Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy
Abstract
:1. Background
2. A General Description of Solid-State Fermentation
2.1. Predominant Features of SSF
2.2. The Challenge in Solid-State Fermentation—Underdevelopment of Bioreactors
3. Potential Role of Solid-State Fermentation in a Circular Bioeconomy
3.1. The Role of Enzymes in the Bioconversion of Organic Residues
3.2. Sequential Solid-State Fermentation and Submerged-Liquid Fermentation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Gómez, J.P.; Manan, M.A.; Webb, C. Solid-state fermentation of food industry wastes. In Food Industry Wastes, 2nd ed.; Kosseva, M.R., Webb, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–161. [Google Scholar] [CrossRef]
- Pandey, A. Recent process developments in solid-state fermentation. Process Biochem. 1992, 27, 109–117. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Larroche, C. Current Developments in Solid-State Fermentation; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- SciVerse Scopus, Scopus-Analyze Search Results. 2021. Available online: https://www.scopus.com/term/analyzer.uri?sid=a1d81e2ded735f2ba3d07d032dc4da30&origin=resultslist&src=s&s=%28TITLE-ABS-KEY+%28solid+state+fermentation%29+AND+TITLE-ABS-KEY+%28solid-state+fermentation%29%29&sort=plf-f&sdt=b&sot=b&sl=87&count=7442&analyzeRes (accessed on 12 February 2021).
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- W.I.P. Organization, WIPO IP Portal. 2021. Available online: https://patentscope.wipo.int/search/en/result.jsf?_vid=P21-KNFZ6E-29561 (accessed on 13 April 2021).
- Costa, J.A.V.; Treichel, H.; Kumar, V.; Pandey, A. Advances in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Modern microbial solid state fermentation technology for future biorefineries for the production of added-value products. Biofuel Res. J. 2017, 4, 730–740. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Oviedo-Lopera, J.C.; Zartha-Sossa, J.W.; Zapata-Ruiz, D.L.; Bohorquez-Naranjo, I.; Morales-Arevalo, K.S. Systematic Review and Study of S Curves for Biomass Quantification in Solid-state Fermentation (SSF) and Digital Image Processing (DIP) Applied to Biomass Measurement in Food Processes. Recent Pat. Biotechnol. 2020, 14, 194–202. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Pitol, L.O.; Biz, A.; Finkler, A.T.J.; de Lima Luz, L.F., Jr.; Krieger, N. Design and Operation of a Pilot-Scale Packed-Bed Bioreactor for the Production of Enzymes by Solid-State Fermentation. In Solid State Fermentation. Advances in Biochemical Engineering/Biotechnology; Steudler, S., Werner, A., Cheng, J., Eds.; Springer: Berlin, Germany, 2019; Volume 169, pp. 27–50. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Manan, M.A.; Webb, C. Design Aspects of Solid State Fermentation as Applied to Microbial Bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Ashok, A.; Doriya, K.; Rao, D.R.M.; Kumar, D.S. Design of solid state bioreactor for industrial applications: An overview to conventional bioreactors. Biocatal. Agric. Biotechnol. 2017, 9, 11–18. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2020, 27, 100407. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2020, 124566. [Google Scholar] [CrossRef]
- Verduzco-Oliva, R.; Gutierrez-Uribe, J.A. Beyond enzyme production: Solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustainability 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Medeiros, A.B.P.; Larroche, C.; Pandey, A. Production of Organic Acids by Solid-state Fermentation. In Current Developments in Solid-State Fermentation; Springer: New York, NY, USA, 2009; pp. 205–229. [Google Scholar] [CrossRef]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative Production of Bioproducts From Organic Waste Through Solid-State Fermentation, Front. Sustain. Food Syst. 2019, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Yazid, N.A.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Kosseva, M.R. Recovery of Commodities from Food Wastes Using Solid-State Fermentation. In Food Industry Wastes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 77–102. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Raimbault, M. General and microbiological aspects of solid substrate fermentation, Electron. J. Biotechnol. 1998, 1, 174–188. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process. Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E. Why solid-state fermentation seems to be resistant to catabolite repression? Food Technol. Biotechnol. 2006, 44, 397–406. [Google Scholar]
- Shinkawa, S.; Mitsuzawa, S. Feasibility study of on-site solid-state enzyme production by Aspergillus oryzae, Biotechnol. Biotechnol. Biofuels 2020, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- de Castro, R.J.S.; Sato, H.H. Enzyme Production by Solid State Fermentation: General Aspects and an Analysis of the Physicochemical Characteristics of Substrates for Agro-industrial Wastes Valorization. Waste Biomass Valorization 2015, 6, 1085–1093. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst. Microbiol. Biomanufacturing 2020, 1, 142–165. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Pérez-Rivero, C.; Webb, C. Investigating a non-destructive alternative for a preliminary evaluation of fungal growth in solid state fermentations. J. Microbiol. Methods 2019, 160, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, A.; Renaud, R.; Almanza, S.; Maratray, J.; Diez, M.; Desgranges, C. Solid state fermentation reactors: From lab scale to pilot plant. Biotechnol. Adv. 1993, 11, 591–597. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Luz, L.F.d.; Krieger, N.; Berovič, M. Bioreactors for Solid-State Fermentation. In Comprehensive Biotechnology, 2nd ed.; Moo-Yong, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 347–360. [Google Scholar] [CrossRef]
- Lüth, P.; Eiben, U. Solid-State Fermenter and Method for Solid-State Fermentation, US6620614B1. 2003. Available online: https://patents.google.com/patent/US6620614?oq=forced+aeration+beauveria (accessed on 10 May 2021).
