Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism Maintenance and Pre-Culture Conditions
2.2. Conditioning of the Residual Brewing Yeast (RBY)
2.3. Microbial Acclimatization and Cultivation
2.4. Logistic Approach for the Modeling of the Cell Growth
2.5. Biofilm Separation and Purification
2.6. Analyses
2.7. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8. Scanning Electron Microscopy (SEM)
2.9. Thermogravimetric Analysis (TGA)
3. Results
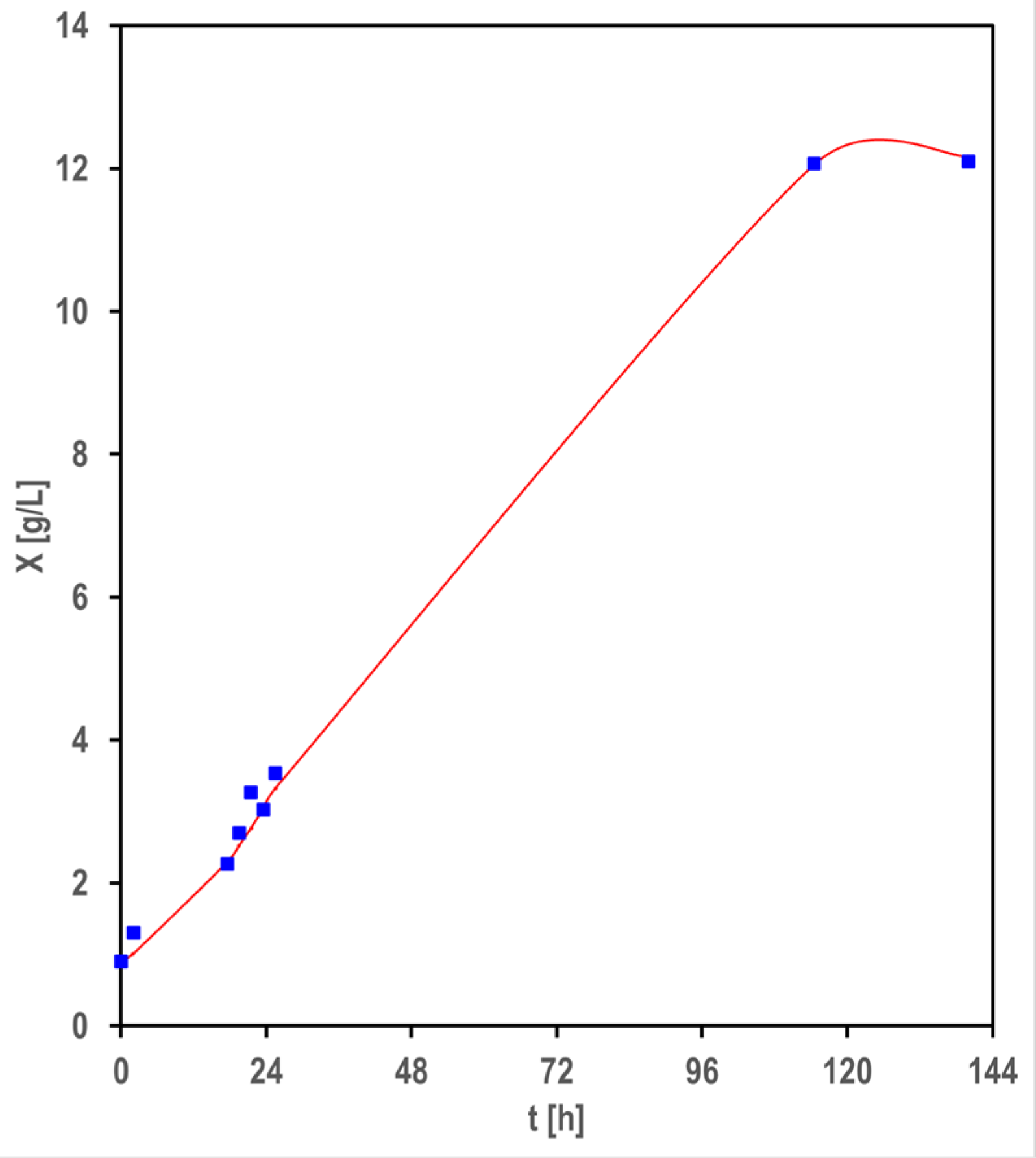
3.1. Biomass and Biofilm Production
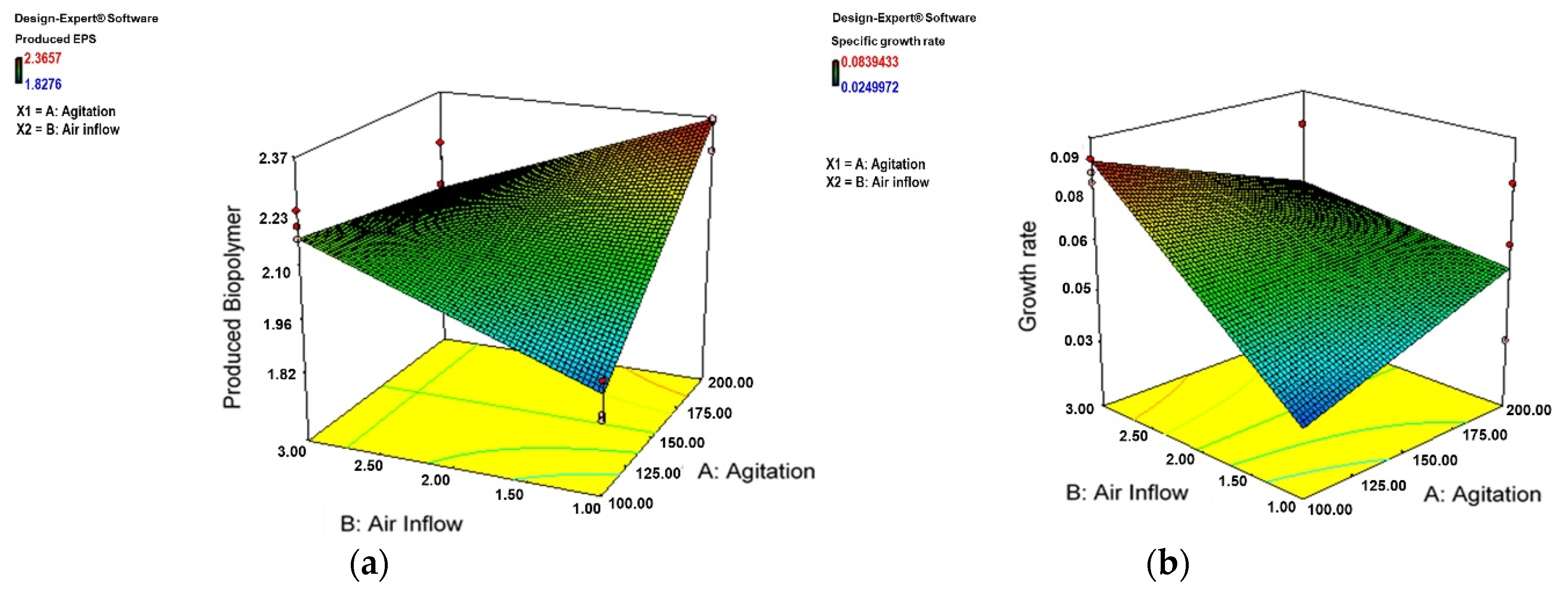
3.2. Effect of Aeration and Agitation on Biomass and Biofilm Production
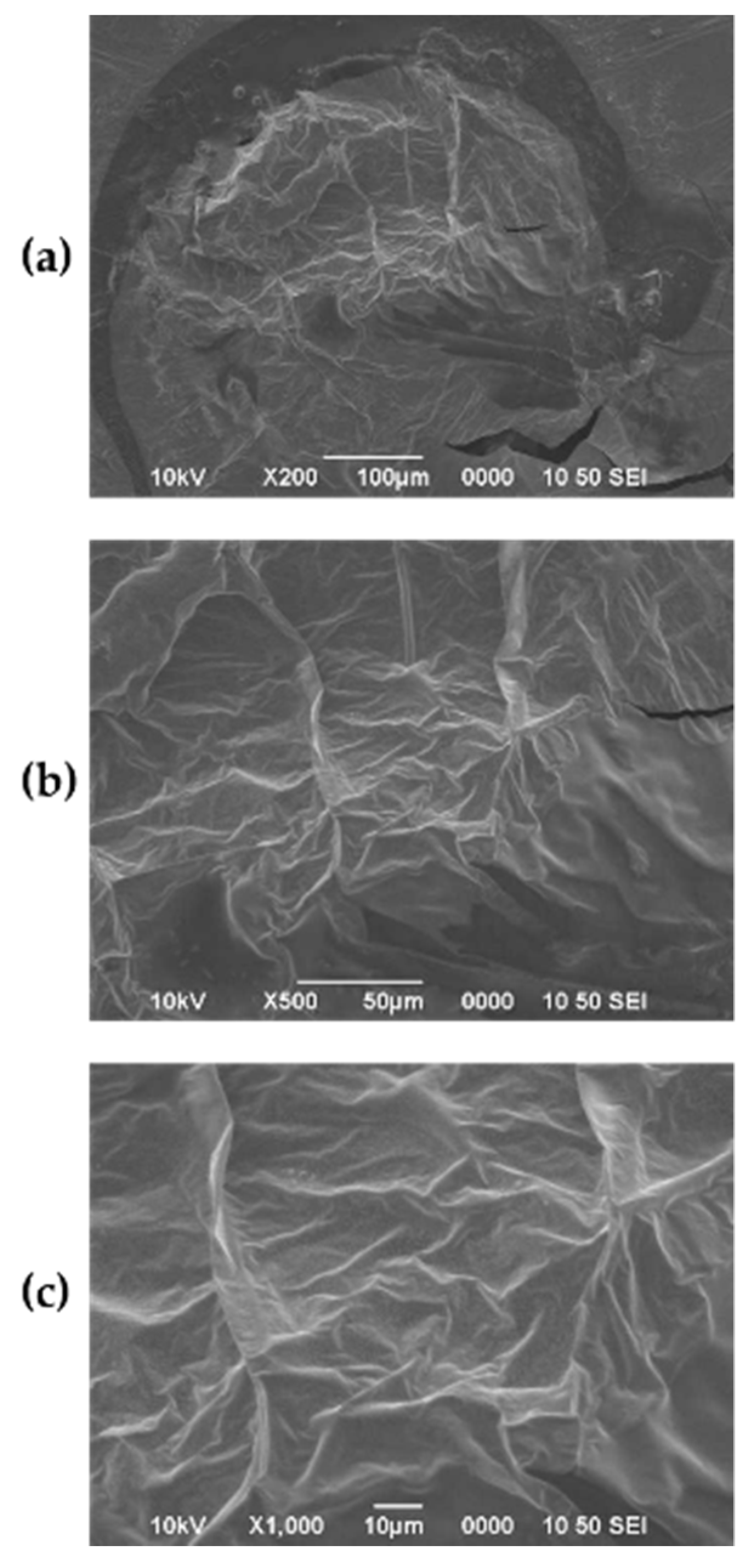
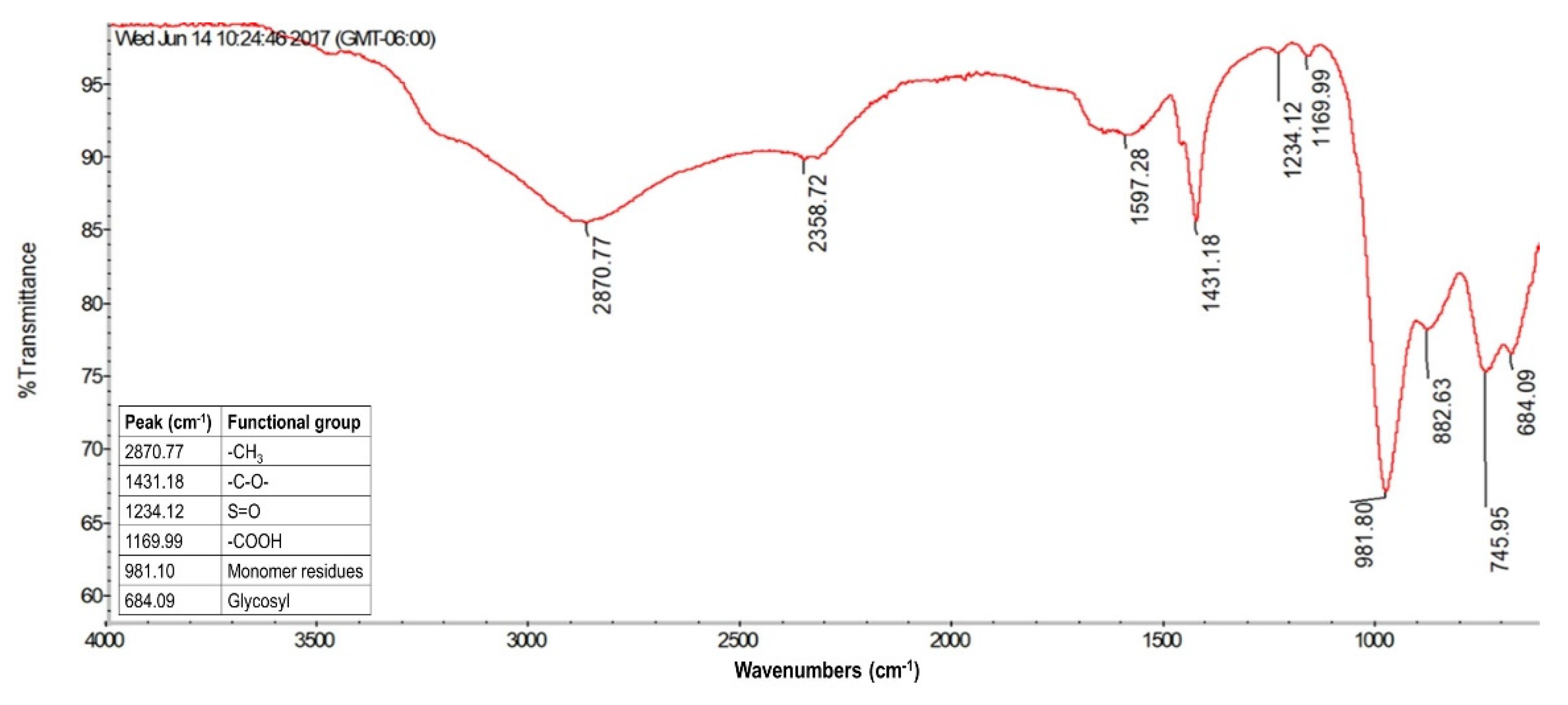
3.3. Characterization of the Biofilm
3.3.1. SEM
3.3.2. FTIR
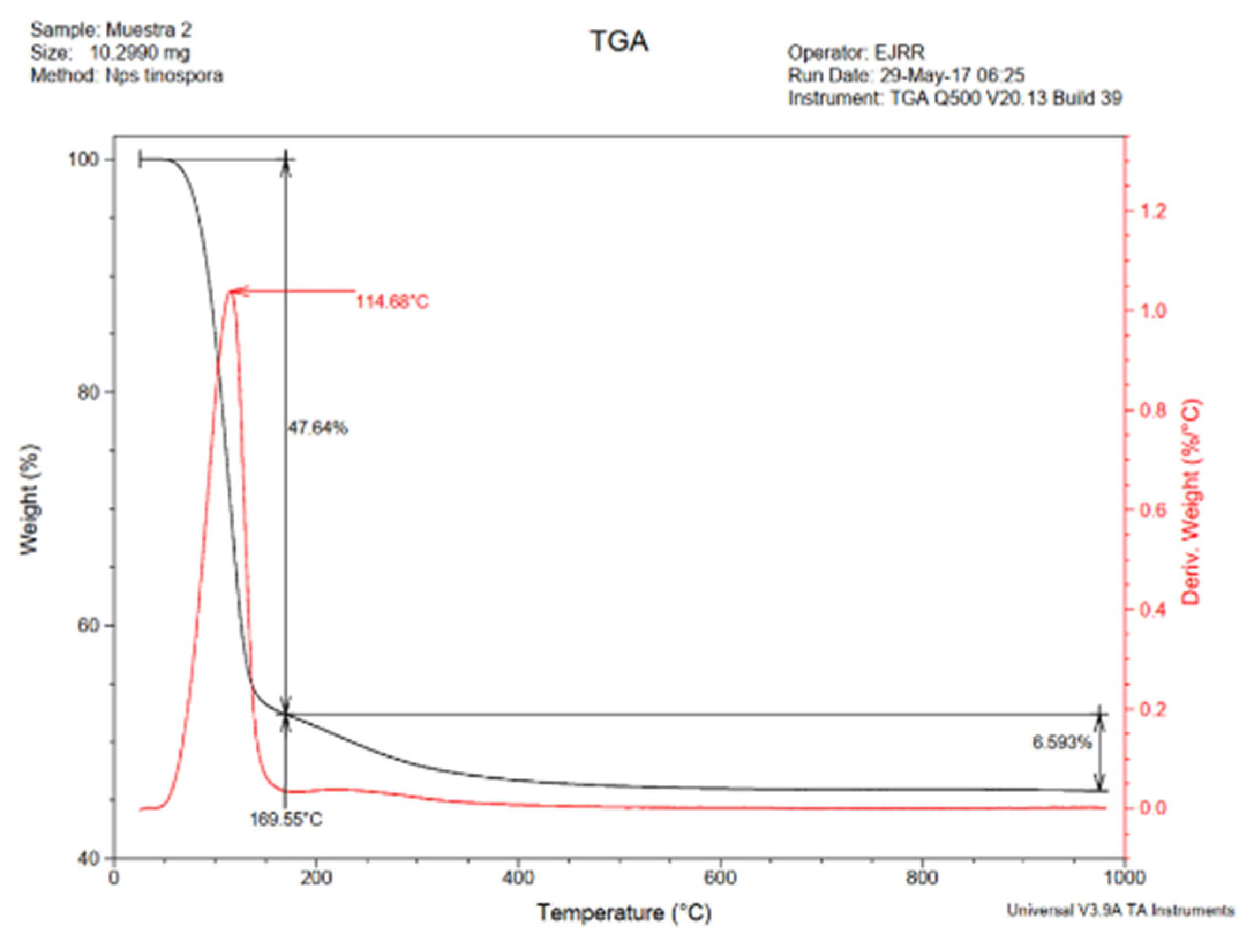
3.3.3. TGA Analysis
4. Discussion
4.1. Biomass and Biofilm Production
4.2. Logistic Approach for Modeling the Cell Growth
4.3. Effect of Aeration and Agitation on Biomass and Biofilm Production
4.4. Characterization of the Biofilm and Preliminary Evaluation of Potential Applications
4.5. Modeling As A Tool for Optimizing Bioconversion of Brewery Residues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Byerlee, D.; Fanzo, J. The SDG of zero hunger 75 years on: Turning full circle on agriculture and nutrition. Glob. Food Sec. 2019, 21, 52–59. [Google Scholar] [CrossRef]
