, S.; Jin, Y.S. Combining C6 and C5 sugar metabolism for enhancing microbial bioconversion. Curr. Opin. Chem. Biol. 2015, 29, 49–57. [Google Scholar] [CrossRef]
- Patiño, M.A.; Ortiz, J.P.; Velásquez, M.; Stambuk, B.U. d-Xylose consumption by nonrecombinant Saccharomyces cerevisiae: A review. Yeast 2019, 36, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Jin, Y.S. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: A review and perspective. Microb. Cell Factories 2017, 16, 82. [Google Scholar] [CrossRef]
- Maleszka, R.; Schneider, H. Fermentation of d-xylose, xylitol, and d-xylulose by yeasts. Can. J. Microbiol. 1982, 28, 360–363. [Google Scholar] [CrossRef]
- Toivola, A.; Yarrow, D.; Van Den Bosch, E.; Van Dijken, J.P.; Scheffers, W.A. Alcoholic fermentation of d-xylose by yeasts. Appl. Environ. Microbiol. 1984, 47, 1221–1223. [Google Scholar] [CrossRef]
- Harner, N.K.; Wen, X.; Bajwa, P.K.; Austin, G.D.; Ho, C.Y.; Habash, M.B.; Trevors, J.T.; Lee, H. Genetic improvement of native xylose-fermenting yeasts for ethanol production. J. Ind. Microbiol. Biotechnol. 2015, 42, 1–20. [Google Scholar] [CrossRef]
- Smiley, K.L.; Bolen, P.L. Demonstration of d-xylose reductase and d-xylitol dehydrogenase in Pachysolen tannophilus. Biotechnol. Lett. 1982, 4, 607–610. [Google Scholar] [CrossRef]
- Bettiga, M.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F. Comparing the xylose reductase/xylitol dehydrogenase and xylose isomerase pathways in arabinose and xylose fermenting Saccharomyces cerevisiae strains. Biotechnol. Biofuels 2008, 1, 16. [Google Scholar] [CrossRef]
- Li, X.; Park, A.; Estrela, R.; Kim, S.-R.; Jin, Y.-S.; Cate, J.H.D. Comparison of xylose fermentation by two high-performance engineered strains of Saccharomyces cerevisiae. Biotechnol. Rep. 2016, 9, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Fonseca, C.; Spencer-Martins, I.; Gorwa-Grauslund, M. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef]
- De Sales, B.B.; Scheid, B.; Gonçalves, D.L.; Knychala, M.M.; Matsushika, A.; Bon, E.P.S.; Stambuk, B.U. Cloning novel sugar transporters from Scheffersomyces (Pichia) stipitis allowing d-xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol. Lett. 2015, 37, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Suh, S.O.; Marshall, C.J.; Blackwell, M. Morphological and ecological similarities: Wood-boring beetles associated with novel xylose-fermenting yeasts, Spathaspora passalidarum gen. sp. nov. and Candida jeffriesii sp. nov. Mycol. Res. 2006, 110, 1232–1241. [Google Scholar] [CrossRef]
- Cadete, R.M.; Santos, R.O.; Melo, M.A.; Mouro, A.; Gonçalves, D.L.; Stambuk, B.U.; Rosa, C.A. Spathaspora arborariae sp. nov., a d-xylose fermenting yeast species isolated from rotting wood in Brazil. FEMS Yeast Res. 2009, 9, 1338–1342. [Google Scholar] [CrossRef]
- Cadete, R.M.; Melo, M.A.; Zilli, J.E.; Vital, M.J.; Mouro, A.; Prompt, A.H.; Gomes, F.C.; Stambuk, B.U.; Lachance, M.A.; Rosa, C.A. Spathaspora brasiliensis sp. nov., Spathaspora sushii sp. nov., Spathaspora roraimanensis sp. nov. and Spathaspora xylofermentans sp. nov., four novel (d)-xylose-fermenting yeast species from Brazilian Amazonian forest. Antonie Leeuwenhoek 2013, 103, 421–431. [Google Scholar] [CrossRef]