- Mazumdar-Shaw, K.; Suryanarayan, S. Commercialization of a novel fermentation concept. Adv. Biochem. Eng. Biotechnol. 2003, 85, 29–42. [Google Scholar] [CrossRef]
- Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Olejniczak, T. Microbial kinetic resolution of aroma compounds using solid-state fermentation. Catalysts 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Enhancing the bioproduction of value-added aroma compounds via solid-state fermentation of sugarcane bagasse and sugar beet molasses: Operational strategies and scaling-up of the process. Bioresour. Technol. 2018, 363, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, J. Valorising Agro-industrial Wastes within the Circular Bioeconomy Concept: The Case of Defatted Rice Bran with Emphasis on Bioconversion Strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Pérez-Rivero, C.; Venus, J. Valorisation of solid biowastes: The lactic acid alternative. Process. Biochem. 2020, 99, 222–235. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Enzyme mediated multi-product process: A concept of bio-based refinery. Ind. Crops Prod. 2020, 154, 112607. [Google Scholar] [CrossRef]
- Nwobi, A.; Cybulska, I.; Tesfai, W.; Shatilla, Y.; Rodríguez, J.; Thomsen, M.H. Simultaneous saccharification and fermentation of solid household waste following mild pretreatment using a mix of hydrolytic enzymes in combination with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2014, 99, 929–938. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Shaarani, S.M.; Godoy, L.C.; Melikoglu, M.; Vergara, C.S.; Koutinas, A.; Webb, C. Bioconversion of rapeseed meal for the production of a generic microbial feedstock. Enzyme Microb. Technol. 2010, 47, 77–83. [Google Scholar] [CrossRef]
- Lunprom, S.; Phanduang, O.; Salakkam, A.; Liao, Q.; Reungsang, A. A sequential process of anaerobic solid-state fermentation followed by dark fermentation for bio-hydrogen production from Chlorella sp. Int. J. Hydrogen Energy 2019, 44, 3306–3316. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Grand View Research, Enzymes Market Size, Share & Trends Analysis Report by Application (Industrial Enzymes, Specialty Enzymes), By Product (Carbohydrase, Proteases, Lipases), by Source, by Region, and Segment Forecasts, 2020–2027, San Francisco. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/enzymes-industry (accessed on 10 May 2021).
