Influence of Nitrogen Sources on D-Lactic Acid Biosynthesis by Sporolactobacillus laevolacticus DSM 442 Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism
2.3. Culture Conditions
2.4. Enzymatic Hydrolysis of Waste, Agricultural and Food Raw Materials
2.5. Influence of Various Nitrogen Sources on D-LA Biosynthesis
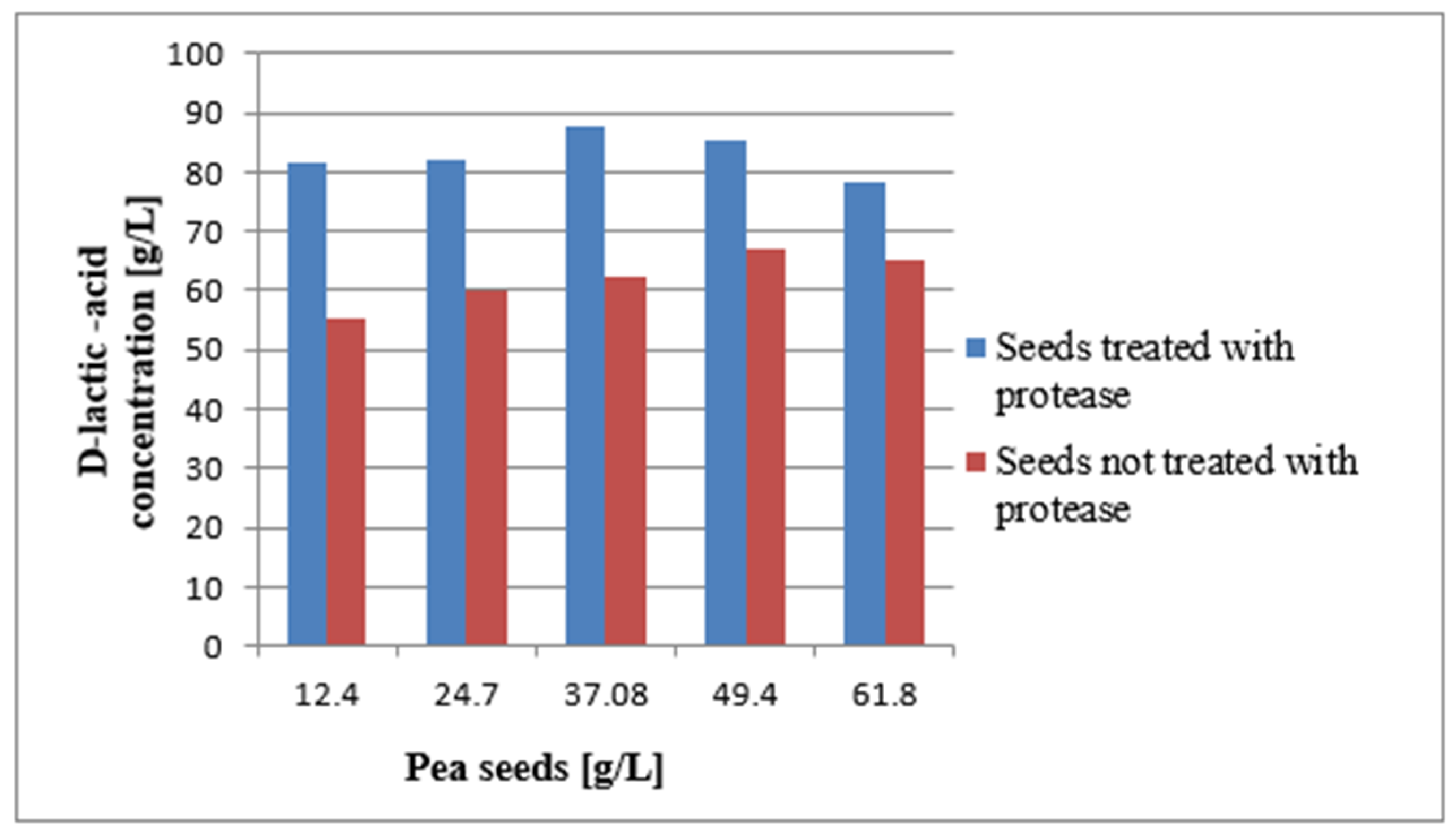
2.6. Influence of Pea Seeds Hydrolysate and Unhydrolysed Pea Seeds on D-LA Biosynthesis
2.7. Batch Fermentation
2.8. Fed-Batch Fermentation
2.9. Analysis of Biosynthesis Products
3. Results
3.1. Effects of Various Nitrogen Sources on D-LA Biosynthesis
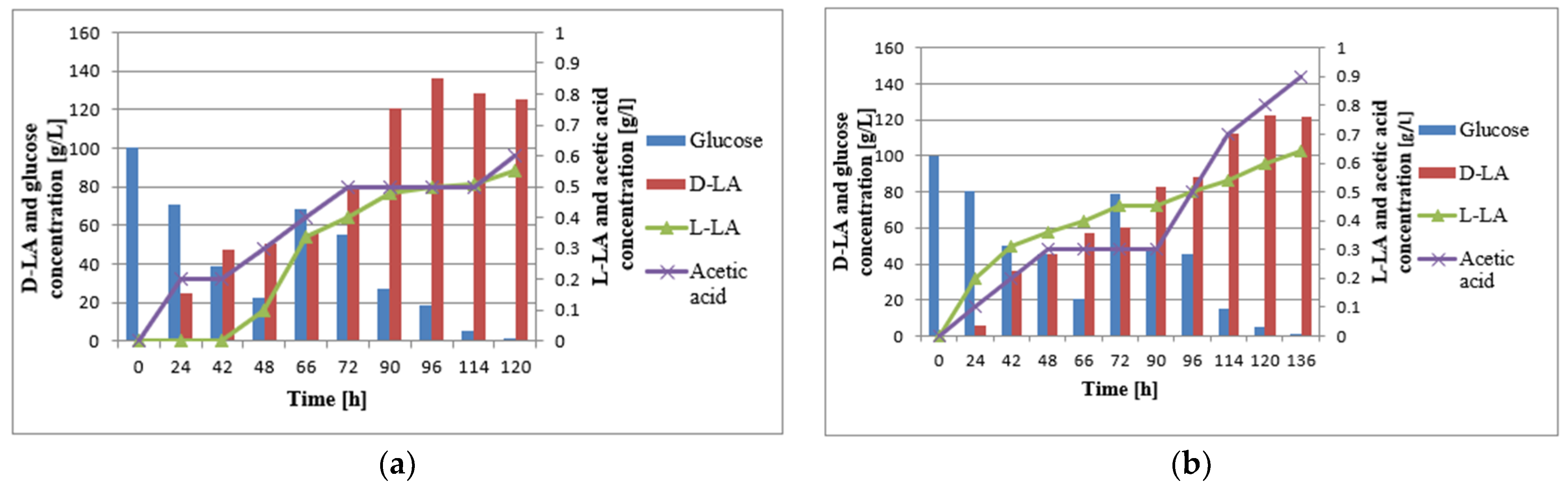
3.2. Batch Fermentation
3.3. Fed-Batch Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawai, H.; Na, K.; Sasaki, N.; Mimitsuka, T.; Minegishi, S.; Yamada, S.; Shimizu, M.K.; Yonehara, T. Membrane-integrated fermentation system for improving the optical purity of D-lactic acid produced during continuous fermentation. Biosci. Biotechnol. Biochem. 2011, 75, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.; Pometto, A. Enhanced production of D(-)-lactic acid by mutants of Lactobacillus delbrueckii ATCC 964. J. Ind. Microbiol. 1992, 11, 23–28. [Google Scholar] [CrossRef]
- Zheng, H.; Gong, J.; Chen, T.; Chen, X.; Zhao, X. Strain improvement of Sporolactobacillus inulinus ATCC 15538 for acid tolerance and production of D-lactic acid by genome shuffling. Appl. Microbiol. Biotechnol. 2010, 85, 1541–1549. [Google Scholar] [CrossRef]
- Michalczyk, A.; Cieniecka-Rosłonkiewicz, A.; Garbaczewska, S.; Morytz, B.; Białek, A. Badania nad wykorzystaniem biomasy skrobiowej jako źródła węgla w procesie fermentacji D-mleczanowej metodą SHF i SSF. Przem. Chem. 2020, 99, 99–102. [Google Scholar] [CrossRef]
- Smerilli, M.; Neureiter, M.; Wurz, S.; Haas, C.; Frühauf, S.; Fuchs, W. Direct fermentation of potato starch and potato residues to lactic acid by Geobacillus stearothermophilus under non-sterile conditions. J. Chem. Technol. Biotechnol. 2015, 90, 648–657. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Yu, X.; Chen, H.; Sun, Y.; Chen, G. Pretreatment of corn stover by solid acid for D-lactic acid fermentation. Bioresour. Technol. 2017, 239, 490–495. [Google Scholar] [CrossRef]