- Hou, X. Anaerobic xylose fermentation by Spathaspora passalidarum. Appl. Microbiol. Biotechnol. 2012, 94, 205–214. [Google Scholar] [CrossRef]
- Cadete, R.M.; de Las Heras, A.M.; Sandström, A.G.; Ferreira, C.; Gírio, F.; Gorwa-Grauslund, M.F.; Rosa, C.A.; Fonseca, C. Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 167. [Google Scholar] [CrossRef]
- Bruinenberg, P.M.; de Bot, P.H.M.; van Dijken, J.P.; Scheffers, W.A. The role of redox balances in the anaerobic fermentation of xylose by yeasts. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 287–292. [Google Scholar] [CrossRef]
- Wohlbach, D.J.; Kuo, A.; Sato, T.K.; Potts, K.M.; Salamov, A.A.; Labutti, K.M.; Sun, H.; Clum, A.; Pangilinan, J.L.; Lindquist, E.A.; et al. Comparative genomics of xylose-fermenting fungi for enhanced biofuel production. Proc. Natl. Acad. Sci. USA 2011, 108, 13212–13217. [Google Scholar] [CrossRef]
- Mamoori, Y.I.; Yahya, A.G.I.; Al-Jelawi, M.H. Expression of xylose reductase enzyme from Spathaspora passalidarum in Saccharomyces cerevisiae. Iraqi J. Sci. 2013, 54, 316–323. [Google Scholar]
- Mamoori, Y.I.; Al-Jelawi, M.H.; Yahya, A.G.I. Cloning and expression of xylitol dehydrogenase enzyme from Spathaspora passalidarum in Saccharomyces cerevisiae. J. Al-Nahrain Univer. Sci. 2014, 17, 123–131. [Google Scholar] [CrossRef]
- Selim, K.A.; Easa, S.M.; El-Diwany, A.I. The xylose metabolizing yeast Spathaspora passalidarum is a promising genetic treasure for improving bioethanol production. Fermentation 2020, 6, 33. [Google Scholar] [CrossRef]
- Mussatto, S.I. Application of xylitol in food formulations and benefits for health. In d-Xylitol, Fermentative Production, Application and Commercialization; da Silva, S., Chandel, A., Eds.; Springer: Heidelberg, Germany, 2012; pp. 309–323. [Google Scholar]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- De Fátima Rodrigues de Souza, R.; Dutra, E.D.; Leite, F.C.B.; Cadete, R.M.; Rosa, C.A.; Stambuk, B.U.; Stamford, T.L.M.; de Morais, M.A., Jr. Production of ethanol fuel from enzyme-treated sugarcane bagasse hydrolysate using d-xylose-fermenting wild yeast isolated from Brazilian biomes. 3 Biotech. 2018, 8, 312. [Google Scholar]
- Entian, K.; Kotter, P. Yeast genetic strain and plasmid collections. Methods Microbiol. 2007, 36, 629–666. [Google Scholar]
- Petracek, M.E.; Longtine, M.S. PCR-based engineering of yeast genome. Methods Enzymol. 2002, 350, 445–469. [Google Scholar] [PubMed]
- Watanabe, S.; Saleh, A.A.; Pack, S.P.; Annaluru, N.; Kodaki, T.; Makino, K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein engineered NADP+-dependent xylitol dehydrogenase. J. Biotechnol. 2007, 130, 316–319. [Google Scholar] [CrossRef]
- Mumberg, D.; Müller, R.; Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 1995, 156, 119–122. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Short Protocols in Molecular Biology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Gietz, D.; St Jean, A.; Woods, R.A.; Schiestl, R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992, 20, 1425. [Google Scholar] [CrossRef]
