Contribution of Grape Skins and Yeast Choice on the Aroma Profiles of Wines Produced from Pinot Noir and Synthetic Grape Musts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Yeast Isolation
2.3. Molecular Biology Techniques
2.4. Yeast Culture Conditions
2.5. Fermentation Media
2.6. Fermentation Trials
2.7. Residual Sugar and Alcohol Analysis
2.8. Volatile Aroma Compound Quantitation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Yeast Species and Strain Typing
3.2. Fermentation Progress
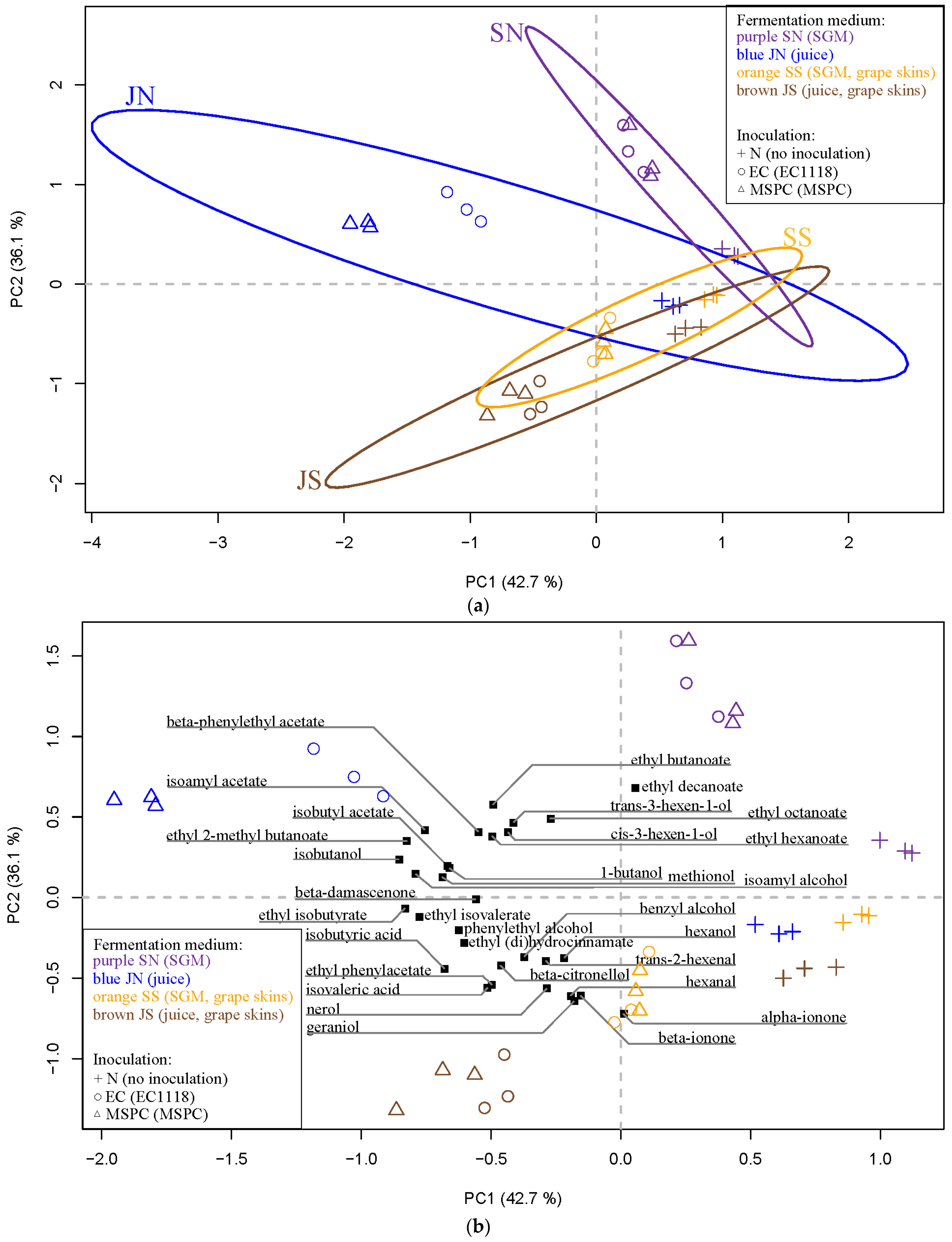
3.3. Analysis of Aroma Compounds Using PERMANOVA and PCA
3.4. Overview of the Aroma Profile for Each Treatment by Compound Class
3.4.1. Acetate Esters and Higher Alcohols
3.4.2. Fatty Acids Ethyl Esters and Fatty Acids
3.4.3. C13-Norisoprenoids and Terpenes
3.4.4. C6-Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2021, 61, 1589–1604. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M.C. Quantification of selected aroma-active compounds in Pinot noir wines from different grape maturities. J. Agric. Food Chem. 2006, 54, 8567–8573. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R.; Frost, A. Aroma composition of 2-year-old New Zealand Pinot Noir wine and its relationship to sensory characteristics using canonical correlation analysis and addition/omission tests. Aust. J. Grape Wine Res. 2015, 21, 376–388. [Google Scholar] [CrossRef]
- Girard, B.; Kopp, G.; Reynolds, G.; Cliff, M. Influence of vinification treatments on aroma constituents and sensory descriptors of Pinot noir wines. Am. J. Enol. Vitic. 1997, 48, 198–206. [Google Scholar]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Asproudi, A.; Ferrandino, A.; Bonello, F.; Vaudano, E.; Pollon, M.; Petrozziello, M. Key norisoprenoid compounds in wines from early-harvested grapes in view of climate change. Food Chem. 2018, 268, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Castro Vázquez, L.; Pérez-Coello, M.S.; Cabezudo, M.D. Effects of enzyme treatment and skin extraction on varietal volatiles in Spanish wines made from Chardonnay, Muscat, Airén, and Macabeo grapes. Anal. Chim. Acta 2002, 458, 39–44. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Contribution of grape skin and fermentation microorganisms to the development of red- and black-berry aroma in merlot wines. J. Int. Sci. Vigne Vin 2011, 45, 27–37. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in sauvignon blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Borneman, A.R.; Forgan, A.H.; Kolouchova, R.; Fraser, J.A.; Schmidt, S.A. Whole genome comparison reveals high levels of inbreeding and strain redundancy across the spectrum of commercial wine strains of Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2016, 6, 957–971. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [Green Version]