- Renneberg, R.; Berkling, V.; Loroch, V. Green Biotechnology. In Biotechnology for Beginners, 2nd ed.; Renneberg, R., Berkling, V., Loroch, V., Eds.; Academic Press: New York, NY, USA, 2017; pp. 233–279. [Google Scholar]
- Roth, F.X. Microorganisms as a source of protein for animal nutrition. In Advances in Agricultural Microbiology, 1st ed.; Subba Rao, N.S., Ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 1982. [Google Scholar]
- Wolf, K. Nonconventional Yeasts in Biotechnology; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Komagata, K.; Nakase, T.; Katsuya, N. Assimilation of hydrocarbons by yeast. I. Preliminary screening. J. Gen. Appl. Microbiol. 1964, 10, 313–321. [Google Scholar] [CrossRef][Green Version]
- Awe, S.; Mikolasch, A.; Hammer, E.; Schauer, F. Degradation of phenylalkanes and characterization of aromatic intermediates acting as growth inhibiting substances in hydrocarbon utilizing yeast Candida maltosa. Int. Biodeter. Biodegrad. 2008, 62, 408–414. [Google Scholar] [CrossRef]
- Dmitriev, V.V.; Crowley, D.; Rogachevsky, V.V.; Negri, C.M.; Rusakova, T.G.; Kolesnikova, S.A.; Akhmetov, L.I. Microorganisms form exocellular structures, trophosomes, to facilitate biodegradation of oil in aqueous media. FEMS Microbiol. Lett. 2011, 315, 134–140. [Google Scholar] [CrossRef][Green Version]