- Grosse, C.; Grigsby, W.J.; Noël, M.; Treu, A.; Thévenon, M.F.; Gérardin, P. Optimizing chemical wood modification with oligomeric lactic acid by screening of processing conditions. J. Wood Chem. Technol. 2019, 39, 385–398. [Google Scholar] [CrossRef]
- Yang, S.; Yu, H.; You, Y.; Li, X.; Jiang, J. Effective lactic acid production from waste paper using Streptococcus thermophilus at low enzyme loading assisted by Gleditsia saponin. Carbohyd Polym. 2018, 200, 122–127. [Google Scholar] [CrossRef]
- Vidra, A.; Tóth, A.J.; Németh, Á. Lactic acid production from cane molasses. Liquid Waste Recovery 2017, 2, 13–16. [Google Scholar] [CrossRef]
- Ghasemi, M.; Najafpour, G.; Rahimnejad, M.; Beigi, P.A.; Sedighi, M.; Hashemiyeh, B. Effect of different media on production of lactic acid from whey by Lactobacillus bulgaricus. Afr. J. Biotechnol. 2009, 8, 81–84. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Fukushima, K.; Chang, Y.-H.; Kimura, Y. Enhanced stereocomplex formation of poly(L-lactic acid) and poly(D-lactic acid) in the presence of stereoblock poly(lactic acid). Macromol. Biosci. 2007, 7, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Naveena, B.J.; Reddy, G. Use of inexpensive nitrogen sources and starch for L-lactic acid production in anaerobic submerged fermentation. Bioresour. Technol. 2007, 98, 498–503. [Google Scholar] [CrossRef]
- Nancib, A.; Nancib, N.; Cherif, D.M.; Boubendir, A.; Fick, M.; Boudrant, J. Joint effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 2005, 96, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G.H. Use of hydrolysates from Atlantic cod (Gadus morhua L.) viscera as a complex nitrogen source for lactic acid bacteria. FEMS Microbiol. Lett. 2005, 248, 65–68. [Google Scholar] [CrossRef][Green Version]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from yellow fin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Hyun, C.K.; Shin, H.K. Utilization of animal blood proteins as nitrogen sources for the cultivation of lactic acid bacteria. Korean J. Appl. Microbiol. Biotechnol. 1997, 25, 218–223. [Google Scholar]