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Conversion of exhausted sugar beet pulp into fermentable sugars from a biorefinery approach. Foods 2020, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jasso, R.M.R.; Gonzalez-Gloria, K.D.; Rosales, M.; Cerda, R.B.; Aguilar, C.N.; Singhania, R.R.; Ruiz, H.A. The enzyme biorefinery platform for advanced biofuels production. Bioresour. Technol. Rep. 2019, 7, 100257. [Google Scholar] [CrossRef]
- Ferreira, R.D.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, J.; Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess. Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ellilä, S.; Fonseca, L.; Uchima, C.; Cota, J.; Goldman, G.H.; Saloheimo, M.; Sacon, V.; Siika-Aho, M. Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol. Biofuels 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Johnson, E. Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioprod. Biorefining 2016, 10, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, R.K.; Christopher, M.; Kooloth-Valappil, P.; Sreeja-Raju, A.; Mathew, R.M.; Sankar, M.; Puthiyamadam, A.; Adarsh, V.-P.; Aswathi, A.; Rebinro, V.; et al. Addressing challenges in production of cellulases for biomass hydrolysis: Targeted interventions into the genetics of cellulase producing fungi. Bioresour. Technol. 2021, 329, 124746. [Google Scholar] [CrossRef]
- Wang, R.; Godoy, L.C.; Shaarani, S.M.; Melikoglu, M.; Koutinas, A.; Webb, C. Improving wheat flour hydrolysis by an enzyme mixture from solid state fungal fermentation. Enzyme Microb. Technol. 2009, 44, 223–228. [Google Scholar] [CrossRef]
- Steudler, S.; Werner, A.; Walther, T. It Is the Mix that Matters: Substrate-Specific Enzyme Production from Filamentous Fungi and Bacteria Through Solid-State Fermentation. In Solid State Fermentation. Advances in Biochemical Engineering/Biotechnology; Steudler, S., Werner, A., Cheng, J., Eds.; Springer: Berlin, Germany, 2019; Volume 169, pp. 51–82. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Bourgine, J.; Thomsen, M. Closing the loop of cereal waste and residues with sustainable technologies: An overview of enzyme production via fungal solid-state fermentation. Sustain. Prod. Consum. 2021, 27, 845–857. [Google Scholar] [CrossRef]
- Farinas, C.S. Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. Renew. Sustain. Energy Rev. 2015, 52, 179–188. [Google Scholar] [CrossRef]
- Cunha, F.M.; Esperança, M.N.; Zangirolami, T.C.; Badino, A.C.; Farinas, C.S. Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour. Technol. 2012, 112, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viesturs, U.E.; Strikauska, S.V.; Leite, M.P.; Berzins, A.J.; Tengerdy, R.P. Combined submerged and solid substrate fermentation for the bioconversion of lignocellulose. Biotechnol. Bioeng. 1987, 30, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; Diaz, A.B.; Wang, R.; Koutinas, A.; Webb, C. Particulate bioprocessing: A novel process strategy for biorefineries. Process. Biochem. 2009, 44, 546–555. [Google Scholar] [CrossRef]