- Van Vleet, J.H.; Jeffries, T.W.; Olsson, L. Deleting the para-nitrophenyl phosphatase (pNPPase), PHO13, in recombinant Saccharomyces cerevisiae improves growth and ethanol production on d-xylose. Metab. Eng. 2008, 10, 360–369. [Google Scholar] [CrossRef]
- Kim, S.R.; Xu, H.; Lesmana, A.; Kuzmanovic, U.; Au, M.; Florencia, C.; Oh, E.J.; Zhang, G.; Kim, K.H.; Jin, Y.S. Deletion of PHO13, encoding haloacid dehalogenase type IIA phosphatase, results in upregulation of the pentose phosphate pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 81, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kim, S.; Sorek, H.; Lee, Y.; Jeong, D.; Kim, J.; Oh, E.J.; Yun, E.J.; Wemmer, D.E.; Kim, K.H.; et al. PHO13 deletion-induced transcriptional activation prevents sedoheptulose accumulation during xylose metabolism in engineered Saccharomyces cerevisiae. Metab. Eng. 2016, 34, 88–96. [Google Scholar] [CrossRef]
- Lobo, F.P.; Gonçalves, D.L.; Alves, S.L., Jr.; Gerber, A.L.; Vasconcelos, A.T.R.; Basso, L.C.; Franco, G.R.; Soares, M.A.; Cadete, R.M.; Rosa, C.A.; et al. Draft genome sequence of the d-xylose-fermenting yeast Spathaspora arborariae UFMG-HM19.1AT. Genome Announc. 2014, 2, e01163-13. [Google Scholar] [CrossRef]
- Walfridsson, M.; Anderlund, M.; Bao, X.; Hahn-Hägerdal, B. Expression of different levels of enzymes from the Pichia stipitis XYL1 and XYL2 genes in Saccharomyces cerevisiae and its effect on product formation during xylose utilization. Appl. Microbiol. Biotechnol. 1997, 48, 218–224. [Google Scholar] [CrossRef]
- Johansson, B.; Christensson, C.; Hobley, T.; Hahn-Hägerdal, B. Xylulokinase overexpression in two strains of Saccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl. Environ. Microbiol. 2001, 67, 4249–4255. [Google Scholar] [CrossRef]
- Zhang, F.; Qiao, D.; Xu, H.; Liao, C.; Li, S.; Cao, Y. Cloning, expression, and characterization of xylose reductase with higher activity from Candida tropicalis. J. Microbiol. 2009, 47, 351–357. [Google Scholar] [CrossRef]
- Lee, J.-K.; Koo, B.-S.; Kim, S.-Y. Cloning and characterization of the xyl1 gene, enconding an NADH-preferring xylose reductase from Candida parapsilosis and its functional expression in Candida tropicalis. Appl. Environ. Microbiol. 2003, 69, 6179–6188. [Google Scholar] [CrossRef]
- Nidetzky, B.; Brüggler, K.; Kratzer, R.; Mayr, P. Multiple forms of xylose reductase in Candida intermedia: Comparison of their functional properties using quantitative structure-activity relationships, steady-state kinetic analysis, and pH studies. J. Agric. Food Chem. 2003, 51, 7930–7935. [Google Scholar] [CrossRef]
- Amore, R.; Kötter, P.; Küster, C.; Ciriacy, M.; Hollenberg, C.P. Cloning and expression in Saccharomyces cerevisiae of the NAD(P)H-dependent xylose reductase-encoding gene (XYL1) from the xylose-assimilating yeast Pichia stipitis. Gene 1991, 109, 89–97. [Google Scholar] [CrossRef]
- Ditzelmuller, G.; Kubicek, C.P.; Wohrer, W.; Rohr, M. Xylose metabolism in Pachysolen tannophilus: Purification and properties of xylose reductase. Can. J. Microbiol. 1984, 30, 1330–1336. [Google Scholar] [CrossRef]
- Kumar, S.; Gummadi, S.N. Purification and biochemical characterization of a moderately halotolerant NADPH dependent xylose reductase from Debaryomyces nepalensis NCYC 3413. Bioresour. Technol. 2011, 102, 9710–9717. [Google Scholar] [CrossRef]