- Paulova, L.; Chmelik, J.; Branska, B.; Patakova, P.; Drahokoupil, M.; Melzoch, K. Comparison of lactic acid production by L. casei in batch, fed-batch and continuous cultivation, testing the use of feather hydrolysate as a complex nitrogen source. Braz. Arch. Biol. Technol. 2020, 63, e20190151. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, P.C.; Lee, E.G.; Chang, Y.K.; Chang, N. Production of lactic acid by Lactobacillus rhamnosus with vitamin supplemented soybean hydrolysate. Enzyme Microb. Technol. 2000, 26, 209–215. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, B.; Li, F.; Xu, K.; Ma, C.; Tao, F.; Li, Q.; Xu, P. Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, Y.Y. Effect of temperature and various nitrogen sources on L(+)-lactic acid production by Lactobacillus casei. Appl. Microbiol. Biotechnol. 1996, 3, 307–313. [Google Scholar] [CrossRef]
- Altaf, M.; Naveena, B.J.; Ready, G. Screening of inexpensive nitrogen sources for production of L(+) lactic acid from starch by amylolytic Lactobacillus amylophilus GV6 in single step fermentation, Food Technol. Biotechnol. 2005, 43, 235–239. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Ju, J.; Yu, B.; Ma, Y. Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM 442 with agricultural waste cottonseed as the sole nitrogen source. Bioresour. Technol. 2013, 142, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Gao, Z.; Sun, J.; Wu, B.; He, B. D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresour. Technol. 2016, 207, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Jiang, A.L.; Mao, Y.Q.; Bo, W.; He, M.-X.; Hu, W.; Chen, J.-H.; Li, W.-J. Efficient L-lactic acid production from purified sweet sorghum juice coupled with soybean hydrolysate as nitrogen source by Lactobacillus thermophilus A69 strain. J. Chem Technol. Biotechnol. 2019, 94, 1752–1759. [Google Scholar] [CrossRef]
- Wee, Y.J.; Reddy, L.V.A.; Ryu, H.W. Fermentative production of L (+)-lactic acid from starch hydrolyzate and corn steep liquor as inexpensive nutrients by batch culture of Enterococcus faecalis RKY1. J. Chem. Technol. Biotechnol. 2008, 83, 1387–1393. [Google Scholar] [CrossRef]
- Yu, L.; Lei, T.; Ren, X.; Pei, X.; Feng, Y. Response surface optimization of l-(+)-lactic acid production using corn steep liquor as an alternative nitrogen source by Lactobacillus rhamnosus CGMCC 1466. Biochem. Eng. J. 2008, 39, 496–502. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Qi, B.; Chen, X.; Su, Z.; Wan, Y. Optimizing l-(+)-lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochem. Eng. J. 2010, 52, 212–219. [Google Scholar] [CrossRef]