- Chrzanowski, Ł.; Kaczorek, E.; Olszanowski, A. The ability of Candida maltosa for hydrocarbon and emulsified hydrocarbon degradation. Pol. J. Environ. Stud. 2006, 47–51. [Google Scholar]
- Echenique, P. Screening y Selección de Microorganismos que Posean Fuerte Capacidad de Formación de un Biofilm Estable en Condiciones Optimizadas de Cultivo. Bachelor’s Thesis, Universidad Mayor de San Andrés, La Paz, Bolivia, 2013. [Google Scholar]
- Romero, D.X.; Cárdenas, O.V.; Álvarez, M.T. Detection of ALS3 and EAP1 gene expression in Candida albicans and Candida maltosa biofilms by FISH. J. Adv. Biotechnol. 2016, 6, 848–857. [Google Scholar] [CrossRef]
- Singh, R.; Paul, D.; Jain, R.K. Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef]
- Nicolella, C.; Van Loosdrecht, M.C.M.; Heijnen, J.J. Wastewater treatment with particulate biofilm reactors. J. Biotechnol. 2000, 80, 1–33. [Google Scholar] [CrossRef]
- Williams, P.A. (Ed.) Renewable Resources for Functional Polymers and Biomaterials: Polysaccharides, Proteins and Polyesters, 1st ed.; Polymer Chemistry Series; RSC Publishing: London, UK, 2011. [Google Scholar]