- Ko, B.S.; Jung, H.C.; Kim, J.H. Molecular cloning and characterization of NAD+-dependent xylitol dehydrogenase from Candida tropicalis ATCC 20913. Biotechnol. Prog. 2006, 22, 1708–1714. [Google Scholar] [CrossRef]
- Biswas, D.; Datt, M.; Aggarwal, M.; Mondal, A.K. Molecular cloning, characterization and engineering of xylitol dehydrogenase from Debaryomyces hansenii. Appl. Microbiol. Biotechnol. 2013, 97, 1613–1623. [Google Scholar] [CrossRef]
- Guo, J.; Huang, S.; Chen, Y.; Guo, X.; Xiao, D. Heterologous expression of Spathaspora passalidarum xylose reductase and xylitol dehydrogenase genes improved xylose fermentation ability of Aureobasidium pullulans. Microb. Cell Factories 2018, 17, 64. [Google Scholar] [CrossRef]
- Krahulec, S.; Klimacek, M.; Nidetzky, B. Analysis and prediction of the physiological effects of altered coenzyme specificity in xylose reductase and xylitol dehydrogenase during xylose fermentation by Saccharomyces cerevisiae. J. Biotechnol. 2012, 158, 192–202. [Google Scholar] [CrossRef]
- Reshamwala, S.M.S.; Lali, A.M. Exploiting the NADPH pool for xylitol production using recombinant Saccharomyces cerevisiae. Biotechnol. Prog. 2020, e2972. [Google Scholar] [CrossRef]
- Gonçalves, D.L.; Matsushika, A.; de Sales, B.B.; Goshima, T.; Bon, E.P.S.; Stambuk, B.U. Xylose and xylose/glucose co-fermentation by recombinant Saccharomyces cerevisiae strains expressing individual hexose transporters. Enzyme Microb. Technol. 2014, 63, 13–20. [Google Scholar] [CrossRef]
- Rao, R.S.; Bhadra, B.; Shivaji, S. Isolation and characterization of xylitol-producing yeasts from the gut of colleopteran insects. Curr. Microbiol. 2007, 55, 441–446. [Google Scholar] [CrossRef]
- Pappu, J.S.; Gummadi, S.N. Multi response optimization for enhanced xylitol production by Debaryomyces nepalensis in bioreactor. 3 Biotech. 2016, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, J.; Zhao, S.; He, M.; Hu, G.; Ge, X.; Peng, N. Single-cell protein and xylitol production by a novel yeast strain Candida intermedia FL023 from lignocellulosic hydrolysates and xylose. Appl. Biochem. Biotechnol. 2018, 185, 163–178. [Google Scholar] [CrossRef]
- Carneiro, C.V.G.C.; de Paula e Silva, F.C.; Almeida, J.R.M. Xylitol production: Identification and comparison of new producing yeasts. Microorganisms 2019, 7, 484. [Google Scholar] [CrossRef]
- Barbosa, M.F.S.; de Medeiros, M.B.; de Mancilha, I.M.; Schneider, H.; Lee, H. Screening of yeasts for production of xylitol from d-xylose and some factors which affect xylitol yield in Candida guilliermondii. J. Ind. Microbiol. 1988, 3, 241–251. [Google Scholar] [CrossRef]
- Sampaio, F.C.; Passos, F.M.L.; Passos, F.J.V.; de Faveri, D.; Perego, P.; Converti, A. Xylitol crystallization from culture media fermented by yeasts. Chem. Eng. Process. 2006, 45, 1041–1046. [Google Scholar] [CrossRef]
- Martínez, E.A.; Canettieri, E.V.; Bispo, J.A.C.; Giulietti, M.; de Almeida e Silva, J.B.; Converti, A. Strategies for xylitol purification and crystallization: A review. Sep. Sci. Technol. 2015, 50, 2087–2098. [Google Scholar] [CrossRef]
| Strains, Plasmids and Primers | Relevant Features, Genotype or Sequence | Source |
