Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Source of DOW
2.3. Sample Preparation
2.4. Extract and Quantitative Analysis of Adenosine, HEA, and Polysaccharides
2.5. Animals Grouping and Experiment Schedule
2.6. Surgery for i.c.v. Aβ40 Infusion
2.7. Apparatus of Water Maze
2.8. Morris Water Maze Task
2.9. Preparation of Brains
2.10. Enzyme-Linked Immunosorbent Assay
2.11. Statistical Analysis
3. Results
3.1. Effects of DOW and MgCl2 on C. cicadae and the Production of Functional Components
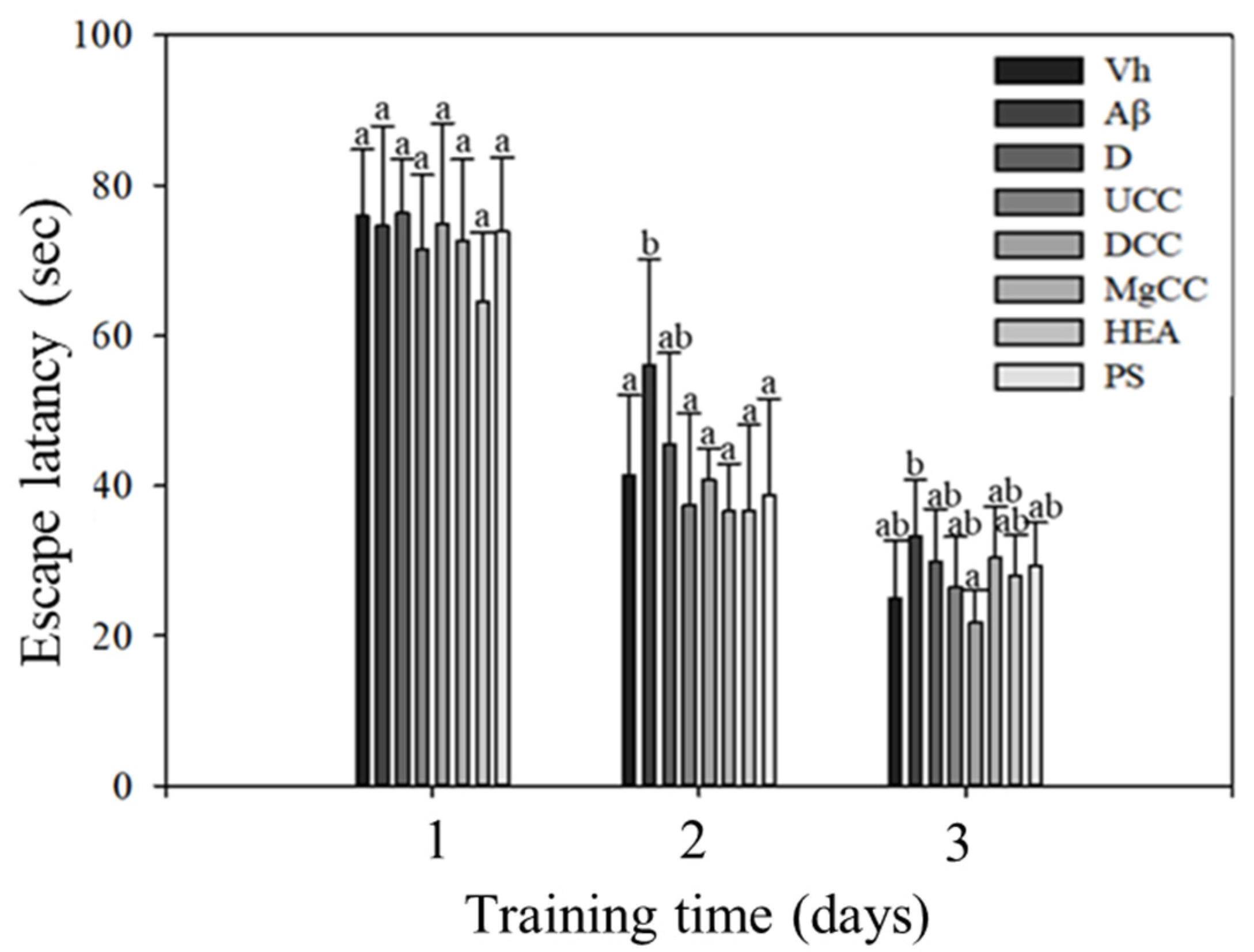
3.2. Effects of DOW-Cultivated C. cicadae Fermentation Products on the Memory Tests and Spatial Learning of Rats with Brain Infusions of Aβ40 and STZ
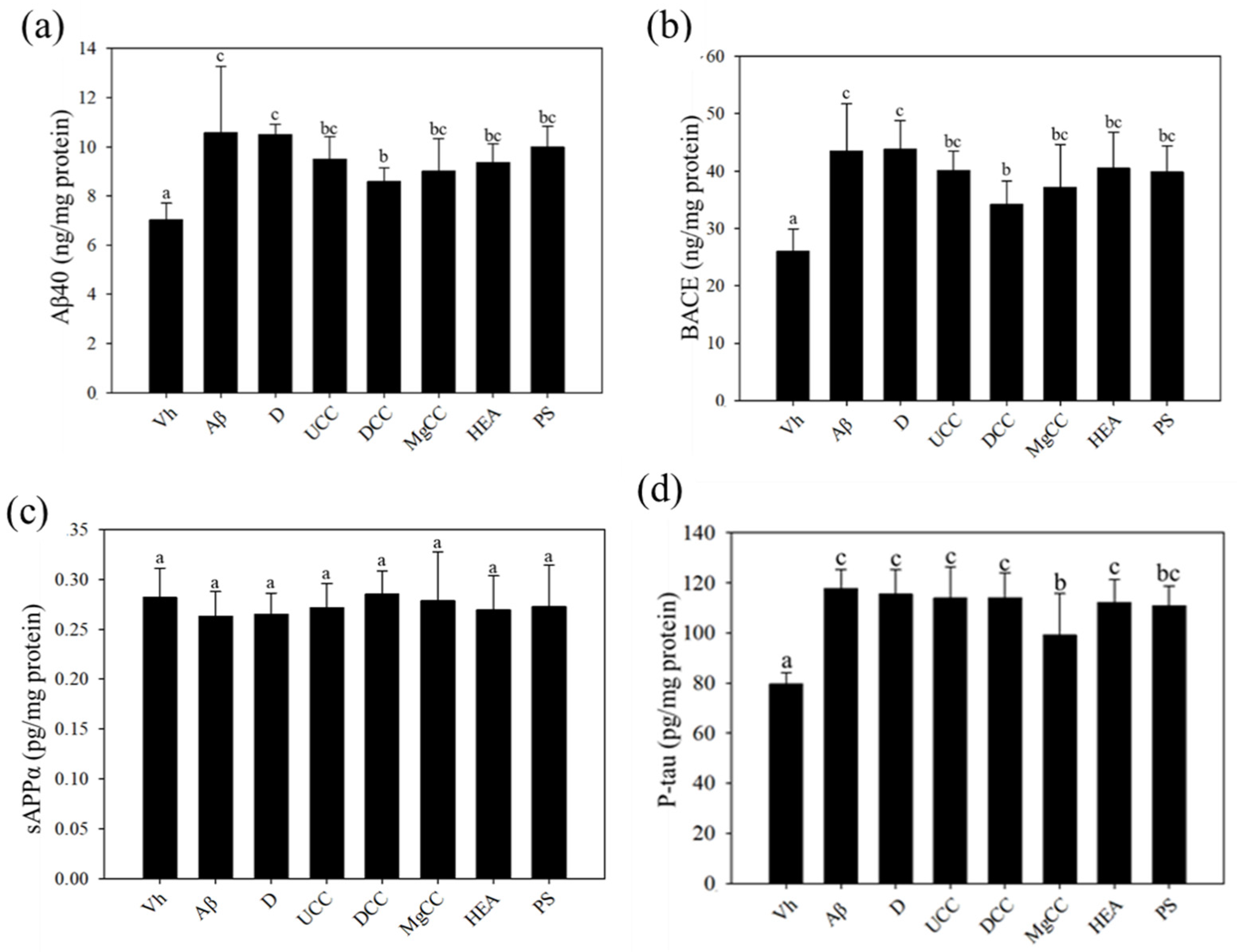
3.3. C. cicadae Fermentation Products and the Expression of Aβ40 and BACE Proteins in the Hippocampus of Rats with Brain Infusions of Aβ40 and STZ
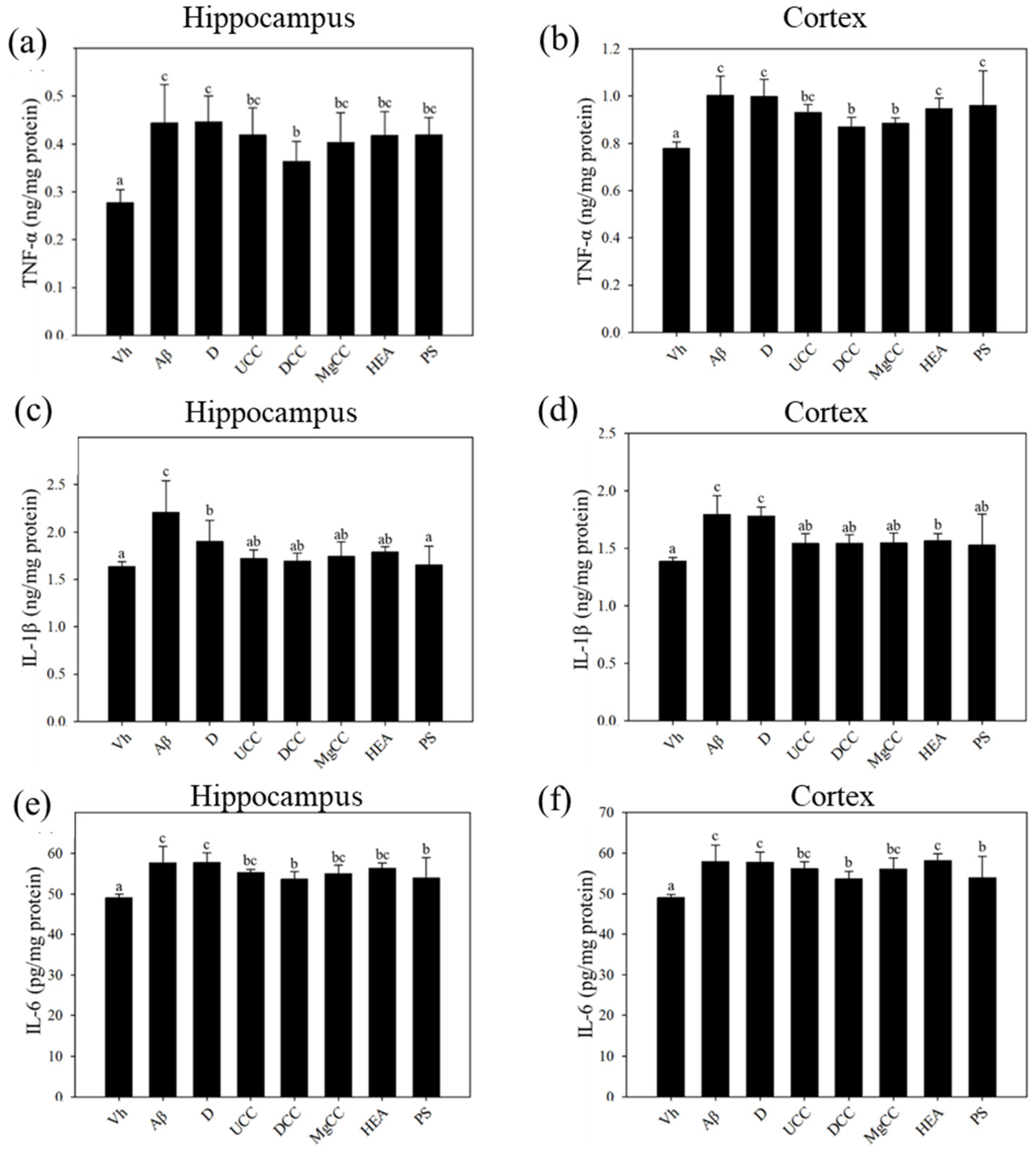
3.4. Effects of C. cicadae Fermentation Products on the Expression of TNF-α, IL-6, and IL-1β in the Hippocampus of Rats with Brain Infusions of Aβ40 and STZ
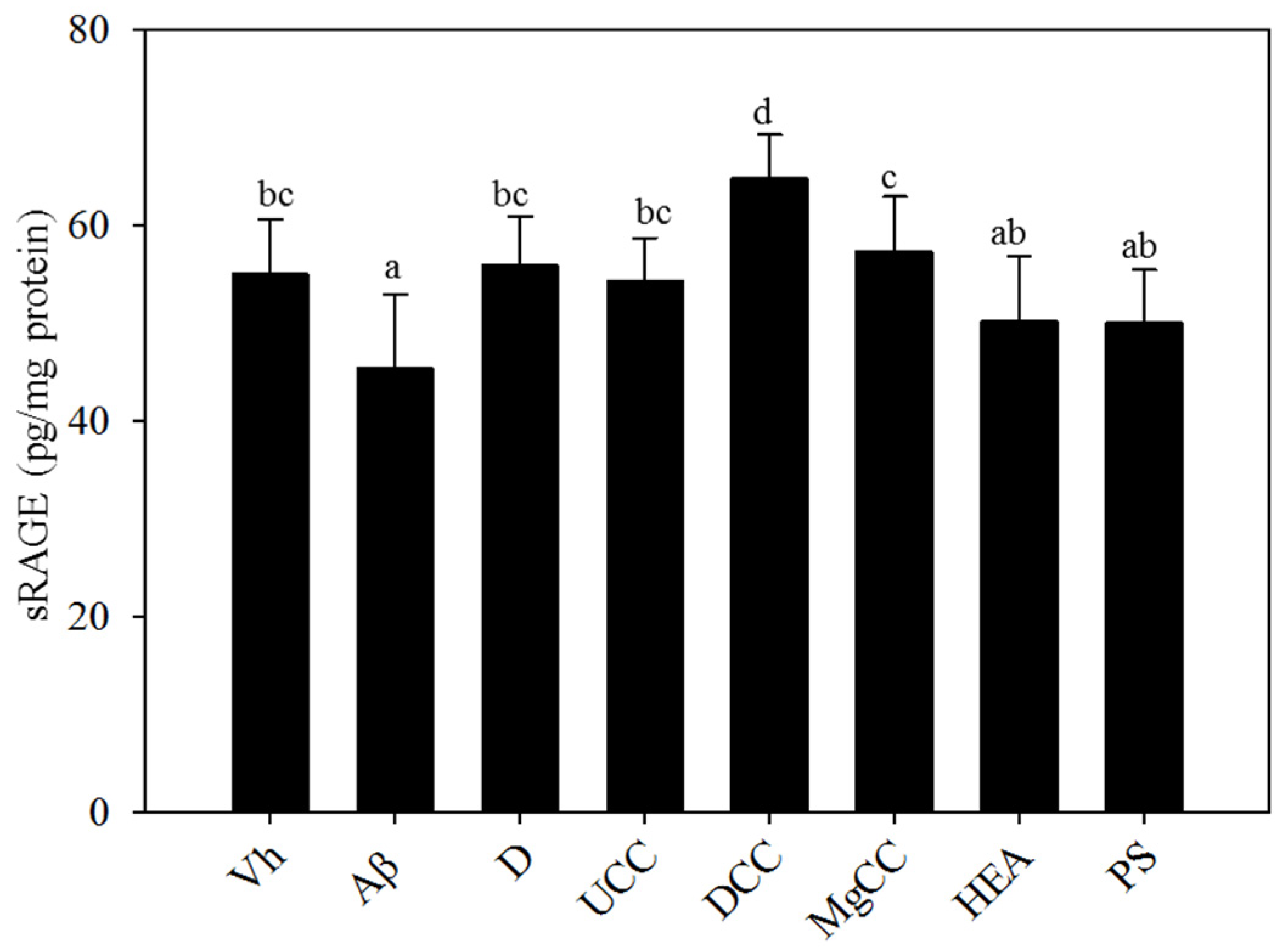
3.5. Effects of C. cicadae Fermentation Products on the Expression of sRAGE in the Hippocampus of Rats with Brain Infusions of Aβ40 and STZ
3.6. Effects of C. cicadae Fermentation Products on Mg2+ in the Blood Serum, Hippocampus, and Cortex of Rats with Brain Infusions of Aβ40 and STZ
