Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Selection of ICA Producer
3.2. Effect of Growth-Limiting Component of Cultivation Media
3.3. Effect of Nitrogen Source
3.4. Effect of pH
3.5. Effect of EAF Concentration
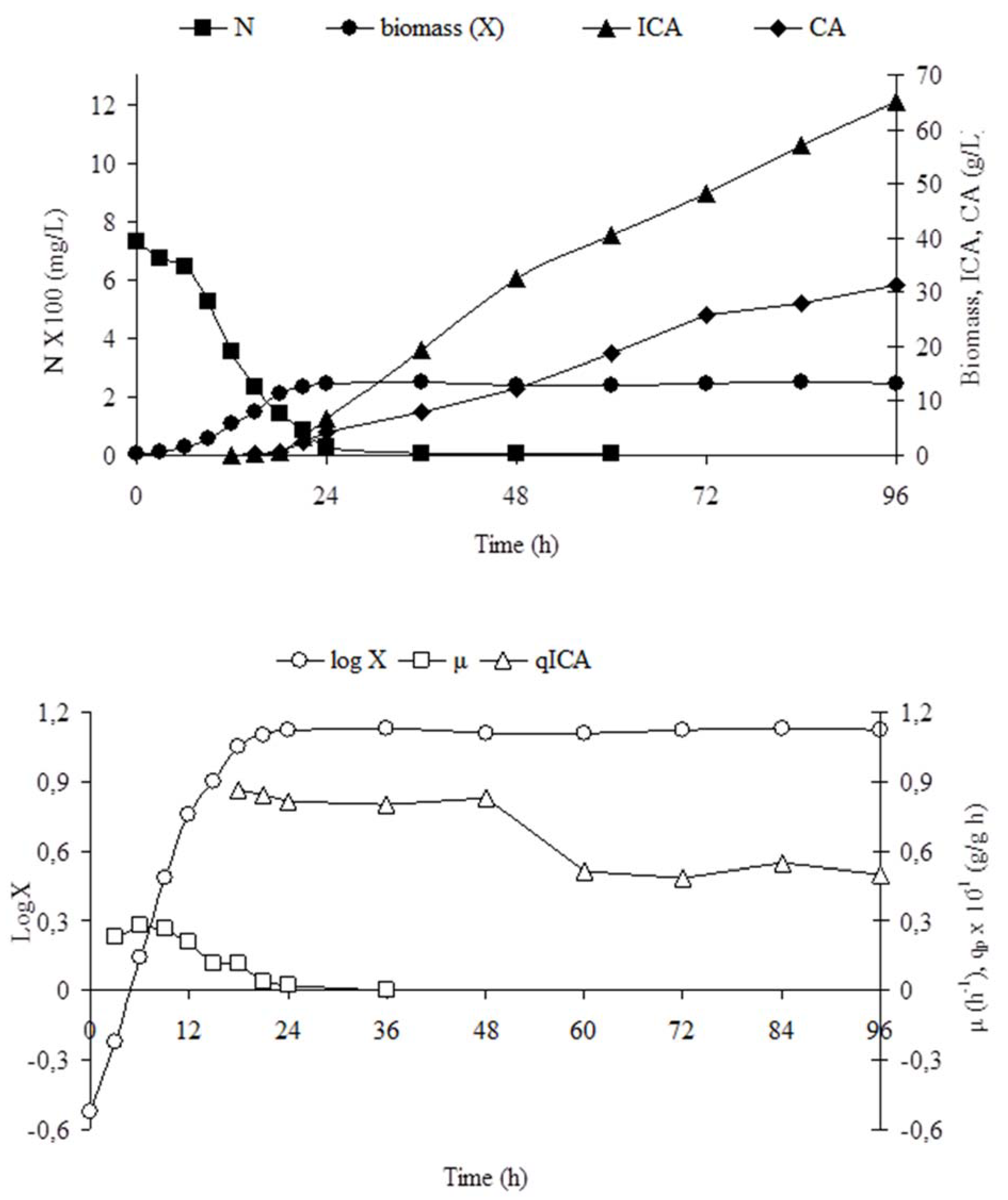
3.6. Dynamics of Yeast Growth and Acid Excretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heretsch, P.; Thomas, F.; Aurich, A.; Krautscheid, H.; Sicker, D.; Giannis, A. Syntheses with a chiral building block from the citric acid cycle: (2R,3S)-isocitric acid by fermentation of sunflower oil. Angew. Chem. Int. Ed. Engl. 2008, 47, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Shaikh, Y.B.; Borhade, A.S.; Dhondge, A.P.; Chavhan, S.W.; Desai, M.P.; Birhade, D.R.; Dhatrak, N.R.; Gannimani, R. The efficient synthesis of (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol and its isomers. Tetrahedron Asymmetry 2010, 21, 2394–2398. [Google Scholar] [CrossRef]
- Aurich, A.; Specht, R.; Müller, R.A.; Stottmeister, U.; Yovkova, V.; Otto, C.; Holz, M.; Barth, G.; Heretsch, P.; Thomas, F.A.; et al. Microbiologically produced carboxylic acids used as building blocks in organic synthesis. In Reprogramming Microbial Metabolic Pathways. Subcellular Biochemistry; Wang, X., Chen, J., Quinn, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 64, pp. 391–423. [Google Scholar] [CrossRef]
- Aurich, A.; Hofmann, J.; Oltrogge, R.; Wecks, M.; Glaser, R.; Blömer, L.; Mauersberger, S.; Roland, A.; Müller, R.A.; Sicker, D.; et al. Improved isolation of microbiologically produced (2R,3S)-isocitric acid by adsorption on activated carbon and recovery with methanol. Org. Process. Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Moore, G.L.; Stringham, R.W.; Teager, D.S.; Yue, T.Y. Practical synthesis of the bicyclic darunavir side chain: (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol from monopotassium isocitrate. Org. Process. Res. Dev. 2017, 21, 98–106. [Google Scholar] [CrossRef]
- Yang, J.; Kim, M.J.; Yoon, W.; Kim, E.Y.; Kim, H.; Lee, Y.; Min, B.; Kang, K.S.; Son, J.H.; Park, H.T.; et al. Isocitrate protects DJ-1 null dopaminergic cells from oxidative stress through NADP+-dependent isocitrate dehydrogenase (IDH). PLoS Genet. 2017, 13, e1006975. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.V.; Inozemtsev, A.N. Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol. 2019, 103, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Bullin, K.; Hennig, L.; Herold, R.; Krautscheid, H.; Richter, K.; Sicker, D. An optimized method for an (2R,3S)-isocitric acid building block. Mon. Chem. 2019, 150, 247–253. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.V.; Lunina, J.N.; Inozemtsev, A.N. Microbiological production of isocitric acid from biodiesel waste and its effect on spatial memory. Microorganisms 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fickers, P.; Cheng, H.; Sze, K.; Lin, C. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Vickery, H.B. A suggested new nomenclature for the isomers of isocitric acid. J. Biol. Chem. 1962, 237, 1739–1741. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef]
- Finogenova, T.V.; Shishkanova, N.V.; Fausek, E.A.; Eremina, S.S. Biosynthesis of isocitric acid from ethanol by yeasts. Appl. Microbiol. Biotechnol. 1991, 36, 231–235. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shamin, R.V.; Stepanova, N.N.; Morgunov, G.I.; Lunina, J.N.; Allayarov, R.K.; Samoilenko, V.A.; Morgunov, I.G. Fermentation conditions and media optimization for isocitric acid production from ethanol by Yarrowia lipolytica. Biomed. Res. Int. 2018, e2543210. [Google Scholar] [CrossRef] [Green Version]
- Kamzolova, S.V.; Dedyukhina, E.G.; Samoilenko, V.A.; Lunina, J.N.; Puntus, I.F.; Allayarov, R.K.; Chiglintseva, M.N.; Mironov, A.A.; Morgunov, I.G. Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 2013, 97, 9133–9144. [Google Scholar] [CrossRef]
- Laptev, I.A.; Filimonova, N.A.; Allayarov, R.K.; Kamzolova, S.V.; Samoilenko, V.A.; Sineoky, S.P.; Morgunov, I.G. New recombinant strains of the yeast Yarrowia lipolytica with overexpression of the aconitate hydratase gene for the obtainment of isocitric acid from rapeseed oil. Appl. Biochem. Microbiol. 2016, 52, 699–704. [Google Scholar] [CrossRef]
- Hapeta, P.; Rakicka-Pustułka, M.; Juszczyk, P.; Robak, M.; Rymowicz, W.; Lazar, Z. Overexpression of citrate synthase increases isocitric acid biosynthesis in the heast Yarrowia lipolytica. Sustainability 2020, 12, 7364. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Effects of medium components on isocitric acid production by Yarrowia lipolytica yeast. Fermentation 2020, 6, 112. [Google Scholar] [CrossRef]
