Mechanisms of Metabolic Adaptation in Wine Yeasts: Role of Gln3 Transcription Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Genetic Manipulation
2.2. Growth Media and Conditions
2.3. Biochemical Determinations
2.4. Western Blot and Zymogram
3. Results
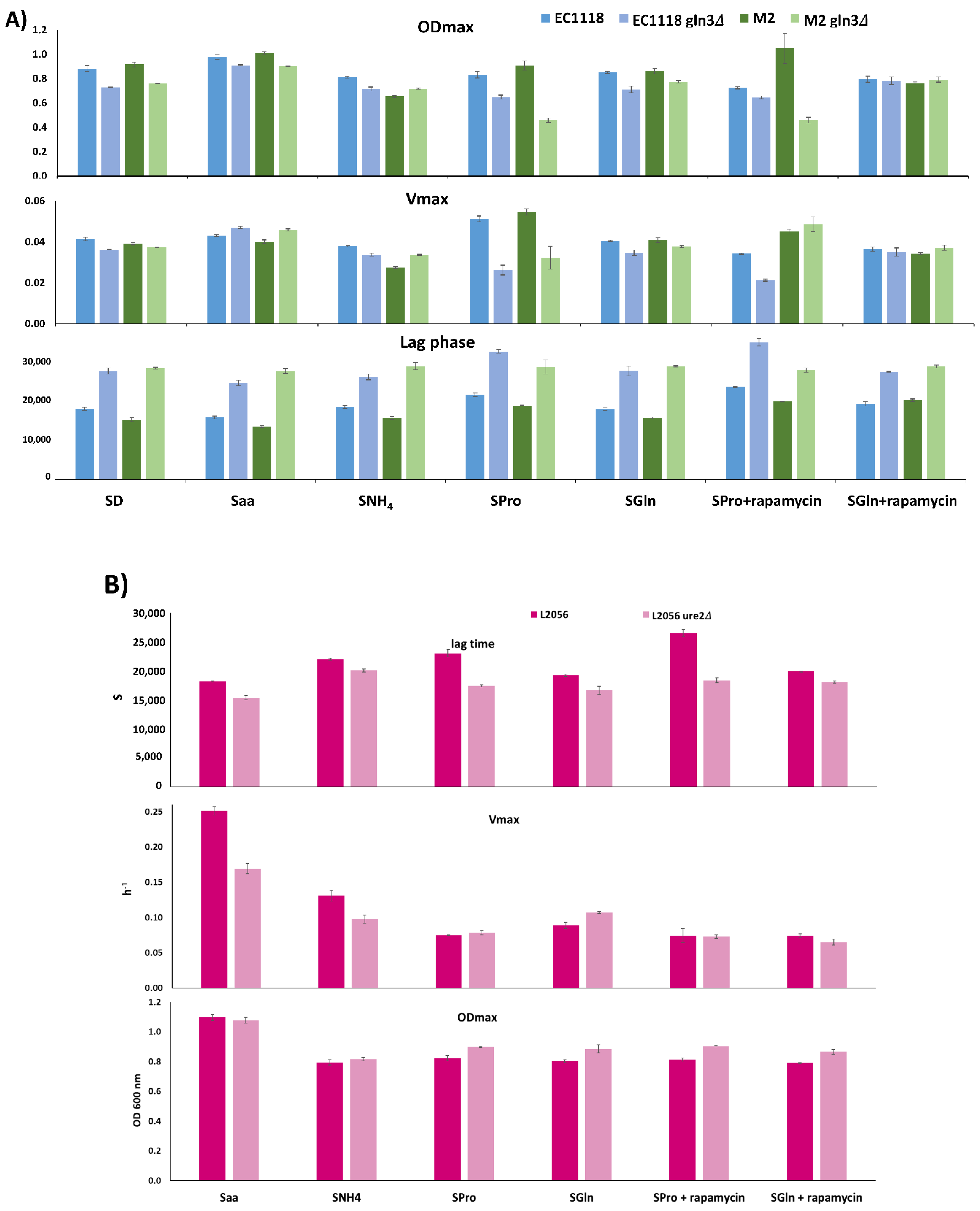
3.1. Quantitative Analysis of Carbon Metabolism in Food-related S. cerevisiae Strains
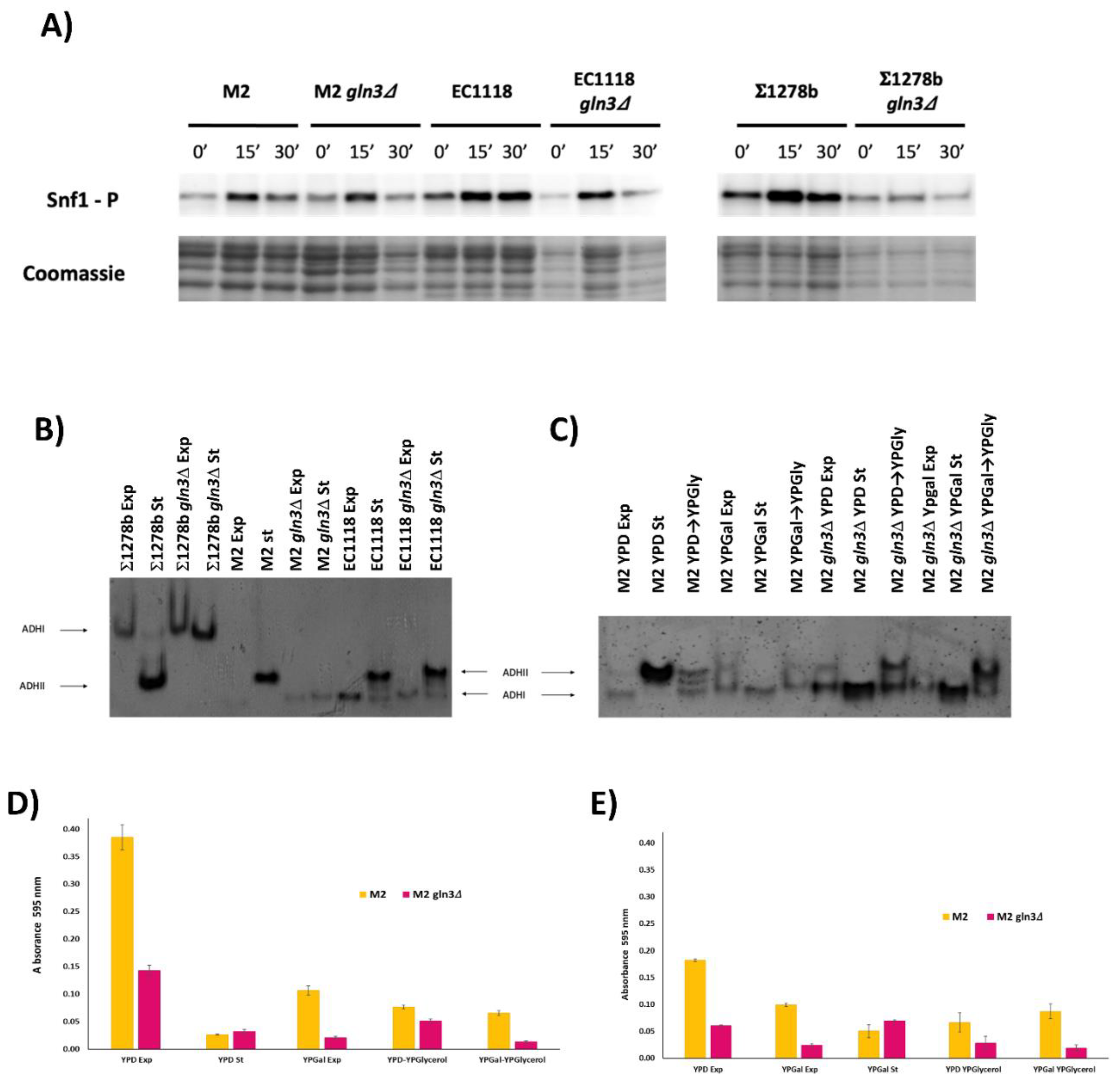
3.2. Gln3 Has a Complex Role in Carbon Metabolism in Commercial Wine Yeasts
3.3. Molecular Markers of GLN3 Deletion
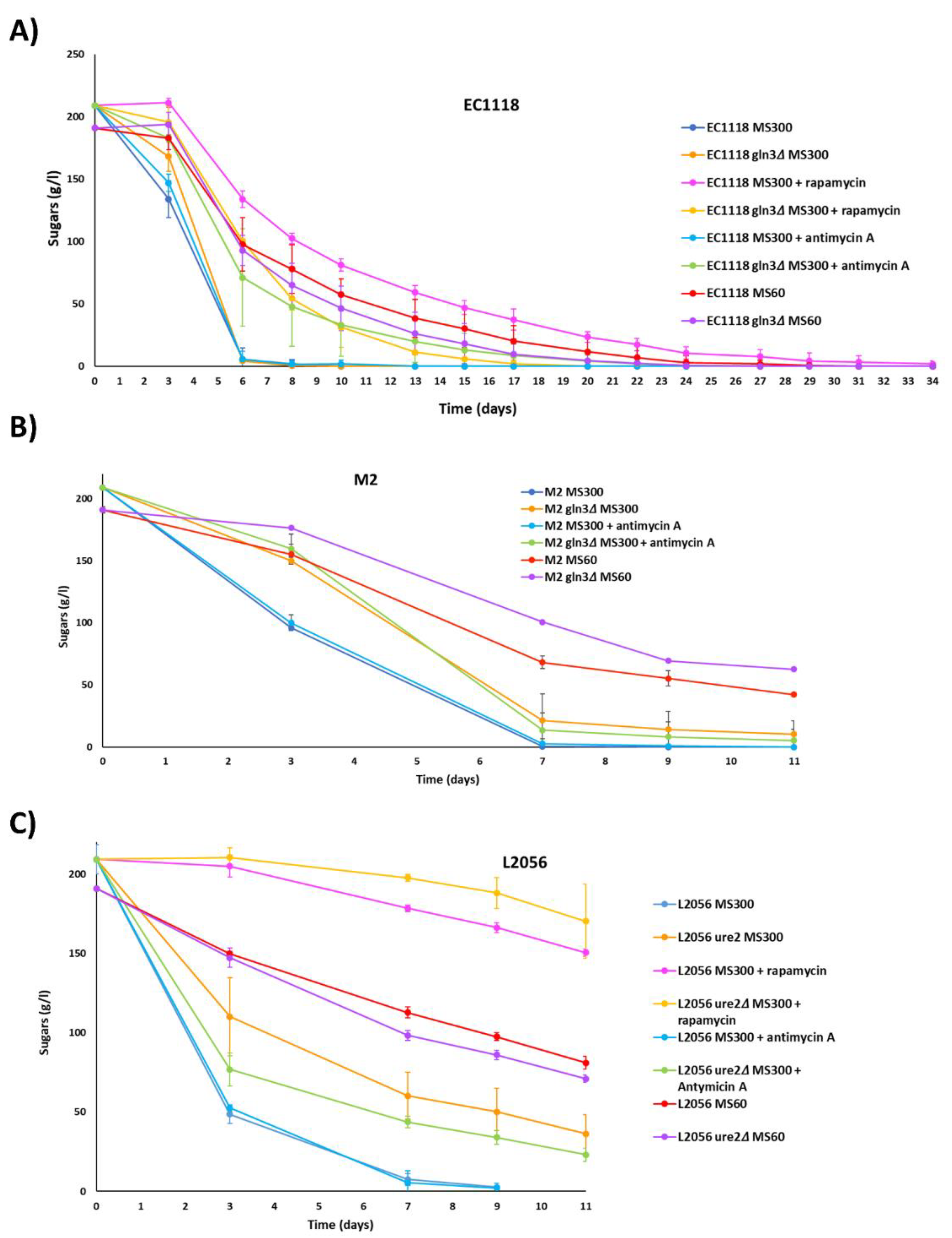
3.4. Role of GLN3 in Winemaking Conditions
3.5. GLN3 Relevance in Nitrogen Metabolism in Industrial Wine Yeasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matallana, E.; Aranda, A. Biotechnological impact of stress response on wine yeast. Lett. Appl. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessi-Perez, E.I.; Molinet, J.; Martinez, C. Disentangling the genetic bases of Saccharomyces cerevisiae nitrogen consumption and adaptation to low nitrogen environments in wine fermentation. Biol. Res. 2020, 53, 2. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, E.; Guillamón, J.M. Mechanisms of yeast adaptation to wine fermentations. Prog. Mol. Subcell. Biol. 2019, 58, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299. [Google Scholar] [CrossRef] [Green Version]
- Piskur, J.; Rozpedowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrado, R.; Gamero, E.; Gómez-Pastor, R.; Garre, E.; Aranda, A.; Matallana, E. Yeast biomass, an optimised product with myriad applications in the food industry. Trends Food Sci. Technol. 2015, 46, 167–175. [Google Scholar] [CrossRef]