3.7. Effects of C. cicadae Fermentation Products on the Expression of MAGTI Protein in the Hippocampus of Rats with Brain Infusions of Aβ40 and STZ
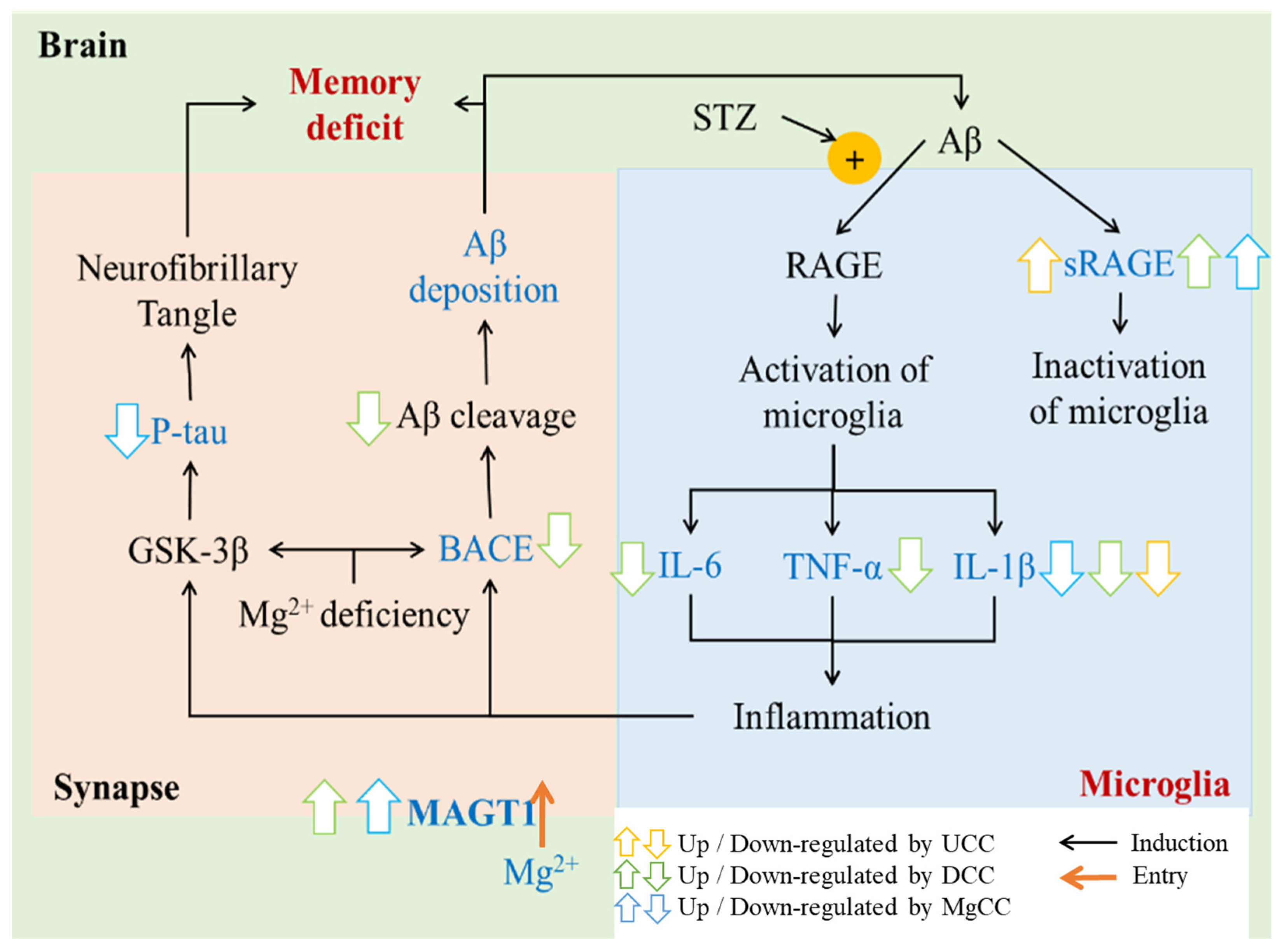
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, M.Y.; Chen, C.C.; Lee, L.Y.; Lin, T.W.; Kuo, C.F. N(6)-(2-Hydroxyethyl)adenosine in the Medicinal Mushroom Cordyceps cicadae Attenuates Lipopolysaccharide-Stimulated Pro-inflammatory Responses by Suppressing TLR4-Mediated NF-kappaB Signaling Pathways. J. Nat. Prod. 2015, 78, 2452–2460. [Google Scholar] [CrossRef]
- Zheng, R.; Zhu, R.; Li, X.; Li, X.; Shen, L.; Chen, Y.; Zhong, Y.; Deng, Y. N6-(2-Hydroxyethyl) Adenosine From Cordyceps cicadae Ameliorates Renal Interstitial Fibrosis and Prevents Inflammation via TGF-beta1/Smad and NF-kappaB Signaling Pathway. Front. Physiol. 2018, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Wang, X.; Feng, Z.; Cui, H.; Zhu, Z.; Xia, C.; Han, X.; Liu, W.J.; Liu, Y.N. Cordyceps cicadae Prevents Renal Tubular Epithelial Cell Apoptosis by Regulating the SIRT1/p53 Pathway in Hypertensive Renal Injury. Evid. Based Complement. Altern. Med. 2020, 2020, 7202519. [Google Scholar] [CrossRef]