- Finogenova, T.V.; Shishkanova, N.V.; Ermakova, I.T.; Kataeva, I.A. Properties of Candida lipolytica mutants with the modified glyoxylate cycle and their ability to produce citric and isocitric acid. II. Synthesis of citric and isocitric acid by mutants and peculiarities of their enzyme systems. Appl. Microbiol. Biotechnol. 1986, 23, 378–383. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, B. Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.; Förster, A.; Mauersberger, S.; Barth, G. Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 81, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasheva, E.Y.; Scarcia, P.; Yuzbashev, T.V.; Messina, E.; Kosikhina, I.M.; Palmieri, L.; Shutov, A.V.; Taratynova, M.O.; Amaro, R.L.; Palmieri, F.; et al. Engineering Yarrowia lipolytica for the selective and high-level production of isocitric acid through manipulation of mitochondrial dicarboxylate-tricarboxylate carriers. Metab. Eng. 2021, 65, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, I.T.; Shishkanova, N.V.; Melnikova, O.F.; Finogenova, T.V. Properties of Candida lipolytica mutants with the modified glyoxylate cycle and their ability to produce citric and isocitric acid. I. Physiological, biochemical and cytological characteristics of mutants grown on glucose or hexadecane. Appl. Microbiol. Biotechnol. 1986, 23, 372–377. [Google Scholar] [CrossRef]
- Da Silva, L.V.; Tavares, C.B.; Amaral, P.F.F.; Coehlo, M.A.Z. Production of citric acid by Yarrowia lipolytica in different crude oil concentrations and in different nitrogen sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Barth, G.; Gaillardin, C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 1997, 19, 219–237. [Google Scholar] [CrossRef]
- Rywińska, A.; Wojtatowicz, M.; Rymowicz, W. Citric acid biosynthesis by Yarrowia lipolytica A-101-1.31 under deficiency of various medium macrocomponents. Electron. J. Pol. Agric. Univ. 2006, 9, 15. [Google Scholar]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia Lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Citric acid and itaconic acid accumulation: Variations of the same story? Appl. Microbiol. Biotechnol. 2019, 103, 2889–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotech. 2006, 44, 185–195. [Google Scholar]