- Meneses, F.J.; Henschke, P.A.; Jiranek, V. A Survey of industrial strains of Saccharomyces cerevisiae reveals numerous altered patterns of maltose and sucrose utilisation. J. Inst. Brew. 2002, 108, 310–321. [Google Scholar] [CrossRef]
- Vallejo, B.; Peltier, E.; Garrigós, V.; Matallana, E.; Marullo, P.; Aranda, A. Role of Saccharomyces cerevisiae nutrient signaling pathways during winemaking: A phenomics approach. Front. Bioeng. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Beier, D.R.; Sledziewski, A.; Young, E.T. Deletion analysis identifies a region, upstream of the ADH2 gene of Saccharomyces cerevisiae, which is required for ADR1-mediated derepression. Mol. Cell Biol 1985, 5, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- De Virgilio, C. The essence of yeast quiescence. FEMS Microbiol. Rev. 2012, 36, 306–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, B.; Matallana, E.; Aranda, A. Saccharomyces cerevisiae nutrient signaling pathways show an unexpected early activation pattern during winemaking. Microb. Cell Fact. 2020. [Google Scholar] [CrossRef]
- Bertram, P.G.; Choi, J.H.; Carvalho, J.; Chan, T.F.; Ai, W.; Zheng, X.F. Convergence of TOR-nitrogen and Snf1-glucose signaling pathways onto Gln3. Mol. Cell Biol. 2002, 22, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Grijalva-Vallejos, N.; Aranda, A.; Matallana, E. Evaluation of yeasts from Ecuadorian chicha by their performance as starters for alcoholic fermentations in the food industry. Int. J. Food Microbiol. 2020, 317, 108462. [Google Scholar] [CrossRef]