- De la Torre, I.; Ladero, M.; Santos, V.E. Production of d-lactic acid by Lactobacillus delbrueckii ssp. delbrueckii from orange peel waste: Techno-economical assessment of nitrogen sources. Appl. Microbiol. Biotechnol. 2018, 102, 10511–10521. [Google Scholar] [CrossRef]
- Lund, B.; Norddahl, B.; Ahring, B. Production of lactic acid from whey using hydrolysed whey protein as nitrogen source. Biotechnol. Lett. 1992, 14, 851–856. [Google Scholar] [CrossRef]
- Amrane, A.; Prigent, Y. Lactic acid production from lactose in batch culture: Analysis of the data with the help of a mathematical model; relevance for nitrogen source and preculture assessment. Appl. Microbiol. Biotechnol. 1994, 40, 644–649. [Google Scholar] [CrossRef]
- Nancib, N.; Nancib, A.; Boudjelal, A.; Benslimane, C.; Blanchard, F.; Boudrant, J. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 2001, 78, 149–153. [Google Scholar] [CrossRef]
- Clifford, C.E.; Scott, V.B.; Alan, B.K. A Powerful Kjeldahl nitrogen method using peroxymonosulfuric acid. J. Agric. Food Chem. 1985, 33, 1117–1123. [Google Scholar]
- Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of Lactobacill. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Okano, K.; Hama, S.; Kihara, M.; Noda, H.; Tanaka, T.; Kondo, A. Production of optically pure D-lactic acid from brown rice using metabolically engineered Lactobacillus plantarum. Appl Microbiol Biotechnol. 2017, 101, 1869–1875. [Google Scholar] [CrossRef]
- Sreenath, H.K.; Moldes, A.B.; Koegel, R.G.; Straub, R.J. Lactic acid production from agriculture residues. Biotechnol. Lett. 2001, 23, 179–184. [Google Scholar] [CrossRef]
- Moldes, A.B.; Torrado, A.; Converti, A.; Domínguez, J.M. Complete bioconversion of hemicellulosic sugars from agricultural residues into lactic acid by Lactobacillus pentosus. Biotechnol. Appl. Biochem. 2006, 135, 219–227. [Google Scholar] [CrossRef]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Schepers, A.W.; Thibault, J.; Lacroix, C. Lactobacillus helveticus growth and lactic acid production during pH-controlled batch cultures in whey permeate/yeast extract medium. Part I. multiplefactor kinetic analysis. Enzyme Microb. Technol. 2002, 30, 176–186. [Google Scholar] [CrossRef]
- Hsieh, C.M.; Yang, F.C.; Iannott, E.L. The effect of soy protein hydrolyzates on fermentation by Lactobacillus amylovorus. Process Biochem. 1999, 34, 173–179. [Google Scholar] [CrossRef]