- Hall, H.; Zhou, Q.; Qian, M.C.; Osborne, J.P. Impact of yeasts present during prefermentation cold maceration of Pinot noir grapes on wine volatile aromas. Am. J. Enol. Vitic. 2017, 68, 81–90. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the usefulness of aroma networks to explain wine aroma properties: A case study of Portuguese wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petronilho, S.; Rudnitskaya, A.; Coimbra, M.A.; Rocha, S.M. Comprehensive study of variety oenological potential using statistic tools for the efficient use of non-renewable resources. Appl. Sci. 2021, 11, 4003. [Google Scholar] [CrossRef]
- Jiranek, V.; Eglinton, J.M.; Gockowiak, H.; Langridge, P.; Henschke, P.A. Nitrogen: A critical regulator of fermentation. In Proceedings of the Eighth Australian Wine Industry Technical Conference, Melbourne, VIC, Australia, 25–29 October 1992; pp. 133–141. [Google Scholar]
- Rodríguez-Bencomo, J.J.; Cabrera-Valido, H.M.; Pérez-Trujillo, J.P.; Cacho, J. Bound aroma compounds of Marmajuelo and Malvasía grape varieties and their influence on the elaborated wines. Eur. Food Res. Technol. 2011, 233, 413–426. [Google Scholar] [CrossRef]
- Fuxman Bass, J.I.; Reece-Hoyes, J.S.; Walhout, A.J.M. Zymolyase-Treatment and Polymerase Chain Reaction Amplification from Genomic and Plasmid Templates from Yeast. Cold Spring Harb Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Evol. Microbiol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a Medium for Simple Extraction of DNA for PCR-Based Typing from Forensic Material. BioTechniques 2013, 54, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Richards, K.D.; Goddard, M.R.; Gardner, R.C. A database of microsatellite genotypes for Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 2009, 96, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Harsch, M.J.; Gardner, R.C. Yeast genes involved in sulfur and nitrogen metabolism affect the production of volatile thiols from Sauvignon Blanc musts. Appl. Microbiol. Biotechnol. 2013, 97, 223–235. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of Alcoholic Fermentation Kinetics: Its Variability and Significance. American J. Enol. Vitic. 1990, 41, 319. [Google Scholar]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Jouanneau, S.; Weaver, R.J.; Nicolau, L.; Herbst-Johnstone, M.; Benkwitz, F.; Kilmartin, P.A. Subregional survey of aroma compounds in Marlborough Sauvignon blanc wines. Aust. J. Grape Wine Res. 2012, 18, 329–343. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H. Vegan: Community Ecology Package. 2013. R-Package Version 2.0-10. Available online: http://CRAN.R-project.org/package=vegan (accessed on 20 August 2021).