- Guldener, U.; Heck, S.; Fielder, T.; Beinhauer, J.; Hegemann, J.H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996, 24, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Delneri, D.; Tomlin, G.C.; Wixon, J.L.; Hutter, A.; Sefton, M.; Louis, E.J.; Oliver, S.G. Exploring redundancy in the yeast genome: An improved strategy for use of the cre-loxP system. Gene 2000, 252, 127–135. [Google Scholar] [CrossRef]
- Generoso, W.C.; Gottardi, M.; Oreb, M.; Boles, E. Simplified CRISPR-Cas genome editing for Saccharomyces cerevisiae. J. Microbiol. Methods 2016, 127, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzym. 2002, 350, 87–96. [Google Scholar]
- Adams, A.; Kaiser, C.; Cold Spring Harbor Laboratory. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 1997th ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1998; ISBN 0879695080. [Google Scholar]
- Riou, C.; Nicaud, J.M.; Barre, P.; Gaillardin, C. Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation. Yeast 1997, 13, 903–915. [Google Scholar] [CrossRef]
- Viana, T.; Loureiro-Dias, M.C.; Prista, C. Efficient fermentation of an improved synthetic grape must by enological and laboratory strains of Saccharomyces cerevisiae. AMB Express 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robyt, J.F.; Whelan, W.J. Reducing value methods for maltodextrins. I. Chain-length dependence of alkaline 3,5-dinitrosalicylate and chain-length independence of alkaline copper. Anal. Biochem. 1972, 45, 510–516. [Google Scholar] [CrossRef]