- Gientka, I.; Bzducha-Wróbel, A.; Stasiak-Różańska, L.; Bednarska, A.A.; Błażejak, S. The exopolysaccharides biosynthesis by Candida yeast depends on carbon sources. Electron. J. Biotechnol. 2016, 22, 31–37. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.C.; De Aguiar, P.F.; Sérvulo, E.F.C. Production of Bacillus sphaericus entomopathogenic biomass using brewery residues. Appl. Biochem. Biotechnol. 2006, 131, 659–667. [Google Scholar] [CrossRef]
- Brochier, M.A.; Carvalho, S. Aspectos ambientais, produtivos e econômicos do aproveitamento de resíduo úmido de cervejaria na alimentação de cordeiros em sistema de confinamento (Environmental, productive and economic aspects of use of brewery residue as food of lamb feedlots in finishing phase). Ciência Agrotecnologia 2009, 33, 1392–1399. (In Portuguese) [Google Scholar]
- Butt, K.R. Utilisation of solid paper-mill sludge and spent brewery yeast as a feed for soil-dwelling earthworms. Bioresour. Technol. 1993, 44, 105–107. [Google Scholar] [CrossRef]
- Gonçalves, G.D.C.; Nakamura, P.K.; Furtado, D.F.; Veit, M.T. Utilization of brewery residues to produces granular activated carbon and bio-oil. J. Clean. Prod. 2017, 168, 908–916. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Moncada, J.; Roberto, I.C.; Cardona, C.A. Techno-economic analysis for brewer’s spent grains use on a biorefinery concept: The Brazilian case. Bioresour. Technol. 2013, 148, 302–310. [Google Scholar] [CrossRef]