- Webb, C.; Koutinas, W.R.; Wang, R. Developing a sustainable bioprocessing strategy based on a generic feedstock. Adv. Biochem. Eng. Biotechnol. 2004, 87, 195–268. [Google Scholar] [CrossRef]
- Webb, C.; Wang, R. Development of a Generic Fermentation Feedstock from Whole Wheat Flour. In Cereals; Springer: Boston, MA, USA, 1997; pp. 205–218. [Google Scholar] [CrossRef]
- Du, C.; Lin, S.K.C.; Koutinas, A.; Wang, R.; Dorado, P.; Webb, C. A wheat biorefining strategy based on solid-state fermentation for fermentative production of succinic acid. Bioresour. Technol. 2008, 99, 8310–8315. [Google Scholar] [CrossRef]
- Dorado, M.P.; Lin, S.K.C.; Koutinas, A.; Du, C.; Wang, R.; Webb, C. Cereal-based biorefinery development: Utilisation of wheat milling by-products for the production of succinic acid. J. Biotechnol. 2009, 143, 51–59. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.-Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Sun, Z.; Leung, C.C.J.; Han, W.; Lau, K.Y.; Li, M.; Lin, C.S.K. Valorisation of bakery waste for succinic acid production. Green Chem. 2013, 15, 690–695. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of By-Products from Sunflower-Based Biodiesel Production Processes for the Production of Fermentation Feedstock. Waste Biomass Valorization 2013, 4, 529–537. [Google Scholar] [CrossRef]
- García, I.L.; López, J.A.; Dorado, M.P.; Kopsahelis, N.; Alexandri, M.; Papanikolaou, S.; Villar, M.A.; Koutinas, A.A. Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresour. Technol. 2013, 130, 16–22. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Valorisation of food waste via fungal hydrolysis and lactic acid fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016, 217, 129–136. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Khonngam, T.; Salakkam, A. Bioconversion of sugarcane bagasse and dry spent yeast to ethanol through a sequential process consisting of solid-state fermentation, hydrolysis, and submerged fermentation. Biochem. Eng. J. 2019, 150, 107284. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J. Clean. Prod. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- Cunha, F.M.; Esperança, M.N.; Florencio, C.; Vasconcellos, V.M.; Farinas, C.S.; Badino, A.C. Three-phasic fermentation systems for enzyme production with sugarcane bagasse in stirred tank bioreactors: Effects of operational variables and cultivation method. Biochem. Eng. J. 2015, 97, 32–39. [Google Scholar] [CrossRef]