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Sauvignon Blanc aroma and sensory profile modulation from high fining rates. Aust. J. Grape Wine Res. 2017, 23, 359–367. [Google Scholar] [CrossRef]
- Mardia, K.V. Multi-dimensional multivariate Gaussian Markov random fields with application to image processing. J. Multivar. Anal. 1988, 24, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Goddard, M.R. Quantifying the complexities of Saccharomyces cerevisiae’s ecosystem engineering via fermentation. Ecology 2008, 89, 2077–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Lee, S.A.; Bradbury, J.E.; Warren, R.N.; Sheth, H.; Hooks, D.O.; Richards, K.D.; Gardner, R.C. Yeasts isolated from New Zealand vineyards and wineries. Aust. J. Grape Wine Res. 2010, 16, 491–496. [Google Scholar] [CrossRef]
- Delfini, C.; Gaia, P.; Schellino, R.; Strano, M.; Pagliara, A.; Ambro, S. Fermentability of grape must after inhibition with dimethyl dicarbonate (DMDC). J. Agric. Food Chem. 2002, 50, 5605–5611. [Google Scholar] [CrossRef]
- Henderson, C.M.; Lozada-Contreras, M.; Jiranek, V.; Longo, M.L.; Block, D.E. Ethanol production and maximum cell growth are highly correlated with membrane lipid composition during fermentation as determined by lipidomic analysis of 22 Saccharomyces cerevisiae strains. Appl. Environ. Microbiol. 2013, 79, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.A.; Carmona, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Pre-bottling use of dehydrated waste grape skins to improve colour, phenolic and aroma composition of red wines. Food Chem. 2013, 136, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, A.M.; Dambergs, R.G.; Close, D.C. Grape skins as supplements for color development in Pinot noir wine. Food Res. Int. 2020, 133, 1–10. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Ouellet, E.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casassa, L.F.; Sari, S.E.; Bolcato, E.A.; Diaz-Sambueza, M.A.; Catania, A.A.; Fanzone, M.L.; Raco, F.; Barda, N. Chemical and Sensory Effects of Cold Soak, Whole Cluster Fermentation, and Stem Additions in Pinot noir Wines. Am. J. Enol. Vitic. 2019, 70, 19. [Google Scholar] [CrossRef]
- Song, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef]
- Bi, F.; Ali, A.; Iqbal, S.; Arman, M.; Mahmood, U.-H. Chemical Esterification of Fusel Oil Alcohol for the Production of Flavor and Fragrance Esters. J. Chem. Soc. Pak. 2008, 30, 919. [Google Scholar]
- Stribny, J.; Gamero, A.; Perez-Torrado, R.; Querol, A. Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 2015, 205, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Deed, R.C.; Fedrizzi, B.; Gardner, R.C. Influence of Fermentation Temperature, Yeast Strain, and Grape Juice on the Aroma Chemistry and Sensory Profile of Sauvignon Blanc Wines. J. Agric. Food Chem. 2017, 65, 8902–8912. [Google Scholar] [CrossRef]
- Abd Ellah, A.E.; Mohamed, K.M.; Backheet, E.Y.; Mohamed, M.H. Cinnamyl alcohol, benzyl alcohol, and flavonoid glycosides from Sanchezia nobilis. Chem. Nat. Compd. 2014, 50, 823–826. [Google Scholar] [CrossRef]