- González-García, S.; Morales, P.C.; Gullón, B. Estimating the environmental impacts of a brewery waste–based biorefinery: Bio-ethanol and xylooligosaccharides joint production case study. Ind. Crops Prod. 2018, 123, 331–340. [Google Scholar] [CrossRef]
- Mathias, T.R.S.; de Mello, P.P.M.; Sérvulo, E.F.C. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar]
- Ivanova, V.; Antontceva, E.; Harbah, R.; Meledina, T.; Shamtsyan, M. Residual brewing yeasts as a source of beta-glucans. E3S Web Conf. 2020, 164, 06027. [Google Scholar] [CrossRef]
- Rashid, N.F.M.; Azemi, M.A.F.M.; Amirul, A.A.A.; Wahid, M.; Bhubalan, K. Simultaneous production of biopolymer and biosurfactant by genetically modified Pseudomonas aeruginosa UMTKB-5. IPCBEE 2015, 90, 16–21. [Google Scholar]
- Soto, L.R.; Byrne, E.; van Niel, E.W.; Sayed, M.; Carrasco, C.; Hatti-Kaul, R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef]
- Shuler, M.L.; Kargı, F.; DeLisa, M. Bioprocess Engineering: Basic Concepts, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wang, D.; Xu, Y.; Hu, J.; Zhao, G. Fermentation kinetics of different sugars by apple wine yeast Saccharomyces cerevisiae. J. Inst. Brew. 2004, 110, 340–346. [Google Scholar] [CrossRef]
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Aberna Ebenezer Selvakumari, I.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermotolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293, 122060. [Google Scholar] [CrossRef]
- Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutierrez, F.; Vega-Baudrit, H.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides production by cultivating a bacterial isolate from the hypersaline environment of Salar de Uyuni (Bolivia) in pretreatment liquids of steam-exploded quinoa stalks and enzymatic hydrolyzates of Curupaú sawdust. Fermentation 2021, 7, 33. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Anjum, N.; Ahmad, A.; Khan, S.T. Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir–Part II. Food Hydrocoll. 2013, 30, 343–350. [Google Scholar] [CrossRef]
- Asahi, Y.; Miura, J.; Tsuda, T.; Kuwabata, S.; Tsunashima, K.; Noiri, Y.; Hayashi, M. Simple observation of Streptococcus mutans biofilm by scanning electron microscopy using ionic liquids. AMB Express 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Kielak, A.; Castellane, T.; Campanharo, J.; Colnago, L.; Costa, O.; Silva, M.; van Veen, J.; Lemos, E.; Kuramae, E. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications. Sci. Rep. 2016, 7, 41193. [Google Scholar] [CrossRef]
- Tipson, R.S. Infrared Spectroscopy of Carbohydrates: A Review of the Literature. NBS Monograph; United States Government Printing Office: Washington, DC, USA, 1968.