- Ishida, N.; Suzuki, T.; Tokuhiro, K.; Nagamori, E.; Onishi, T.; Saitoh, S.; Kitamoto, K.; Takahashi, H. D-Lactic acid production by metabolically engineered Saccharomyces cerevisiae. J. Biosci. Bioeng. 2006, 101, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Benthin, S.; Villadsen, J. Production of optically pure D-lactate by Lactobacillus bulgaricus and purification by crystallization and liquid/liquid extraction. Appl. Microbiol. Biotechnol. 1995, 42, 826–829. [Google Scholar] [CrossRef]
- Okino, S.; Suda, M.; Fujikura, K.; Inui, M.; Yukawa, H. Production of D-lactic acid by Corynebacterium glutamicumunder oxygen deprivation. Biotechnol. Bioprocess Eng. 2008, 78, 449–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Praveen, V.D. Lactic acid biosynthesis from biomass-derived sugars via Lactobacillus delbrueckii fermentation. Biotechnol. Bioprocess Eng. 2013, 36, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Mimitsuka, T.; Na, K.; Morita, K.; Sawai, H.; Minegishi, S.; Henmi, M.; Yamada, K.; Shimizu, S.; Yonehara, T. A membrane-integrated fermentation reactor system: Its effects in reducing the amount of sub-raw materials for D-lactic acid continuous fermentation by Sporolactobacillus laevolacticus. Biosci. Biotechnol. Biochem. 2012, 76, 67–72. [Google Scholar] [CrossRef]
- De Boer, J.P.; De Mattos, M.J.; Neijsseld, O.M. D(-)Lactic acid production by suspended and aggregated continuous cultures of Bacillus laevolacticus. Biosci. Biotechnol. Biochem. 1990, 34, 149–153. [Google Scholar] [CrossRef]

| Type of Raw Material | Total Nitrogen Content (%) (w/w) | Quantity of Substrate Used in the Production Medium (g/L) |
|---|---|---|
| (NH4)2SO4 | 21 a | 2.4 |
| NH4NO3 | 35 a | 1.4 |
| Urea | 47 a | 1.1 |
| Yeast extract | 10 b | 5.0 |
| Meat extract | 10 b | 5.0 |
| Peptone Bacteriological | 12 b | 4.2 |
| Casein tryptone | 10 b | 5.0 |
| Yeast cake | 8.5 c | 5.9 |
| Corn steep liquor | 1.9 c | 27.0 |
| Baker’s yeast | 2.7 c | 18.5 |
| Wheat middling | 10 c | 5.0 |
| Hazelnuts | 4.1 c | 12.3 |
| Pumpkin seeds | 5.5 c | 9.0 |
| Flax seeds | 3.2 c | 15.6 |
| Sunflower seeds | 2.3 c | 21.7 |
| Alfalfa seeds | 9.1 c | 5.5 |
| Lupine seeds | 6.5 c | 7.8 |
| Pea seeds | 4.1 c | 12.3 |
| Soybean seeds | 8.1 c | 6.2 |