- Miranda-Lopez, R.; Libbey, L.M.; Watson, B.T.; McDaniel, M.R. Identification of additional odor-active compounds in Pinot noir wines. Am. J. Enol. Vitic. 1992, 43, 90–92. [Google Scholar]
- Ravaglia, S.; Delfini, C. Production of medium chain fatty acids and their ethyl esters by yeast strains isolated from musts and wines. Ital. J. Food Sci. 1993, 5, 21–36. [Google Scholar]
- Bardi, L.; Cocito, C.; Marzona, M. Saccharomyces cerevisiae cell fatty acid composition and release during fermentation without aeration and in absence of exogenous lipids. Int. J. Food Microbiol. 1999, 47, 133–140. [Google Scholar] [CrossRef]
- Diaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C. Prediction of Prefermentation Nutritional Status of Grape Juice: The Formol Method. In Food Microbiology Protocols; Spencer, J.F.T., de Ragout Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 283–296. [Google Scholar]
- Giuliano, G.; Al-Babili, S.; von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Palomo, E.S.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; Viñas, M.A.G.; Cabezudo, M.D. Contribution of free and glycosidically-bound volatile compounds to the aroma of muscat “a petit grains” wines and effect of skin contact. Food Chem. 2006, 95, 279–289. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M. Aroma compounds in Oregon Pinot Noir wine determined by aroma extract dilution analysis (AEDA). Flavour Fragr. J. 2005, 20, 22–29. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Albertin, W.; Setati, M.E.; Miot-Sertier, C.; Mostert, T.T.; Colonna-Ceccaldi, B.; Coulon, J.; Girard, P.; Moine, V.; Pillet, M.; Salin, F.; et al. Hanseniaspora uvarum from Winemaking Environments Show Spatial and Temporal Genetic Clustering. Front. Microbiol. 2015, 6, 1569. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Beloqui, A.A.; Bertrand, A.; Doazan, J.P. An analytical method for studying the volatile compounds of Merlot noir clone wines. Am. J. Enol. Viticulture 1998, 49, 44–48. [Google Scholar]
- Falcão, L.D.; de Revel, G.; Perello, M.-C.; Riquier, L.; Rosier, J.-P.; Uberti, A.A.A.; Bordignon-Luiz, M.T. Volatile profile characterization of young Cabernet-Sauvignon wines from a new grape growing region in Brazil. Oeno One 2008, 42, 133–145. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Robinson, A.L.; Johnson, A.J.; Ebeler, S.E. Direct hydrolysis and analysis of glycosidically bound aroma compounds in grapes and wines: Comparison of hydrolysis conditions and sample preparation methods. Aust. J. Grape Wine Res. 2014, 20, 361–377. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Santiago-Gómez, M.P.; Thanh, H.T.; De Coninck, J.; Cachon, R.; Kermasha, S.; Belin, J.-M.; Gervais, P.; Husson, F. Modeling hexanal production in oxido-reducing conditions by the yeast Yarrowia lipolytica. Process Biochem. 2009, 44, 1013–1018. [Google Scholar] [CrossRef]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, C.Q.; Yan, G.L. Effects of Adding Unsaturated Fatty Acids on Fatty Acid Composition of Saccharomyces cerevisiae and Major Volatile Compounds in Wine. South Afr. J. Enol. Vitic. 2015, 30, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.T.; Duan, C.Q.; Yan, G.L. Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation. Molecules 2019, 24, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | SC | SU | SU | SU | SC | HU | SU | SU | SU | SU | SU | - |