- Moeller, L.; Strehlitz, B.; Aurich, A.; Zehnsdorf, A.; Bley, T. Optimization of citric acid production from glucose by Yarrowia Lipolytica. Eng. Life Sci. 2007, 7, 504–511. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywinska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nicaud, J.M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef]
| Strain | Acids (g/L) | ICA (% of Total Acids) | ||||||
|---|---|---|---|---|---|---|---|---|
| ICA | AA | CA | KGA | SA | MA | FA | ||
| Aciculoconidium aculeatum VKM Y-1301 | 0 | 0 | 0.11 | 0 | 0 | 0.11 | 0.06 | - |
| Babjeviella inositovora VKM Y-2494 | 0.10 | 0.09 | 0.13 | 0.08 | 0 | 0.04 | 0.05 | 22.7 |
| Blastobotrys adeninivorans VKM Y-2676 | 0 | 0.11 | 0.33 | 0.16 | 0 | 0.11 | 0.11 | - |
| Candida intermedia | 0.08 | 0.12 | 0.01 | 0.12 | 0 | 0.12 | 0.12 | 17.8 |
| C. saitoana 127 | 0.10 | 0 | 0.45 | 0.07 | 0 | 0.1 | 0.07 | 13.9 |
| C. utilis VKM Y-33 | 0 | 0.14 | 1.05 | 0.42 | 0.32 | 0.45 | 0.10 | - |
| C. zeylanoides VKM Y-14 | 0.50 | 0.12 | 1.0 | 0.10 | 0.32 | 0.44 | 0.07 | 20.2 |
| C. zeylanoides VKM Y-2324 | 0.38 | 0.12 | 0.55 | 0.13 | 0.25 | 0.22 | 0.06 | 23.0 |
| C. valida VKM Y-1493 | 0.14 | 0.12 | 0 | 0 | 0.52 | 0.12 | 0.01 | 15.6 |
| Diutina catenulata VKM Y-5 | 0.15 | 0.15 | 0.21 | 1.50 | 0.33 | 0.62 | 0.08 | 5.1 |
| D. rugosa VKM Y-67 | 0 | 0.14 | 0.15 | 0 | 0.2 | 0 | 0.07 | - |
| Kluyveromyces wickerhamii VKM Y-589 | 0 | 0.62 | 0.18 | 0.08 | 0.21 | 0.10 | 0.05 | - |
| Kregervanrija fluxuum VKM Y-240 | 0.31 | 0.11 | 0 | 0.10 | 0.32 | 0.15 | 0.08 | 31.3 |
| Meyerozyma guilliermondii | 0.20 | 0.53 | 0.45 | 0.09 | 0.15 | 0.12 | 0.05 | 13.0 |
| Pichia besseyi VKM Y-2084 | 0 | 0.12 | 0.43 | 0.45 | 0.40 | 0.32 | 0.03 | - |
| P. media VKM Y-1381 | 0.20 | 0.10 | 0.40 | 0.10 | 0.33 | 0.10 | 0.04 | 16.3 |
| P. membranifaciens VKM Y-292 | 0 | 0.13 | 0.15 | 0 | 0.13 | 0 | 0.07 | - |
| Sugiyamaella paludigena VKM Y-2443 | 0.08 | 0.55 | 0 | 0.40 | 0.55 | 0.32 | 0.07 | 4.2 |
| Torulaspora candida 420 | 0.10 | 0.45 | 0.32 | 0.10 | 0 | 0.10 | 0.08 | 9.3 |
| T. globosa VKM Y-93 | 0.10 | 0.10 | 0 | 0.10 | 0.10 | 0.10 | 0.10 | 20.0 |
| Wickerhamomyces anomalus VKM Y-118 | 0 | 0.15 | 0 | 0.10 | 0.43 | 0.12 | 0.07 | - |
| Yarrowia lipolytica 12a | 0.15 | 0.15 | 0 | 0.09 | 0.52 | 0.15 | 0.06 | 14.2 |
| Y. lipolytica VKM Y-47 | 0 | 0.10 | 0.52 | 0.45 | 0.40 | 0.30 | 0.10 | - |