- Ke, B.J.; Lee, C.L. Cordyceps Cicadae NTTU 868 Mycelium Prevents CCl4-induced Hepatic Fibrosis in BALB/c Mice Via Inhibiting the Expression of Pro-inflammatory and Pro-fibrotic Cytokines. J. Funct. Foods 2018, 43, 214–223. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.; Wang, D.; Yu, X. Neuroprotective effects of adenosine isolated from Cordyceps cicadae against oxidative and ER stress damages induced by glutamate in PC12 cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.J.; Lee, C.L. Using submerged fermentation to fast increase N6-(2-hydroxyethyl)-adenosine, adenosine and polysaccharide productions of Cordyceps cicadae NTTU 868. AMB Express 2019, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.B.; Chang, J.; Zhu, Y.; Sun, X. Chemical Constituents of Cordyceps cicadae. Nat. Prod. Commun. 2015, 10, 2145–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Hamada, A.; Cui, T.; Yokota, J.; Yamamoto, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; Kaneda, R.; Tsutsui, Y.; et al. Pharmacological activity of deep-sea water: Examination of hyperlipemia prevention and medical treatment effect. Biol. Pharm. Bull. 2003, 26, 1552–1559. [Google Scholar] [CrossRef]
- Wang, L.C.; Kuo, I.U.; Tsai, T.Y.; Lee, C.L. Antrodia camphorata-fermented product cultured in deep ocean water has more liver protection against thioacetamide-induced fibrosis. Appl. Microbiol. Biotechnol. 2013, 97, 9955–9967. [Google Scholar] [CrossRef]