| B | SU | SU | SU | HU | SU | SU | SU | SU | SC | HU | SU | SC |
| C | SU | SU | HU | SU | SC | HU | SU | SU | SU | SC | SU | SU |
| D | SU | HU | SC | SU | SU | HU | SU | HU | SU | HU | HU | SU |
| E | HU | SC | HU | SU | SU | SC | HU | HU | SC | HU | SU | HU |
| F | SU | SU | HU | SU | SC | SU | SU | SC | HU | HU | SC | - |
| G | SU | SU | SC | SU | SU | SC | SC | SU | SU | SC | HU | - |
| H | SU | SU | SC | HU | SU | SU | SC | SU | HU | SU | - | - |
| JN-EC | JN-MSPC | JN-N | JS-EC | JS-MSPC | JS-N | SN-EC | SN-MSPC | SN-N | SS-EC | SS-MSPC | SS-N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| viable cells after sterilization (CFU mL−1) | 10 | 10 | 10 | 1.65 × 104 | 1.65 × 104 | 1.65 × 104 | - | - | - | 7.00 × 103 | 7.00 × 103 | 7.00 × 103 |
| length of fermentation (h) | 167 | 262 | 262 | 262 | 262 | 262 | 262 | 262 | - | 262 | 262 | 262 |
| fermentation time at half total weight loss (h) | 70 | 70 | 75 | 53 | 60 | 82 | 70 | 70 | - | 60 | 70 | 96 |
| total weight loss (g) | 11.8 ± 0.25 d | 11.6 ± 0.26 d | 4.61 ± 0.35 b | 17.8 ± 0.52 e | 17.6 ± 0.48 e | 10.9 ± 0.41 cd | 10.8 ± 0.02 cd | 11.0 ± 0.35 cd | 0.72 ± 0.26 a | 17.1 ± 0.11 e | 16.6 ± 0.62 e | 9.71 ± 1.02 c |
| total residual sugar (g L−1) | 0.10 ± 0.02 a | 0.27 ± 0.16 a | 113 ± 23.5 c | 0.21 ± 0.07 a | 0.29 ± 0.25 a | 81.4 ± 5.84 b | 1.26 ± 0.45 a | 7.56 ± 2.78 a | 186 ± 9.4 d | 0.12 ± 0.05 a | 0.10 ± 0.02 a | 87.2 ± 4.14 bc |
| residual D-glucose (g L−1) | 0.01 ± 0.02 d | 0.12 ± 0.14 d | 58.4 ± 12.1 b | 0.09 ± 0.07 d | 0.09 ± 0.01 d | 41.6 ± 2.81 c | 0.14 ± 0.03 d | 0.54 ± 0.47 d | 94.2 ± 4.2 a | 0.07 ± 0.01 d | 0.09 ± 0.06 d | 44.0 ± 1.75 c |
| residual D-fructose (g L−1) | 0.10 ± 0.03 c | 0.14 ± 0.05 c | 54.2 ± 11.5 b | 0.12 ± 0.03 c | 0.20 ± 0.25 c | 39.8 ± 3.52 b | 1.12 ± 0.48 c | 7.02 ± 2.32 c | 91.8 ± 5.9 a | 0.06 ± 0.05 c | 0.02 ± 0.04 c | 43.2 ± 2.60 b |
| ethanol (% v/v) | 14.4 ± 0.22 e | 13.7 ± 0.42 de | 5.36 ± 0.19 b | 10.9 ± 0.22 c | 11.2 ± 0.23 c | 4.81 ± 0.15 b | 14.3 ± 0.30 de | 13.4 ± 0.18 d | 1.53 ± 0.09 a | 11.3 ± 0.27 c | 11.1 ± 0.34 c | 4.78 ± 0.05 b |
| JN-EC | JN-MSPC | JN-N | JS-EC | JS-MSPC | JS-N | SN-EC | SN-MSPC | SN-N | SS-EC | SS-MSPC | SS-N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate Esters (µg L−1) | ||||||||||||
| isobutyl acetate | 67.5 ± 2.3 e | 98.6 ± 4.3 f | 29 ± 12 d | 10.3 ± 0.7 abc | 15.1 ± 0.4 bc | 21.1 ± 8.6 cd | 9.7 ± 3.5 abc | 6.2 ± 1.3 ab | ND | 6.7 ± 0.4 ab | 6.0 ± 0.4 ab | 6.1 ± 0.7 ab |
| isoamyl acetate | 738 ± 154 d | 747 ± 62 d | 66 ± 13 abc | 69.7 ± 7.3 abc | 140 ± 7.9 abc | 66 ± 32 abc | 201 ± 46 c | 175 ± 27 bc | 1.4 ± 0.2 a | 67.0 ± 3.6 abc | 102 ± 11 abc | 24.2 ± 5.6 ab |