- Herlet, J.; Kornberger, P.; Roessler, B.; Glanz, J.; Schwarz, W.H.; Liebl, W.; Zverlov, V.V. A new method to evaluate temperature vs. pH activity profiles for biotechnological relevant enzymes. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Solid state fermentation of waste bread pieces by Aspergillus awamori: Analysing the effects of airflow rate on enzyme production in packed bed bioreactors. Food Bioprod. Process. 2015, 95, 63–75. [Google Scholar] [CrossRef]
- Lopes, A.M.; Filho, E.X.F.; Moreira, L.R.S. An update on enzymatic cocktails for lignocellulose breakdown. J. Appl. Microbiol. 2018, 125, 632–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Fact. 2016, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
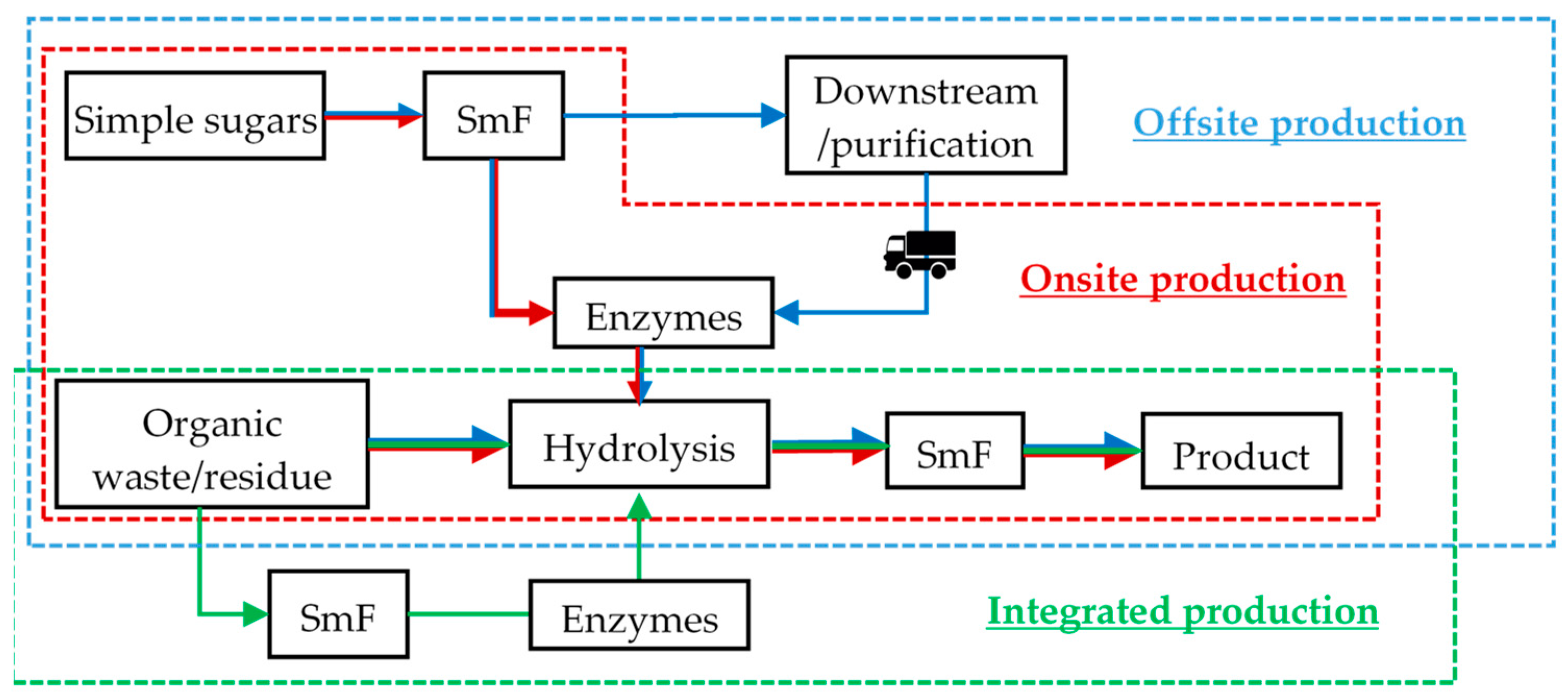


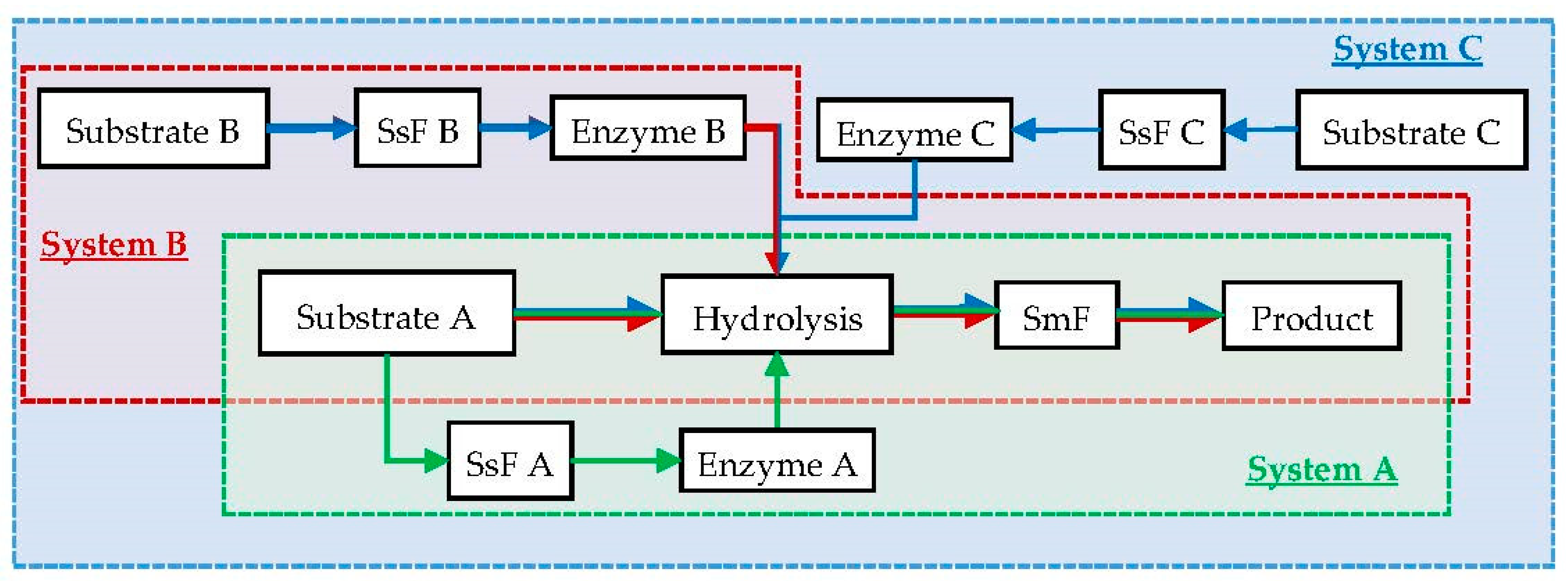
| Characteristic | SmF | SsF |
|---|---|---|
| Culture medium (cost) | High | Low |
| Energy requirement | High | Low |
| Yield | Smaller | Greater |
| Wastewater generation | High | Low |
| Space required (bioreactor volume) | Large | Small |
| O2 mass transfer | Low | High |
| Contamination risk | High | Low |
| Temperature control | Easy | Difficult |
| Online control of parameters | Easy | Difficult |
| Nutrient and product regulation | Easy | Difficult |
| Product recovery and purification | Easy | Less easy |
| Technology development level | High | Low |
| Large scale bioreactors | Available | Limited availability |
| Fermentation time | Shorter | Longer |
| SsF | Hydrolysis | SmF | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate | Organism | Product | Enzyme Addition | Substrate | Product | Substrate | Microorganism | Product | |
| Wheat straw * | T. reesei or C. versicolor | Reducing sugars for SmF | - | - | - | Extract from the SsF | E. fibuliger | Protein | [64] |
| Wheat grains | A. awamori 2B.361U2/1 | Generic feedstock | - | - | - | Extract from the SsF | W. eutropha | PHB | [65] |
| S. cerevisiae | Ethanol | ||||||||
| Wheat bran | A. awamori | Glucoamylases | Enzyme extract | Gluten-free flour | Glucose stream (140 g L−1) | Mixed glucose and nitrogen streams | A. succinogenes ATCC 55618 | Succinic acid | [68] |
| A. oryzae | Proteases | Gluten | Nitrogen stream (3.5 g L−1) | ||||||
| Wheat bran | A. awamori | Glucoamylases | SsF solids | Wheat flour milling by-product | Hydrolysate (120 g L−1 sugars, 300 mg L−1 FAN) | Wheat flour milling by-product hydrolysates | A. succinogenes ATCC 55618 | Succinic acid | [69] |
| A. oryzae | Proteases | ||||||||
| Waste bread | A. awamori | Glucoamylases | SsF solids | Waste bread | Hydrolysate (over 100 g L−1 glucose, 490 mg L−1 FAN) | Bread suspension hydrolysate | A. succinogenes | Succinic acid | [70] |
| A. oryzae | Proteases | ||||||||
| Bakery wastes (cake, pastry) | A. awamori | Glucoamylases | SsF solids | Pastry | Hydrolysate (44 g L−1 glucose, 715 mg L−1 FAN) | Pastry and cake hydrolysates | A. succinogenes ATCC 55618 | Succinic acid | [71] |