| Source of Nitrogen | Optimal Fermentation Time [h] | Unreacted Glucose Content [g/L] (a) | D-LA Concentration [g/L] (a) | D-LA Yield [%] (b) |
|---|---|---|---|---|
| (NH4)2SO4 | 96 | 63.4, 0.26 | 33.0, 0.56 | 33 |
| NH4NO3 | 96 | 89.3, 0.59 | 8.9, 0.22 | 9 |
| Yeast extract | 96 | 12.8, 1.55 | 83.6, 2.65 | 84 |
| Urea | 120 | 79.4, 0.69 | 17.9, 1.29 | 18 |
| Meat extract | 96 | 62.8, 1.09 | 34.6, 2.09 | 35 |
| Peptone Bacteriological | 96 | 55.3, 1,45 | 36.0, 0.09 | 36 |
| Casein tryptone | 96 | 54.8, 1.22 | 42.9, 0.45 | 43 |
| Yeast cake | 168 | 45.2, 1.25 | 51.3, 0.78 | 51 |
| Corn steep liquor | 168 | 60.2, 1.65 | 36.9, 0.09 | 37 |
| Baker’s yeast | 168 | 50.2, 1.59 | 46.2, 2.51 | 46 |
| Wheat middling | 144 | 52.8, 1.22 | 44.1, 1.65 | 44 |
| Hazelnuts | 120 | 60.7, 1.68 | 34.8, 1.89 | 35 |
| Pumpkin seeds | 120 | 60.5, 2.09 | 35.5, 0.99 | 36 |
| Flax seeds | 120 | 66.2, 1.98 | 30.1, 0.89 | 30 |
| Sunflower seeds | 120 | 59.4, 0.98 | 37.6, 0.78 | 38 |
| Alfalfa seeds | 120 | 36.2, 0.88 | 61.0, 1.89 | 61 |
| Lupine seeds | 120 | 21.20, 0.5 | 74.4, 0.89 | 74 |
| Pea seeds | 120 | 15.3, 0.23 | 81.5, 2.90 | 82 |
| Soybean seeds | 120 | 34.9, 0.89 | 63.6, 3.09 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalczyk, A.K.; Garbaczewska, S.; Morytz, B.; Białek, A.; Zakrzewski, J. Influence of Nitrogen Sources on D-Lactic Acid Biosynthesis by Sporolactobacillus laevolacticus DSM 442 Strain. Fermentation 2021, 7, 78. https://doi.org/10.3390/fermentation7020078
Michalczyk AK, Garbaczewska S, Morytz B, Białek A, Zakrzewski J. Influence of Nitrogen Sources on D-Lactic Acid Biosynthesis by Sporolactobacillus laevolacticus DSM 442 Strain. Fermentation. 2021; 7(2):78. https://doi.org/10.3390/fermentation7020078
Chicago/Turabian StyleMichalczyk, Alicja Katarzyna, Sylwia Garbaczewska, Bolesław Morytz, Arkadiusz Białek, and Jerzy Zakrzewski. 2021. "Influence of Nitrogen Sources on D-Lactic Acid Biosynthesis by Sporolactobacillus laevolacticus DSM 442 Strain" Fermentation 7, no. 2: 78. https://doi.org/10.3390/fermentation7020078
APA StyleMichalczyk, A. K., Garbaczewska, S., Morytz, B., Białek, A., & Zakrzewski, J. (2021). Influence of Nitrogen Sources on D-Lactic Acid Biosynthesis by Sporolactobacillus laevolacticus DSM 442 Strain. Fermentation, 7(2), 78. https://doi.org/10.3390/fermentation7020078