- Lung, T.Y.; Liao, L.Y.; Wang, J.J.; Wei, B.L.; Huang, P.Y.; Lee, C.L. Metals of Deep Ocean Water Increase the Anti-Adipogenesis Effect of Monascus-Fermented Product via Modulating the Monascin and Ankaflavin Production. Mar. Drugs 2016, 14, 106. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, C.L. Higher Anti-Liver Fibrosis Effect of Cordyceps militaris-Fermented Product Cultured with Deep Ocean Water via Inhibiting Proinflammatory Factors and Fibrosis-Related Factors Expressions. Mar. Drugs 2017, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L. The advantages of deep ocean water for the development of functional fermentation food. Appl. Microbiol. Biotechnol. 2015, 99, 2523–2531. [Google Scholar] [CrossRef]
- Hung, Y.P.; Wang, J.J.; Wei, B.L.; Lee, C.L. Effect of the salts of deep ocean water on the production of cordycepin and adenosine of Cordyceps militaris-fermented product. AMB Express 2015, 5, 140. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Lee, C.L.; Tsai, T.Y.; Wang, J.J.; Pan, T.M. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl. Microbiol. Biotechnol. 2006, 70, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E. The Growth of the Surface Area of Human Body; University of Minnesota Press: Minneapolis, MN, USA, 1935. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set; Elsevier Academic: London, UK, 2005. [Google Scholar]