| Y. lipolytica 68 | 0.06 | 0.10 | 0.07 | 0.10 | 0.10 | 0.10 | 0.10 | 11.3 |
| Y. lipolytica 69 | 1.11 | 0.15 | 0.92 | 0.50 | 0.35 | 0.41 | 0.12 | 32.3 |
| Y. lipolytica VKM Y-57 | 1.42 | 0.10 | 0.72 | 0.07 | 0.35 | 0.21 | 0.06 | 49.5 |
| Y. lipolytica VKM Y-2412 | 1.75 | 0.10 | 1.50 | 0.09 | 0.24 | 0.10 | 0.20 | 46.3 |
| Y. lipolytica 374/4 | 2.10 | 0.15 | 1.63 | 0.08 | 0.14 | 0.11 | 0.02 | 50.0 |
| Y. lipolytica 571 | 0.55 | 0.10 | 0.30 | 0.10 | 0.10 | 0.10 | 0.10 | 44.0 |
| Y. lipolytica 581 | 0.65 | 0.10 | 0.72 | 0.10 | 0.10 | 0.10 | 0.10 | 36.7 |
| Y. lipolytica 585 | 0.06 | 0.08 | 0.10 | 0 | 0.30 | 0.10 | 0.08 | 9.4 |
| Y. lipolytica 607 | 2.30 | 0.10 | 1.50 | 0.10 | 0.45 | 0.10 | 0.10 | 50.5 |
| Y. lipolytica VKM-2373 | 5.51 | 0.5 | 4.24 | 0.10 | 0.13 | 0.12 | 0.08 | 53.5 |
| Y. lipolytica UV/NNG | 4.61 | 1.17 | 3.18 | 1.15 | 0.12 | 0.10 | 0.10 | 44.6 |
| Y. lipolytica ACO1 no. 20 | 3.73 | 1.0 | 2.65 | 0.65 | 0.10 | 0.10 | 0.10 | 45.3 |
| Parameters | Full Medium (mg/L) N—630, P—246, S—186, Mg—140, Ca—68, Fe—0.05 | Limiting Component (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| N (63.0) | P (2.5) | S (1.9) | Mg (0.28) | Ca (0.136) | Fe (0.001) | ||
| Biomass (g/L) | 8.9 | 2.33 ± 0.40 | 1.77 ± 0.15 | 1.67 ± 0.15 | 2.10 ± 0.20 | 1.03 ± 0.21 | 1.05 ± 0.25 |
| ICA (g/L) | 0 | 6.33 ± 0.32 | 5.27 ± 0.15 | 5.65 ± 0.25 | 2.37 ± 0.21 | 0 | 0 |
| CA (g/L) | 0 | 3.70 ± 0.20 | 3.43 ± 0.31 | 4.37 ± 0.21 | 0.70 ± 0.10 | 0 | 0 |
| AA (g/L) | 0 | Tr | Tr | Tr | Tr | 0 | 1.25 ± 0.32 |
| ICA/CA ratio | - | 1.7:1 | 1.5:1 | 1.3:1 | 3.4:1 | - | - |
| YICA (g/g) | - | 0.32 | 0.26 | 0.30 | 0.11 | - | - |
| Parameters | Nitrogen Concentration (63 mg/L) | ||||
|---|---|---|---|---|---|
| (NH4)2SO4 | (NH2)2CO | NH4CL | NH4NO3 | CH3COONH4 | |
| Biomass (g/L) | 2.33 ± 0.04 | 2.35 ± 0.31 | 1.63 ± 0.16 | 1.76 ± 0.14 | 1.19 ± 0.03 |
| ICA (g/L) | 6.33 ± 0.32 | 5.90 ± 0.15 | 5.01 ± 0.28 | 4.43 ± 0.39 | 2.03 ± 0.15 |
| CA (g/L) | 3.70 ± 0.20 | 3.52 ± 0.19 | 3.07 ± 0.21 | 2.93 ± 0.15 | 1.77 ± 0.21 |
| AA (g/L) | 0.5 | Tr | 0.5 | Tr | Tr |
| ICA/CA ratio | 1.7:1 | 1.7:1 | 1.6:1 | 1.5:1 | 1.2:1 |
| YICA (g/g) | 0.32 | 0.30 | 0.25 | 0.22 | 0.10 |
| Parameters | Without Titration | Titration | ||
|---|---|---|---|---|
| 10 wt% NaOH | 2 wt% CaCO3 | 3 wt% CaCO3 | ||
| Initial pH | 6.0 | 6.0 | 6.0 | 6.5 |