| ethyl phenylacetate | 1.72 ± 0.24 cd | 1.95 ± 0.45 d | 0.21 ± 0.08 ab | 5.79 ± 1.42 f | 3.67 ± 0.41 e | 0.32 ± 0.13 abc | 0.20 ± 0.03 ab | 0.31 ± 0.10 abc | 0.03 ± 0.02 a | 2.61 ± 0.74 de | 1.53 ± 0.14 bcd | 0.18 ± 0.02 ab |
| β-phenylethyl acetate | 76 ± 11 e | 75 ± 14 e | 13.8 ± 1.1 ab | 14.5 ± 0.3 ab | 25.4 ± 0.7 bcd | 18.0 ± 6.8 abc | 44.9 ± 2.2 d | 38 ± 10 cd | 0.4 ± 0.1 a | 13.8 ± 0.7 ab | 25.3 ± 1.0 bcd | 17 ± 11 ab |
| Alcohols, Aldehydes (µg L−1) | ||||||||||||
| isobutanol | 209,866 ± 45,324 d | 392,892 ± 38,963 e | 19,612 ± 3626 ab | 66,983 ± 4624 bc | 76,305 ± 4358 c | 13,477 ± 504 a | 31,436 ± 6436 abc | 40,452 ± 389 abc | 40 ± 26 a | 48,928 ± 2292 abc | 33,103 ± 4168 abc | 7254 ± 1583 a |
| 1-butanol | 14,822 ± 9199 abc | 96,376 ± 10,656 e | 7766 ± 530 ab | 26,947 ± 449 cd | 21,768 ± 3274 cd | 4259 ± 351 a | 32,471 ± 3441 d | 20,576 ± 902 bcd | 1943 ± 115 a | 26,616 ± 3226 cd | 18,855 ± 1894 bc | 3813 ± 498 a |
| isoamyl alcohol | 416,032 ± 112,652 e | 529,378 ± 40,137 f | 29,211 ± 2358 ab | 180,385 ± 13,726 cd | 218,911 ± 8708 d | 21,719 ± 922 ab | 120,932 ± 12,225 bcd | 104,487 ± 5212 abc | 23.2 ± 3.9 a | 156,540 ± 4185 cd | 147,978 ± 18,883 cd | 15,135 ± 760 a |
| phenylethyl alcohol | 83,025 ± 8821 e | 124,639 ± 17,639 f | 19,817 ± 1597 abc | 81,886 ± 6220 e | 124,654 ± 10,749 f | 18,929 ± 1873 abc | 29,364 ± 3427 bc | 34,830 ± 7805 c | 131 ± 28 a | 58,624 ± 3592 d | 97,529 ± 4470 e | 12,289 ± 1927 ab |
| methionol | 5560 ± 1426 e | 4619 ± 1300 de | 383 ± 168 ab | 2269 ± 1052 abc | 1738 ± 586 abc | 47 ± 37 a | 1158 ± 551 abc | 1139 ± 387 abc | 26.4 ± 0.9 a | 2780 ± 1107 cd | 2573 ± 646 bcd | 111 ± 56 a |
| benzyl alcohol | 1146 ± 16 de | 1048 ± 12 de | 894 ± 84 d | 1332 ± 148 e | 1360 ± 187 e | 1315 ± 227 e | 36.7 ± 7.1 a | 76 ± 73 ab | 17.5 ± 2.1 a | 391 ± 98 bc | 431 ± 28 c | 435 ± 82 c |
| benzaldehyde | 0.71 ± 0.04 abc | 0.56 ± 0.09 abc | 1.86 ± 0.35 d | 1.131 ± 0.34 cd | 0.76 ± 0.20 abc | 4.23 ± 0.54 e | 0.16 ± 0.07 a | 0.24 ± 0.12 ab | 0.34 ± 0.19 ab | 0.40 ± 0.08 abc | 0.26 ± 0.11 ab | 0.97 ± 0.35 bc |
| Fatty Acid Ethyl esters (µg L−1) | ||||||||||||
| ethyl isobutyrate | 44 ± 10 bcd | 137 ± 33 e | 9.7 ± 4.0 ab | 44.8 ± 3.3 cd | 63 ± 19 d | 4.2 ± 0.9 a | 5.4 ± 1.8 a | 7.2 ± 0.4 a | 2.5 ± 3.5 a | 26 ± 10 abc | 19.9 ± 1.9 abc | 2.2 ± 0.3 a |
| ethyl butanoate | 139 ± 21 c | 117 ± 7.3 bc | 11.5 ± 2.4 a | 26.4 ± 1.8 a | 41.3 ± 0.4 a | 3.5 ± 0.3 a | 115 ± 19 bc | 88.4 ± 3.2 b | 28 ± 36 a | 22.4 ± 2.6 a | 28.1 ± 3.3 a | 4.9 ± 0.2 a |
| ethyl 2-methyl butanoate | 2.89 ± 0.121 b | 5.23 ± 0.48 c | 0.82 ± 0.511 a | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ethyl isovalerate | 2.16 ± 0.25 b | 5.16 ± 0.49 c | 0.04 ± 0.07 a | 1.48 ± 0.22 ab | 4.48 ± 1.48 c | 0.12 ± 0.09 a | 0.37 ± 0.13 a | 0.34 ± 0.12 a | 0.78 ± 1.08 ab | 0.74 ± 0.28 ab | 1.19 ± 0.24 ab | ND |
| ethyl hexanoate | 172 ± 46 e | 130 ± 12 cde | 2.5 ± 0.7 a | 81.9 ± 8.1 bc | 107 ± 8.6 bcd | 7.0 ± 1.5 a | 148 ± 28 de | 156 ± 38 de | 0.9 ± 1.0 a | 67.4 ± 6.2 b | 66.0 ± 5.1 b | 1.5 ± 0.3 a |