- Barros, F.; Silva, D.; Sombra, V.; Maciel, J.; Feitosa, J.; Freitas, A.; de Paula, R. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Abou-ElWafa, G.; Shaaban-Dessuuki, S.; Hassan, N. Characterization and antioxidant activity of exopolysaccharide secreted by Nostoc carneum. Int. J. Pharmacol. 2015, 11, 432–439. [Google Scholar] [CrossRef]
- Mahmood, W.; Khan, M.; Yee, T. Effects of reaction temperature on the synthesis and thermal properties of carrageenan ester. J. Phys. Sci. 2014, 25, 123–138. [Google Scholar]
- Rani, R.; Anadharaj, M.; Sabhapathy, P.; Ravindran, A. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int. J. Biol. Macromol. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Daoub, R.; Elmurabak, A.; Misran, M.; Hassan, E.; Osman, M. Characterization and functional properties of some natural Acacia gums. J. Saudi Soc. Agric. Sci. 2016, 17, 241–249. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Pandey, A. Conversion of food and kitchen waste to value-added products. J Environ. Manag. 2019, 241, 619–630. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ballesteros, M.; Negro, M.J. Biorefineries for the valorization of food processing waste. In The Interaction of Food Industry and Environment; Galanakis, C., Ed.; Academic Press: New York, NY, USA, 2020; pp. 155–190. [Google Scholar]
- Munawar, R.A.; Irfan, M.; Nadeem, M.; Syed, Q.A.; Siddique, Z.H. Biosynthesis of single cell biomass of Candida utilis by submerged fermentation. Pak. J. Sc. 2010, 62, 1–5. [Google Scholar]
- Begea, M.; Sirbu, A.; Kourkoutas, Y.; Dima, R. Single-cell protein production of Candida strains in culture media based on vegetal oils. Rom. Biotechnol. Lett. 2012, 17, 7776–7786. [Google Scholar]
- Yunus, F.U.N.; Nadeem, M.; Rashid, F. Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed. J. Inst. Brew. 2015, 121, 553–557. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A biorefinery approach for the production of xylitol, ethanol and polyhydroxybutyrate from brewer’s spent grain. AIMS Agric. Food 2016, 1, 52–66. [Google Scholar] [CrossRef]
- Giavasis, I.; Robertson, I.; McNeil, B.; Harvey, L.M. Simultaneous and rapid monitoring of biomass and biopolymer production by Sphingomonas paucimobilis using Fourier transform-near infrared spectroscopy. Biotechnol. Lett. 2003, 25, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Minkevich, I.G.; Baumann, F.; Rogge, G.; Heinritz, B. Ratio of heat production to oxygen consumption during the cell cycle of Candida maltosa EH 15 grown on ethanol. Acta Biotechnol. 1988, 8, 435–444. [Google Scholar] [CrossRef]
- Kometani, T.; Morita, Y.; Kiyama, Y.; Yoshii, H.; Matsuno, R. Relationship between ethanol consumption rate and prochiral ketone reduction rate in bakers’ yeast. J. Ferm. Bioeng. 1995, 80, 208–210. [Google Scholar] [CrossRef]
- Hofmann, K.H.; Polnisch, E. Activities of gluconeogenetic enzymes in the yeast Candida maltosa during growth on glucose or ethanol. J. Basic Microbiol. 1990, 30, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Sharyshev, A.A.; Peskova, E.B.; Komarova, G.N.; Blinova, T.V. Subcellular organization of aliphatic alcohol metabolism in the yeast Candida maltosa. Appl. Biochem. Microbiol. 1996, 32, 111–118. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, B.; Lv, J.; Jiang, M.G.; Lin, S.J.; Deng, Z.X. Xylitol production from xylose mother liquor: A novel strategy that combines the use of recombinant Bacillus subtilis and Candida maltosa. Microb. Cell Factories 2011, 10, 1–12. [Google Scholar] [CrossRef]
- Domenech, F.; Christen, P.; Paca, J.; Revah, S. Ethanol utilization for metabolite production by Candida utilis strains in liquid medium. Acta Biotechnol. 1999, 19, 27–36. [Google Scholar] [CrossRef]
- Pina, C.; Santos, C.; Couto, J.A.; Hogg, T. Ethanol tolerance of five non-Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae—Influence of different culture conditions. Food Microbiol. 2004, 21, 439–447. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; de Souza Oliveira, R.P.; Guerra, N.P.; Domínguez, J.M. A novel approach to the biorefinery of brewery spent grain. Proc. Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Narayanan, H.; Luna, M.F.; von Stosch, M.; Cruz Bournazou, M.N.; Polotti, G.; Morbidelli, M.; Butté, A.; Sokolov, M. Bioprocessing in the digital age: The role of process models. Biotechnol. J. 2020, 15, 1900172. [Google Scholar] [CrossRef]