- Dukes, B.C.; Butzke, C.E. Rapid determination of primary amino acids in grape juice using an o-phthaldialdehyde/N-acetyl-L-cysteine spectrophotometric Assay. Am. J. Enol. Vitic. 1998, 49, 125–134. [Google Scholar]
- Sánchez, N.S.; Königsberg, M. Using yeast to easily determine mitochondrial functionality with 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide (MTT) assay. Biochem. Mol. Biol. Educ. Bimon. Publ. Int. Union Biochem. Mol. Biol. 2006, 34, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Márquez, I.G.; Ghiyasvand, M.; Massarsky, A.; Babu, M.; Samanfar, B.; Omidi, K.; Moon, T.W.; Smith, M.L.; Golshani, A. Zinc oxide and silver nanoparticles toxicity in the baker’s yeast, Saccharomyces cerevisiae. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Orlova, M.; Barrett, L.; Kuchin, S. Detection of endogenous Snf1 and its activation state: Application to Saccharomyces and Candida species. Yeast 2008, 25, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.M.; Bennetzen, J.; Young, E.T.; Nasmyth, K.; Hall, B.D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature 1980, 283, 214–216. [Google Scholar] [CrossRef]
- Fowler, P.W.; Ball, A.J.; Griffiths, D.E. The control of alcohol dehydrogenase isozyme synthesis in Saccharomyces cerevisiae. Can. J. Biochem. 1972, 50, 35–43. [Google Scholar] [CrossRef]