| ethyl octanoate | 152 ± 45 bcd | 92 ± 13 abc | 3.4 ± 0.5 a | 79 ± 11 ab | 91.8 ± 1.8 abc | 3.7 ± 0.5 a | 256 ± 78 d | 200 ± 97 cd | 1.7 ± 0.4 a | 101 ± 29 abc | 66.5 ± 3.4 ab | 2.4 ± 0.3 a |
| ethyl decanoate | 24.6 ± 7.1 a | 20.2 ± 5.5 a | 0.6 ± 0.2 a | 21.5 ± 3.5 a | 23.9 ± 4.8 a | 1.3 ± 0.3 a | 199 ± 56 b | 208 ± 80 b | 0.8 ± 0.3 a | 28.0 ± 8.3 a | 19.0 ± 7.0 a | 0.5 ± 0.2 a |
| Fatty Acids | ||||||||||||
| isobutyric acid (mg L−1) | 2.8 ± 1.6 ab | 5.9 ± 1.8 c | ND | 6.0 ± 1.1 c | 7.3 ± 0.8 c | 0.8 ± 0.2 a | ND | ND | ND | 3.5 ± 0.8 b | 3.1 ± 0.2 ab | ND |
| isovaleric acid (mg L−1) | ND | 0.66 ± 0.16 b | ND | 0.59 ± 0.36 ab | 0.84 ± 0.23 b | 0.14 ± 0.05 a | ND | ND | ND | 0.53 ± 0.30 ab | 0.65 ± 0.17 b | ND |
| hexanoic acid (mg L−1) | 1.26 ± 0.14 d | 0.65 ± 0.05 b | 0.02 ± 0.01 a | 1.3 ± 0.1 d | 1.0 ± 0.1 c | 0.08 ± 0.01 a | 1.5 ± 0.1 e | 1.20 ± 0.07 d | 0.01 ± 0.01 a | 1.29 ± 0.09 d | 0.95 ± 0.01 c | 0.04 ± 0.01 a |
| octanoic acid (mg L−1) | 0.57 ± 0.24 ab | 0.16 ± 0.07 a | ND | 0.45 ± 0.27 ab | 0.53 ± 0.28 ab | ND | 1.14 ± 0.44 b | 1.16 ± 0.43 b | ND | 0.66 ± 0.28 ab | 0.63 ± 0.16 ab | ND |
| decanoic acid (μg L−1) | 1.7 ± 0.4 d | 1.2 ± 0.5 abcd | 1.2 ± 0.5 abcd | 1.5 ± 0.2 bcd | 1.6 ± 0.3 cd | 1.2 ± 0.3 abcd | 0.56 ± 0.03 abc | 0.44 ± 0.07 ab | 0.20 ± 0.04 a | 1.2 ± 0.3 abcd | 1.6 ± 0.6 cd | 1.3 ± 0.5 bcd |
| Cinnamates (µg L−1) | ||||||||||||
| ethyl (di)hydrocinnamate | 0.22 ± 0.04 cd | 0.32 ± 0.02 d | 0.06 ± 0.02 a | 0.31 ± 0.06 d | 0.26 ± 0.02 cd | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.04 ± 0.01 a | 0.01 ± 0.001 a | 0.20 ± 0.02 bc | 0.18 ± 0.02 bc | 0.09 ± 0.10 ab |
| ethyl cinnamate (trans) | 0.06 ± 0.01 bcde | 0.08 ± 0.01 e | 0.069 ± 0.01 de | 0.07 ± 0.01 de | 0.06 ± 0.02 bcde | 0.06 ± 0.02 cde | 0.04 ± 0.01 abcd | 0.03 ± 0.01 ab | 0.01 ± 0.002 a | 0.04 ± 0.01 abcd | 0.04 ± 0.01 abcd | 0.03 ± 0.003 abc |
| C13-norisoprenoids (µg L−1) | ||||||||||||
| β-damascenone | 3.1 ± 0.1 c | 2.8 ± 0.4 c | 2.6 ± 0.3 c | 1.2 ± 0.1 b | 1.3 ± 0.2 b | 1.1 ± 0.2 b | ND | ND | ND | 0.17 ± 0.03 a | 0.19 ± 0.01 a | 0.16 ± 0.02 a |
| α-ionone | ND | ND | 0.007 ± 0.003 a | 0.016 ± 0.001 cd | 0.026 ± 0.005 d | 0.014 ± 0.004 b | ND | ND | ND | 0.021 ± 0.006 c | 0.022 ± 0.006 cd | 0.012 ± 0.003 b |
| β-ionone | 0.11 ± 0.02 c | 0.09 ± 0.02 c | 0.08 ± 0.01 bc | 0.30 ± 0.04 ef | 0.34 ± 0.04 f | 0.19 ± 0.03 d | 0.01 ± 0.001 ab | 0.02 ± 0.003 ab | 0.01 ± 0.003 a | 0.27 ± 0.04 e | 0.33 ± 0.02 ef | 0.18 ± 0.01 d |
| Terpenes (µg L−1) | ||||||||||||
| cis/trans-rose oxide | 0.16 ± 0.001 ef | 0.08 ± 0.01 bcd | 0.04 ± 0.004 ab | 0.25 ± 0.01 g | 0.18 ± 0.04 f | 0.06 ± 0.01 abc | 0.09 ± 0.01 cd | 0.08 ± 0.01 cd | 0.12 ± 0.02 de | 0.07 ± 0.01 bc | 0.05 ± 0.01 abc | 0.02 ± 0.002 a |
| linalool | 32.7 ± 2.2 d | 30.2 ± 1.1 cd | 15.3 ± 1.1 b | 27.9 ± 2.5 cd | 33.7 ± 4.2 d | 15.1 ± 0.5 b | 26.1 ± 0.6 c | 26.0 ± 0.8 c | 8.6 ± 0.7 a | 25.6 ± 3.5 c | 26.5 ± 1.5 c | 15.7 ± 1.0 b |
| α-terpineol | 4.7 ± 0.3 abc | 7.0 ± 2.2 cd | 5.1 ± 0.3 bc | 5.7 ± 1.1 bc | 9.7 ± 2.9 d | 5.8 ± 0.7 bc | 3.5 ± 0.8 abc | 2.4 ± 0.3 ab | 1.4 ± 0.5 a | 3.5 ± 0.6 ab | 3.9 ± 0.2 abc | 2.4 ± 0.4 ab |