| A. oryzae | Proteases | Cake | Hydrolysate (23.1 g L−1 glucose, 388 mg L−1 FAN) | ||||||
| Nutrient solution + sugarcane bagasse | A. Niger A12 | SsF solids | - | - | - | SsF solids + glucose nutrient solution | A. Niger A12 | Cellulases | [63] |
| Mixed food wastes | A. awamori | Glucoamylases | SsF solids | Mixed food wastes | Hydrolysate (31.9 g L−1glucose, 280 mg L−1 FAN) | Food waste hydrolysate | E. coli | Succininc acid | [72] |
| A. oryzae | Proteases | ||||||||
| Sunflower meal | A. oryzae | Enzyme consortia | SsF solids | Sunflower meal | Nitrogen-rich hydrolysate | Nitrogen-rich hydrolysate + crude glycerol | C. necator DSM 545 | PHA | [73] |
| Rapeseed meal | Rapeseed meal | [74] | |||||||
| Exhausted sugar beet pulp pellets | A. awamori | Hydrolytic enzymes | SsF solids | Exhausted sugar beet pulp pellets | Hydrolysate (66 g L−1 reducing sugars) | - | - | - | [51] |
| Kitchen wastes | A. awamori | Glucoamylases | SsF solids | Kitchen waste | Hydrolysate (100.2 g L−1 glucose, 1081 mg L−1 FAN) | Kitchen waste hydrolysate | L. casei Shirota | Lactic acid | [75] |
| Bakery wastes | A. oryzae | Proteases | Kitchen waste powder | Hydrolysate (97.2 g L−1 glucose, 946.5 mg L−1 FAN) | Kitchen waste powder hydrolysate | ||||
| Wheat milling and flour-rich wastes | A. awamori | Glucoamylases, proteases | SsF solids suspension | Flour-rich waste | Hydrolysate (168.9 g L−1 glucose, 937.2 mg L−1 FAN) | Flour-rich waste hydrolysate | Lipomyces starkeyi DSM 70296 | Microbial oil | [76] |
| Rapeseed meal | A. oryzae | Hydrolytic enzymes | SsF solids suspension | SsF residue | Hydrolysate (2061.2 mg L−1 FAN, 1.8 g L−1 glucose) | Hydrolysate +glucose | S. cerevisiae | Dry yeast cells | [47] |
| Sugarcane bagasse (60%) + dry spent grains (40%) | A. niger TK1 | Hydrolytic enzymes | SsF solids suspension | SsF residue | Hydrolysate (29.7 g L−1 sugars, 585.1 mg L−1 FAN) | Hydrolysate | S. cerevisiae TISTR 5339 | Ethanol | [77] |
| Chlorella sp. TISTR 8411 biomass | Anaerobic granules | Dry residual biomass | - | - | - | Dry residual biomass hydrolysate | Anaerobic granules | Hydrogen | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, J.P.; Venus, J. Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy. Fermentation 2021, 7, 76. https://doi.org/10.3390/fermentation7020076
López-Gómez JP, Venus J. Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy. Fermentation. 2021; 7(2):76. https://doi.org/10.3390/fermentation7020076
Chicago/Turabian StyleLópez-Gómez, José Pablo, and Joachim Venus. 2021. "Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy" Fermentation 7, no. 2: 76. https://doi.org/10.3390/fermentation7020076
APA StyleLópez-Gómez, J. P., & Venus, J. (2021). Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy. Fermentation, 7(2), 76. https://doi.org/10.3390/fermentation7020076