- Perez-Samper, G.; Cerulus, B.; Jariani, A.; Vermeersch, L.; Barrajón Simancas, N.; Bisschops, M.M.M.; van den Brink, J.; Solis-Escalante, D.; Gallone, B.; De Maeyer, D.; et al. The crabtree effect shapes the Saccharomyces cerevisiae lag phase during the switch between different carbon sources. MBio 2018. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.; Bodenmiller, B.; Uotila, A.; Stahl, M.; Wanka, S.; Gerrits, B.; Aebersold, R.; Loewith, R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009, 23, 1929–1943. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.W.; Jin, F.; Hwang, H.; Hwang, S.; Anand, V.; Duncan, M.C.; Huang, J. Insights into TOR function and rapamycin response: Chemical genomic profiling by using a high-density cell array method. Proc. Natl. Acad. Sci. USA 2005, 102, 7215–7220. [Google Scholar] [CrossRef] [Green Version]
- Borneman, A.R.; Forgan, A.H.; Kolouchova, R.; Fraser, J.A.; Schmidt, S.A. Whole genome comparison reveals high levels of inbreeding and strain redundancy across the spectrum of commercial wine strains of Saccharomyces cerevisiae. G3 2016. [Google Scholar] [CrossRef] [Green Version]
- Novo, M.; Bigey, F.; Beyne, E.; Galeote, V.; Gavory, F.; Mallet, S.; Cambon, B.; Legras, J.L.; Wincker, P.; Casaregola, S.; et al. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc. Natl. Acad. Sci. USA 2009, 106, 16333–16338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brice, C.; Sanchez, I.; Bigey, F.; Legras, J.L.; Blondin, B. A genetic approach of wine yeast fermentation capacity in nitrogen-starvation reveals the key role of nitrogen signaling. BMC Genom. 2014, 15, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, J.M.; Barre, P. Improvement of nitrogen assimilation and fermentation kinetics under enological conditions by derepression of alternative nitrogen-assimilatory pathways in an industrial Saccharomyces cerevisiae strain. Appl. Environ. Microbiol. 