| β-citronellol | 32.1 ± 1.2 e | 14.3 ± 0.9 cd | 7.9 ± 3.3 bc | 53.2 ± 5.1 f | 25.7 ± 3.7 e | 4.5 ± 1.3 ab | 6.1 ± 1.0 ab | 0.8 ± 0.3 ab | 0.12 ± 0.03 a | 30.9 ± 4.1 e | 16.6 ± 1.2 d | 2.6 ± 1.1 ab |
| nerol (cis-geraniol) | 9.4 ± 1.2 ab | 6.0 ± 0.9 ab | 6.2 ± 1.5 ab | 16.4 ± 1.0 c | 16.3 ± 4.4 c | 10.2 ± 3.2 ab | ND | ND | ND | 9.5 ± 2.1 ab | 11.1 ± 1.0 bc | 5.6 ± 1.0 a |
| geraniol (trans-geraniol) | ND | ND | ND | 0.16 ± 0.03 a | 0.30 ± 0.13 a | ND | ND | ND | ND | 0.31 ± 0.18 a | 0.31 ± 0.02 a | ND |
| C6-compounds (µg L−1) | ||||||||||||
| hexanal | 5.5 ± 1.9 ab | 5.3 ± 1.4 a | 10.3 ± 2.0 ab | 69 ± 12 d | 53 ± 13 cd | 5.0 ± 1.8 a | 7.7 ± 0.98 ab | 12.2 ± 0.9 ab | 0.60 ± 0.06 a | 55.2 ± 24.3 cd | 31.4 ± 0.9 bc | 12.4 ± 4.6 ab |
| Trans-2-hexenal | 12.4 ± 4.2 ab | 21.3 ± 4.1 bc | 23.1 ± 2.2 cd | 27.4 ± 4.4 cd | 32.5 ± 3.2 d | 20.6 ± 4.7 bc | 5.9 ± 2.1 a | 5.6 ± 5.5 a | 3.1 ± 0.9 a | 9.7 ± 1.5 a | 10.4 ± 0.9 a | 8.4 ± 2.1 a |
| hexanol | 3237 ± 83 c | 3041 ± 410 c | 5626 ± 382 d | 3676 ± 494 c | 3715 ± 154 c | 3360 ± 204 c | 84.2 ± 10.8 a | 87.4 ± 7.1 a | 67 ± 28 a | 1831 ± 124 b | 2155 ± 193 b | 1980 ± 148 b |
| Trans-3-hexen-1-ol | 66.0 ± 9.3 cd | 46.7 ± 5.6 bc | ND | 24.7 ± 2.2 a | 40.1 ± 8.3 ab | ND | 70 ± 16 d | 73.4 ± 7.0 d | ND | 25.5 ± 5.4 a | 26.4 ± 2.0 a | ND |
| Cis-3-hexen−1-ol | 139 ± 11 e | 96 ± 17 d | 1.31 ± 0.40 a | 61.6 ± 2.7 bcd | 90.1 ± 8.5 cd | 3.18 ± 0.10 a | 139 ± 30 e | 143 ± 14 e | ND | 54.2 ± 9.0 b | 59.8 ± 4.4 bc | 0.96 ± 0.22 a |
| Factor | Df | F-statistic | R2 | p-Value |
|---|---|---|---|---|
| inoculation | 2 | 51.972 | 0.300 | <0.001 |
| medium | 3 | 51.346 | 0.445 | <0.001 |
| fermentation completion | 1 | 9.574 | 0.028 | <0.001 |
| inoculation:medium | 5 | 10.936 | 0.158 | <0.001 |
| residuals | 24 | 0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Hawkins, D.; Parish-Virtue, K.; Fedrizzi, B.; Knight, S.J.; Deed, R.C. Contribution of Grape Skins and Yeast Choice on the Aroma Profiles of Wines Produced from Pinot Noir and Synthetic Grape Musts. Fermentation 2021, 7, 168. https://doi.org/10.3390/fermentation7030168
Qiao Y, Hawkins D, Parish-Virtue K, Fedrizzi B, Knight SJ, Deed RC. Contribution of Grape Skins and Yeast Choice on the Aroma Profiles of Wines Produced from Pinot Noir and Synthetic Grape Musts. Fermentation. 2021; 7(3):168. https://doi.org/10.3390/fermentation7030168
Chicago/Turabian StyleQiao, Yifeng, Diana Hawkins, Katie Parish-Virtue, Bruno Fedrizzi, Sarah J. Knight, and Rebecca C. Deed. 2021. "Contribution of Grape Skins and Yeast Choice on the Aroma Profiles of Wines Produced from Pinot Noir and Synthetic Grape Musts" Fermentation 7, no. 3: 168. https://doi.org/10.3390/fermentation7030168
APA StyleQiao, Y., Hawkins, D., Parish-Virtue, K., Fedrizzi, B., Knight, S. J., & Deed, R. C. (2021). Contribution of Grape Skins and Yeast Choice on the Aroma Profiles of Wines Produced from Pinot Noir and Synthetic Grape Musts. Fermentation, 7(3), 168. https://doi.org/10.3390/fermentation7030168