- Lee, C.L.; Kuo, T.F.; Wang, J.J.; Pan, T.M. Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J. Neurosci. Res. 2007, 85, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Kung, Y.H.; Wang, J.J.; Lung, T.Y.; Pan, T.M. Enhanced hypolipidemic effect and safety of red mold dioscorea cultured in deep ocean water. J. Agric. Food Chem. 2011, 59, 8199–8207. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Schlossmacher, M.G.; Hung, A.Y.; Vigo-Pelfrey, C.; Mellon, A.; Ostaszewski, B.L.; Lieberburg, I.; Koo, E.H.; Schenk, D.; Teplow, D.B.; et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 1992, 359, 322–325. [Google Scholar] [CrossRef]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; Drachman, D.A. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Llufriu-Daben, G.; Carrete, A.; Chierto, E.; Mailleux, J.; Camand, E.; Simon, A.; Vanmierlo, T.; Rose, C.; Allinquant, B.; Hendriks, J.J.A.; et al. Targeting demyelination via alpha-secretases promoting sAPPalpha release to enhance remyelination in central nervous system. Neurobiol. Dis. 2018, 109, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Brion, J.P.; Anderton, B.H.; Authelet, M.; Dayanandan, R.; Leroy, K.; Lovestone, S.; Octave, J.N.; Pradier, L.; Touchet, N.; Tremp, G. Neurofibrillary tangles and tau phosphorylation. Biochem. Soc. Symp. 2001, 81–88. [Google Scholar] [CrossRef]