- Jakymec, M.; Morán, H.; Páez, G.; Ferrer, J.; Mármol, Z.; Ramones, E. Kinetic of the Lactic Acid Production by Submerged Fermentation whit Whey as Substrate. Rev. Científica-Fac. Cienc. Vet. 2001, 11, 53–59. [Google Scholar]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. The effect of agitation and aeration on the synthesis and molecular weight of gellan in batch cultures of Sphingomonas paucimobilis. Enz. Microb. Technol. 2006, 38, 101–108. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Martinov, M.; Kuncheva, M.; Panchev, I.; Vlaev, S.; Kambourova, M. Optimization of the aeration and agitation speed of Aeribacillus palidus 418 exopolysaccharide production and the emulsifying properties of the product. Proc. Biochem. 2014, 49, 576–582. [Google Scholar] [CrossRef]
- Rosma, A.; Ooi, K.I. Production of Candida utilis biomass and intracellular protein content: Effect of agitation speed and aeration rate. Malays. J. Microb. 2006, 2, 15–18. [Google Scholar] [CrossRef]
- Dimova, N.D.; Iovkova, Z.S.; Brinkova, M.; Godjevargova, T.I. Production of Candida biomass from hydrolysed agricultural biowaste. Biotechnol. Biotechnol. Equip. 2010, 24, 1577–1581. [Google Scholar] [CrossRef][Green Version]
- Zakeri, A.; Pazouki, M.; Vossougi, M. Development of kinetic model for xanthan production in a laboratory-scale batch fermentor. Iran. J. Sci. Technol. Trans. Sci. 2018, 42, 261–266. [Google Scholar] [CrossRef]
- Khan, T.; Park, J.K.; Kwon, J.H. Functional biopolymers produced by biochemical technology considering applications in food engineering. Korean J. Chem. Eng. 2007, 24, 816–826. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Cutlip, M.B.; Shacham, M. Problem solving in chemical and biochemical engineering with POLYMATH, Excel, and MATLAB. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2008; pp. 1–17. [Google Scholar]
- Rana, S.S.; Janveja, C.; Soni, S.K. Brewer’s spent grain as a valuable substrate for low cost production of fungal cellulases by statistical modeling in solid state fermentation and generation of cellulosic ethanol. Int. J. Food Ferment. Technol. 2013, 3, 41–55. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of antioxidant phenolic compounds from brewer’s spent grain: Optimization and kinetics modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Ingalls, R.G.; Jones, C.L.; Huhnke, R.L.; Khanchi, A. Scenario optimization modeling approach for design and management of biomass-to-biorefinery supply chain system. Bioresour. Technol. 2013, 150, 163–171. [Google Scholar] [CrossRef]
- Sanz, A.; Susmozas, A.; Peters, J.; Dufour, J. Biorefinery modeling and optimization. In Biorefineries. Lecture Notes in Energy; Rabaçal, M., Ferreira, A., Silva, C., Costa, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 57, pp. 123–160. [Google Scholar]
- Ploch, T.; Zhao, X.; Hüser, J.; von Lieres, E.; Hannemann-Tamás, R.; Naumann, U.; Wiechert, W.; Mitsos, A.; Noack, S. Multiscale dynamic modeling and simulation of a biorefinery. Biotechnol. Bioeng. 2019, 116, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Spann, R.; Lantz, A.E.; Gernaey, K.V.; Sin, G. Modeling for process risk assessment in industrial bioprocesses. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–17. [Google Scholar]


| Agitation (rpm) | Aeration (L min−1) | Biofilm (g L−1) | µ a (h−1) | Biomass Productivity b (g L−1·h−1) | (g g−1) | Biomass (g CDW L−1) |
|---|---|---|---|---|---|---|
| 100 | 1 | 1.87 ± 0.04 | 0.027 ± 0.005 | 0.14 ± 0.03 | 0.44 ± 0.02 | 14.17 ± 1.47 |
| 200 | 1 | 2.33 ± 0.04 | 0.057 ± 0.011 | 0.20 ± 0.01 | 0.41 ± 0.02 | 13.17 ± 0.61 |
| 100 | 3 | 2.20 ± 0.03 | 0.080 ± 0.008 | 0.31 ± 0.02 | 0.53 ± 0.01 | 16.97 ± 2.01 |
| 200 | 3 | 2.12 ± 0.07 | 0.067 ± 0.009 | 0.34 ± 0.03 | 0.48 ± 0.02 | 15.47 ± 3.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Copa, V.; Romero-Soto, L.; Romero-Calle, D.; Alvarez-Aliaga, M.T.; Orozco-Gutierrez, F.; Vega-Baudrit, J.; Martín, C.; Carrasco, C. Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4. Fermentation 2021, 7, 84. https://doi.org/10.3390/fermentation7020084
Flores-Copa V, Romero-Soto L, Romero-Calle D, Alvarez-Aliaga MT, Orozco-Gutierrez F, Vega-Baudrit J, Martín C, Carrasco C. Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4. Fermentation. 2021; 7(2):84. https://doi.org/10.3390/fermentation7020084
Chicago/Turabian StyleFlores-Copa, Vidal, Luis Romero-Soto, Danitza Romero-Calle, María Teresa Alvarez-Aliaga, Felipe Orozco-Gutierrez, José Vega-Baudrit, Carlos Martín, and Cristhian Carrasco. 2021. "Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4" Fermentation 7, no. 2: 84. https://doi.org/10.3390/fermentation7020084
APA StyleFlores-Copa, V., Romero-Soto, L., Romero-Calle, D., Alvarez-Aliaga, M. T., Orozco-Gutierrez, F., Vega-Baudrit, J., Martín, C., & Carrasco, C. (2021). Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4. Fermentation, 7(2), 84. https://doi.org/10.3390/fermentation7020084