|---|---|---|
| Yeast strains: | ||
| Sp. arborariae UFMG-HM.19.1AT | Isolated from rotting wood in Minas Gerais, Brazil | [16] |
| Sp. passalidarum UFMG-CM-Y474 | Isolated from rotting wood in Roraima, Brazil | [27] |
| S. cerevisiae CEN.PK2-1C | MATa leu2–3112 ura3–52 trp1–289 his3-Δ1 MAL2–8cSUC2 | [28] |
| S. cerevisiae ASY-1 | Isogenic to CEN.PK2-1C, but KanMX-PADH1::XKS1 | This work |
| S. cerevisiae ASY-2 | Isogenic to ASY-1, but pho13Δ::LoxP-BleR-LoxP | ” |
| Plasmids: | ||
| pFA6a-kanMX6-PADH1 | KanMX6-PADH1 | [29] |
| pUG66 | LoxP-BleR-LoxP | ” |
| pPGK | 2µ URA3 PPGK1-TPGK1 | [30] |
| pTEF-423 | 2µ HIS3 PTEF-TCYC1 | [31] |
| pGPD-423 | 2µ HIS3 PGPD-TCYC1 | ” |
| pPGK-SpXYL1.1 | 2µ URA3 PPGK1-SpXYL1.1-TPGK1 | This work |
| pPGK-SaXYL1 | 2µ URA3 PPGK1-SaXYL1-TPGK1 | ” |
| pGPD-SaXYL1 | 2µ HIS3 PGPD-SaXYL1-TCYC1 | ” |
| pPGK-SpXYL2.2 | 2µ URA3 PPGK1-SpXYL2.2-TPGK1 | ” |
| pTEF-SpXYL2.2 | 2µ HIS3 PTEF-SpXYL2.2-TCYC1 | ” |
| Primers: 1 | ||
| XR-F | ATGAATTCATGGCTACTATTAAATTATCCTCAGGT | This work |
| XR-R | TTGGATCCTTAAACAAAGATTGGAATGTTGTCC | ” |
| XDH-Sp-F | ATGAATTCATGGTTGCTAATCCCTCTTTAGTG | ” |
| XDH-Sp-R | CTGGATCCCTACTCTGGTCCATCAATTAAACACTT | ” |
| Prom_PGK_54_F | ACAGATCATCAAGGAAGTAATTATC | ” |
| Ter_PGK_65_R | TTAGCGTAAAGGATGGGGAAAGAG | ” |
| TEF-XDH-Sp-F | ATAGATCTATGGTTGCTAATCCCTCTTTAGTG | ” |
| TEF-XDH-Sp-R | CTAGATCTCTACTCTGGTCCATCAATTAAACACTT | ” |
| XKS1-Kanr-F | ATTCGGCCAATGCAATCTCAGGCGGACGAATAAGGGGGCCCCAGCTGAAGCTTCGTACGC | ” |
| XKS1-PADH1-R | AAACCTCTCTTGTCTGTCTCTGAATTACTGAACACAACATTGTATATGAGATAGTTG | ” |
| V-XKS1-F | CAAGCGACGCAGGGAATAGCC | ” |
| V-XKS1-R | CTTCGTTCAGTCTCTGTTGTGAGC | ” |
| V-kanr-F | CCGGTTGCATTCGATTCC | ” |
| DE-PHO13-F | CTTATAGCTTGCCCTGACAAAGAATATACAACTCGGGAAACCAGCTGAAGCTTCGTACGC | ” |
| DE-PHO13-R | TTCAAAAAGTAATTCTACCCCTAGATTTTGCATTGCTCCTGCATAGGCCACTAGTGGATC | ” |
| V-PHO13-F | GGAAGTAGATTGTTCGACGC | ” |
| V-PHO13-R | GATACGCCGTTCGATGCAG | ” |
| V-Bler-F | CCTTCTATGAAAGGTTGGGC | ” |
| Specific Activity (U/[mg Protein]) | ||||
|---|---|---|---|---|
| Xylose Reductase | Xylitol Dehydrogenase | |||
| Co-Substrate: | NADPH | NADH | NADP+ | NAD+ |
| Yeast: | ||||
| Sp. arborariae UFMG-HM.19.1AT | 0.455 ± 0.006 | 0.118 ± 0.002 | 0.001 ± 0.0 | 0.25 ± 0.01 |
| Sp. passalidarum UFMG-CM-Y474 | 0.580 ± 0.020 | 0.380 ± 0.010 | 0.003 ± 0.0 | 1.29 ± 0.06 |
| Specific Activity (U/ [mg Protein]) | ||||
|---|---|---|---|---|
| Xylose Reductase | Xylitol Dehydrogenase | |||
| Co-Substrate: | NADPH | NADH | NADP+ | NAD+ |
| Plasmid: | ||||
| pPGK | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| pPGK-SaXYL1 | 2.66 ± 0.02 | 0.81 ± 0.30 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| pPGK-SpXYL1.1 | 3.00 ± 0.17 | 0.05 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| pPGK-SpXYL2.2 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 2.20 ± 0.21 |
| Plasmid: | Substrate: | Km | Vmax (U/[mg Protein]) |
|---|---|---|---|
| pPGK-SaXYL1 | NADH | 12.8 ± 3.0 µM | 1.0 ± 0.04 |
| NADPH | 26.1 ± 11.0 µM | 2.6 ± 0.4 | |
| Xylose (NADH) | 29.5 ± 13.0 mM | 1.0 ± 0.1 | |
| Xylose (NADPH) | 57.5 ± 10.0 mM | 2.8 ± 0.2 | |
| pPGK-SpXYL1.1 | NADPH | 65.9 ± 28.2 µM | 4.7 ± 0.6 |