- Geschwind, D.H. Tau phosphorylation, tangles, and neurodegeneration: The chicken or the egg? Neuron 2003, 40, 457–460. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Xia, S.; Fang, L.; Gou, S. ROS-responsive and multifunctional anti-Alzheimer prodrugs: Tacrine-ibuprofen hybrids via a phenyl boronate linker. Eur. J. Med. Chem. 2020, 112997. [Google Scholar] [CrossRef]
- Shibata, N.; Kobayashi, M. The role for oxidative stress in neurodegenerative diseases. Brain Nerve 2008, 60, 157–170. [Google Scholar]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Choi, J.; Lee, S.; Byun, K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2018, 495, 807–813. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.G.; et al. Elevation of brain magnesium prevents and reverses cognitive deficits and synaptic loss in Alzheimer’s disease mouse model. J. Neurosci. 2013, 33, 8423–8441. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.G.; et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain 2014, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, X.; Yang, J.; Bao, J.; Di, X.; Zhang, G.; Liu, H. Magnesium Sulfate Provides Neuroprotection in Eclampsia-Like Seizure Model by Ameliorating Neuroinflammation and Brain Edema. Mol. Neurobiol. 2017, 54, 7938–7948. [Google Scholar] [CrossRef]
- Xu, Z.P.; Li, L.; Bao, J.; Wang, Z.H.; Zeng, J.; Liu, E.J.; Li, X.G.; Huang, R.X.; Gao, D.; Li, M.Z.; et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, S.; Lee, D.Y.; Lee, C. Increasing anti-Abeta-induced neurotoxicity ability of Antrodia camphorata-fermented product with deep ocean water supplementary. J. Sci. Food Agric. 2016, 96, 4690–4701. [Google Scholar] [CrossRef]
- Ravelli, K.G.; Rosario, B.D.; Camarini, R.; Hernandes, M.S.; Britto, L.R. Intracerebroventricular Streptozotocin as a Model of Alzheimer’s Disease: Neurochemical and Behavioral Characterization in Mice. Neurotox Res. 2017, 31, 327–333. [Google Scholar] [CrossRef]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Lin, S.; Tsai, Y.T.; Hsu, J.H.; Chen, Y.L.; Lin, W.H.; Chen, C.C. Cordyceps cicadae mycelia and its active compound HEA exert beneficial effects on blood glucose in type 2 diabetic db/db mice. J. Sci. Food Agric. 2019, 99, 606–612. [Google Scholar] [CrossRef]


| Culture Water | Dry Mycelium (g/L) | Adenosine (mg/L) | HEA (mg/L) | Intracellular Polysaccharide (mg/L) | Extracellular Polysaccharide (mg/L) | Intracellular Magnesium (mg/L) |
|---|---|---|---|---|---|---|
| UPW | 7.27 ± 0.47 b | 7.15 ± 0.94 b | 0.88 ± 0.08 a | 99.3 ± 8.7 b | 548 ± 104 a | 10.1 ± 0.9 a |
| DOW | 8.07 ± 0.73 c | 6.67 ± 0.48 b | 1.04 ± 0.15 a | 88.0± 12.0 ab | 518 ± 141 a | 161.4 ± 12.0 c |