| Final pH | 2.0 | 5.0 | 6.0 | 6.5 |
| Biomass (g/L) | 1.50 ± 0.10 | 2.33 ± 0.04 | 1.90 ± 0.10 | 1.80 ± 0.10 |
| ICA (g/L) | 1.03 ± 0.15 | 6.33 ± 0.32 | 8.30 ± 0.30 | 9.00 ± 0.26 |
| CA (g/L) | 0.57 ± 0.12 | 3.70 ± 0.20 | 5.53 ± 0.15 | 6.03 ± 0.65 |
| AA (g/L) | 0.5 | 0.5 | 0.1 | 0.1 |
| ICA/CA ratio | 1.8:1 | 1.7:1 | 1.5:1 | 1.5:1 |
| YICA (g/g) | 0.05 | 0.32 | 0.42 | 0.45 |
| Parameters | pH | ||||||
|---|---|---|---|---|---|---|---|
| 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | |
| Biomass (g/L) | 2.76 ± 0.12 | 2.70 ± 0.26 | 2.60 ± 0.10 | 2.45 ± 0.10 | 2.30 ± 0.10 | 2.15 ± 0.10 | 2.00 ± 0.11 |
| ICA (g/L) | 3.04 ± 0.09 | 5.90 ± 0.20 | 6.59 ± 0.19 | 8.00 ± 0.23 | 9.50 ± 0.26 | 9.00 ± 0.10 | 7.31 ± 0.20 |
| CA (g/L) | 1.90 ± 0.17 | 3.67 ± 0.15 | 3.88 ± 0.11 | 4.53 ± 0.13 | 5.16 ± 0.15 | 5.05 ± 0.21 | 4.53 ± 0.13 |
| AA (g/L) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| ICA/CA ratio | 1.6:1 | 1.6:1 | 1.7:1 | 1.8:1 | 1.8:1 | 1.8:1 | 1.6:1 |
| Parameters | EAF Content, Periodically Added to the Cultivation Medium (g/L) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 10 | |
| Biomass (g/L) | 13.0 ± 0.2 | 13.6 ± 1.2 | 13.3 ± 0.4 | 14.5 ± 0.2 | 14.1 ± 1.3 |
| ICA (g/L) | 51.5 ± 5.2 | 56.6 ± 4.4 | 60.7 ± 2.1 | 54.1 ± 3.5 | 22.2 ± 1.1 |
| CA (g/L) | 51.3 ± 2.1 | 33.4 ± 1.2 | 31.1 ± 2.2 | 27.0 ± 2.0 | 16.0 ± 1.1 |
| AA (g/L) | 0.5 | 0.5 | 0.5 | 0.5 | 12.0 ± 1.4 |
| ICA/CA ratio | 1:1 | 1.7:1 | 2:1 | 2:1 | 1.4:1 |
| YICA (g/g) | 0.47 | 0.55 | 0.61 | 0.53 | 0.25 |
| QICA (g/L·h) | 0.78 | 0.81 | 0.89 | 0.81 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica. Fermentation 2021, 7, 146. https://doi.org/10.3390/fermentation7030146
Kamzolova SV, Samoilenko VA, Lunina JN, Morgunov IG. Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica. Fermentation. 2021; 7(3):146. https://doi.org/10.3390/fermentation7030146
Chicago/Turabian StyleKamzolova, Svetlana V., Vladimir A. Samoilenko, Julia N. Lunina, and Igor G. Morgunov. 2021. "Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica" Fermentation 7, no. 3: 146. https://doi.org/10.3390/fermentation7030146
APA StyleKamzolova, S. V., Samoilenko, V. A., Lunina, J. N., & Morgunov, I. G. (2021). Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica. Fermentation, 7(3), 146. https://doi.org/10.3390/fermentation7030146