| Xylose (NADPH) | 53.3 ± 6.1 mM | 4.2 ± 0.1 | |
| pPGK-SpXYL2.2 | NAD+ | 0.52 ± 0.16 mM | 2.4 ± 0.2 |
| Xylitol | 86.0 ± 13.0 mM | 4.3 ± 0.2 |
| Specific Activity (U/[mg Protein]) | |||||
|---|---|---|---|---|---|
| Xylose Reductase | Xylitol Dehydrogenase | ||||
| Co-Substrate: | NADPH | NADH | NADP+ | NAD+ | |
| Strain: | Plasmids: | ||||
| ASY-1 | pPGK-SpXYL1.1 pTEF-SpXYL2.2 | 3.05 ± 0.17 | 0,05 ± 0.01 | 0.01 ± 0.01 | 0.40 ± 0.10 |
| ASY-1 | pPGK-SaXYL1 pTEF-SpXYL2.2 | 2.41 ± 0.25 | 0.89 ± 0.23 | 0.01 ± 0.01 | 0.48 ± 0.03 |
| ASY-2 | pPGK-SaXYL1 pTEF-SpXYL2.2 | 2.37 ± 0.22 | 0.81 ± 0.22 | 0.01 ± 0.01 | 0.48 ± 0.07 |
| ASY-2 | pGPD-SaXYL1 pPGK-SpXYL2.2 | 5.12 ± 1.34 | 2.11 ± 0.66 | 0.01 ± 0.01 | 2.95 ± 0.34 |
| Strains and Plasmids: | Xylose Consumption (%) a | µmax (h−1) | Yp/sethanol (g/g) | Yp/sglycerol (g/g) | Yp/sacetate (g/g) | Yp/sxylitol (g/g) | Qpxylitol (g/L/h) |
|---|---|---|---|---|---|---|---|
| Aerobic growth: | |||||||
| ASY-1 pPGK-SpXYL1.1 pTEF-SpXYL2.2 | 75.7 ± 4.1 | 0.070 ± 0.005 | 0 | 0 | 0 | 0.135 ± 0.025 | 0.040 ± 0.008 |
| ASY-1 pPGK-SaXYL1 pTEF-SpXYL2.2 | 79.9 ± 4.9 | 0.072 ± 0.004 | 0 | 0 | 0 | 0.152 ± 0.035 | 0.058 ± 0.001 |
| ASY-2 pPGK-SaXYL1 pTEF-SpXYL2.2 | 85.5 ± 4.6 | 0.139 ± 0.015 * | 0 | 0 | 0 | 0.230 ± 0.068 | 0.087 ± 0.017 # |
| Batch fermentation: | |||||||
| ASY-2 pPGK-SaXYL1 pTEF-SpXYL2.2 | 87.5 ± 1.6 | NA b | 0.130 ± 0.018 | 0.068 ± 0.036 | 0.013 ± 0.009 | 0.614 ± 0.022 | 0.513 ± 0.033 |
| ASY-2 pGPD-SaXYL1 pPGK-SpXYL2.2 | 85.4 ± 4.3 | NA | 0.123 ± 0.003 | 0.089 ± 0.031 | 0 | 0.572 ± 0.041 | 0.370 ± 0.095 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, A.; dos Santos, A.A.; Agnolo, D.D.; Gubert, G.F.; Bon, E.P.S.; Rosa, C.A.; Fonseca, C.; Stambuk, B.U. Combining Xylose Reductase from Spathaspora arborariae with Xylitol Dehydrogenase from Spathaspora passalidarum to Promote Xylose Consumption and Fermentation into Xylitol by Saccharomyces cerevisiae. Fermentation 2020, 6, 72. https://doi.org/10.3390/fermentation6030072
Mouro A, dos Santos AA, Agnolo DD, Gubert GF, Bon EPS, Rosa CA, Fonseca C, Stambuk BU. Combining Xylose Reductase from Spathaspora arborariae with Xylitol Dehydrogenase from Spathaspora passalidarum to Promote Xylose Consumption and Fermentation into Xylitol by Saccharomyces cerevisiae. Fermentation. 2020; 6(3):72. https://doi.org/10.3390/fermentation6030072
Chicago/Turabian StyleMouro, Adriane, Angela A. dos Santos, Denis D. Agnolo, Gabriela F. Gubert, Elba P. S. Bon, Carlos A. Rosa, César Fonseca, and Boris U. Stambuk. 2020. "Combining Xylose Reductase from Spathaspora arborariae with Xylitol Dehydrogenase from Spathaspora passalidarum to Promote Xylose Consumption and Fermentation into Xylitol by Saccharomyces cerevisiae" Fermentation 6, no. 3: 72. https://doi.org/10.3390/fermentation6030072
APA StyleMouro, A., dos Santos, A. A., Agnolo, D. D., Gubert, G. F., Bon, E. P. S., Rosa, C. A., Fonseca, C., & Stambuk, B. U. (2020). Combining Xylose Reductase from Spathaspora arborariae with Xylitol Dehydrogenase from Spathaspora passalidarum to Promote Xylose Consumption and Fermentation into Xylitol by Saccharomyces cerevisiae. Fermentation, 6(3), 72. https://doi.org/10.3390/fermentation6030072