| MgCl2 | 5.27 ± 0.59 a | 1.58 ± 0.05 a | 1.32 ± 0.15 b | 74.7± 14.7 a | 554 ± 131 a | 132.0 ± 14.7 b |
| Group | Mg2+ Concentration | ||
|---|---|---|---|
| Serum (mg/dL) | Hippocampus (μg/g) | Cortex (μg/g) | |
| Vh | 5.08 ± 0.44 a | 2.24 ± 0.06 c | 1.45 ± 0.19 b |
| Aβ | 3.92 ± 0.50 b | 1.75 ± 0.15 a | 1.25 ± 0.05 a |
| D | 4.10 ± 0.49 b | 1.87 ± 0.16 ab | 1.32 ± 0.07 ab |
| UCC | 3.83 ± 0.66 b | 1.87 ± 0.22 ab | 1.33 ± 0.07 ab |
| DCC | 4.02 ± 0.74 b | 2.03 ± 0.18 b | 1.45 ± 0.11 b |
| MgCC | 3.73 ± 0.30 b | 1.92 ± 0.14 ab | 1.39 ± 0.08 b |
| HEA | 3.90 ± 0.33 b | 1.88 ± 0.09 ab | 1.32 ± 0.12 ab |
| PS | 3.87 ± 0.40 b | 1.74 ± 0.11 a | 1.25 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Z.; Lee, C.-L. Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. https://doi.org/10.3390/fermentation7010039
Wu Y-Z, Lee C-L. Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation. 2021; 7(1):39. https://doi.org/10.3390/fermentation7010039
Chicago/Turabian StyleWu, Yan-Zhong, and Chun-Lin Lee. 2021. "Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain" Fermentation 7, no. 1: 39. https://doi.org/10.3390/fermentation7010039
APA StyleWu, Y.-Z., & Lee, C.-L. (2021). Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation, 7(1), 39. https://doi.org/10.3390/fermentation7010039