1998, 64, 3831–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deed, N.K.; van Vuuren, H.J.; Gardner, R.C. Effects of nitrogen catabolite repression and di-ammonium phosphate addition during wine fermentation by a commercial strain of S. cerevisiae. Appl. Microbiol. Biotechnol. 2011, 89, 1537–1549. [Google Scholar] [CrossRef]
- Tate, J.J.; Tolley, E.A.; Cooper, T.G. Sit4 and PP2A dephosphorylate nitrogen catabolite repression-sensitive Gln3 when TorC1 is up- as well as down-regulated. Genetics 2019. [Google Scholar] [CrossRef]
- Dufour, M.; Zimmer, A.; Thibon, C.; Marullo, P. Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Appl. Microbiol. Biotechnol. 2013, 97, 5893–5905. [Google Scholar] [CrossRef]
- Reimand, J.; Vaquerizas, J.M.; Todd, A.E.; Vilo, J.; Luscombe, N.M. Comprehensive reanalysis of transcription factor knockout expression data in Saccharomyces cerevisiae reveals many new targets. Nucleic Acids Res. 2010, 38, 4768–4777. [Google Scholar] [CrossRef] [Green Version]
- Young, E.T.; Yen, K.; Dombek, K.M.; Law, G.L.; Chang, E.; Arms, E. Snf1-independent, glucose-resistant transcription of Adr1-dependent genes in a mediator mutant of Saccharomyces cerevisiae. Mol. Microbiol. 2009, 74, 364–383. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Heitman, J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 1998, 17, 1236–1247. [Google Scholar] [CrossRef] [Green Version]
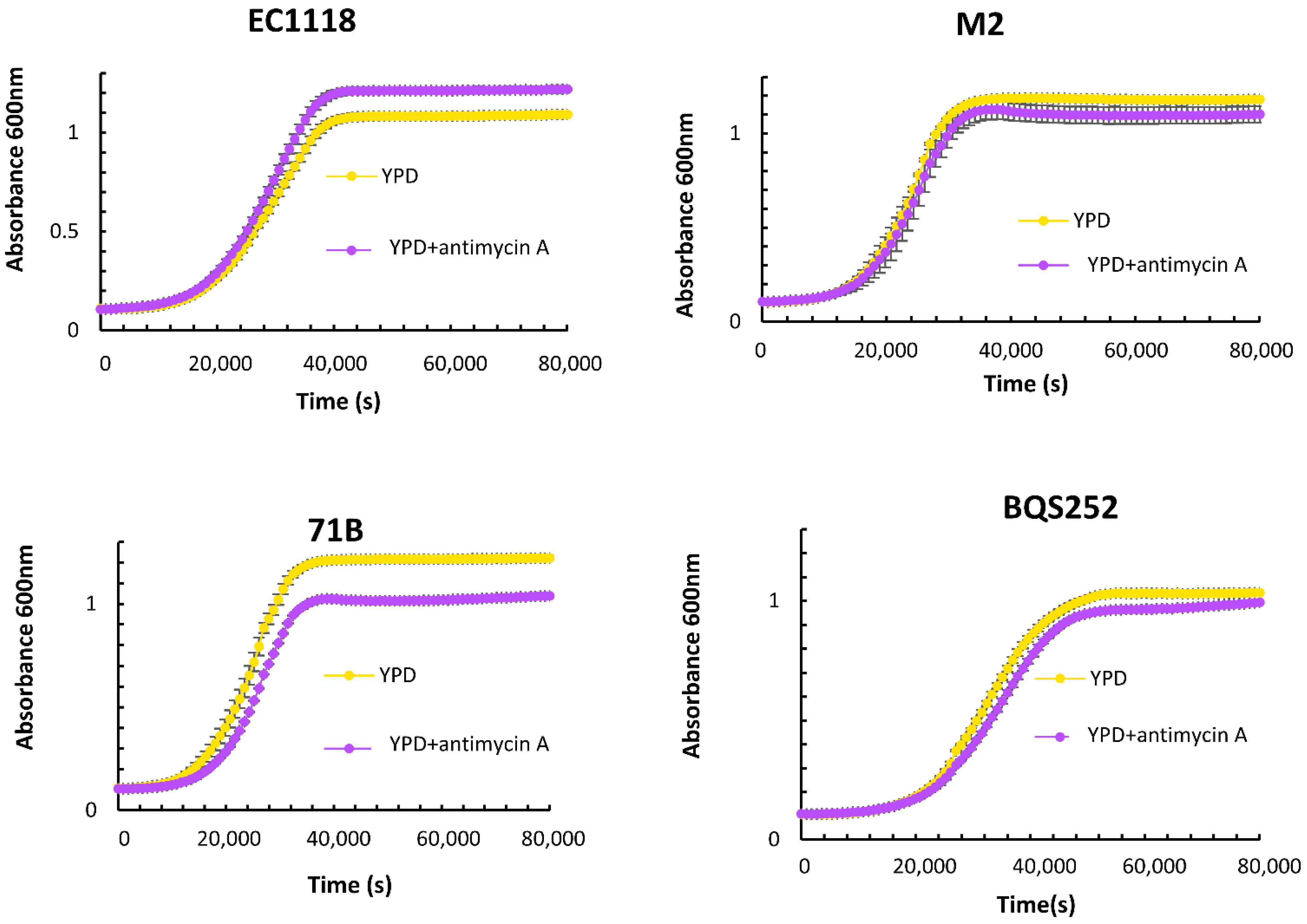
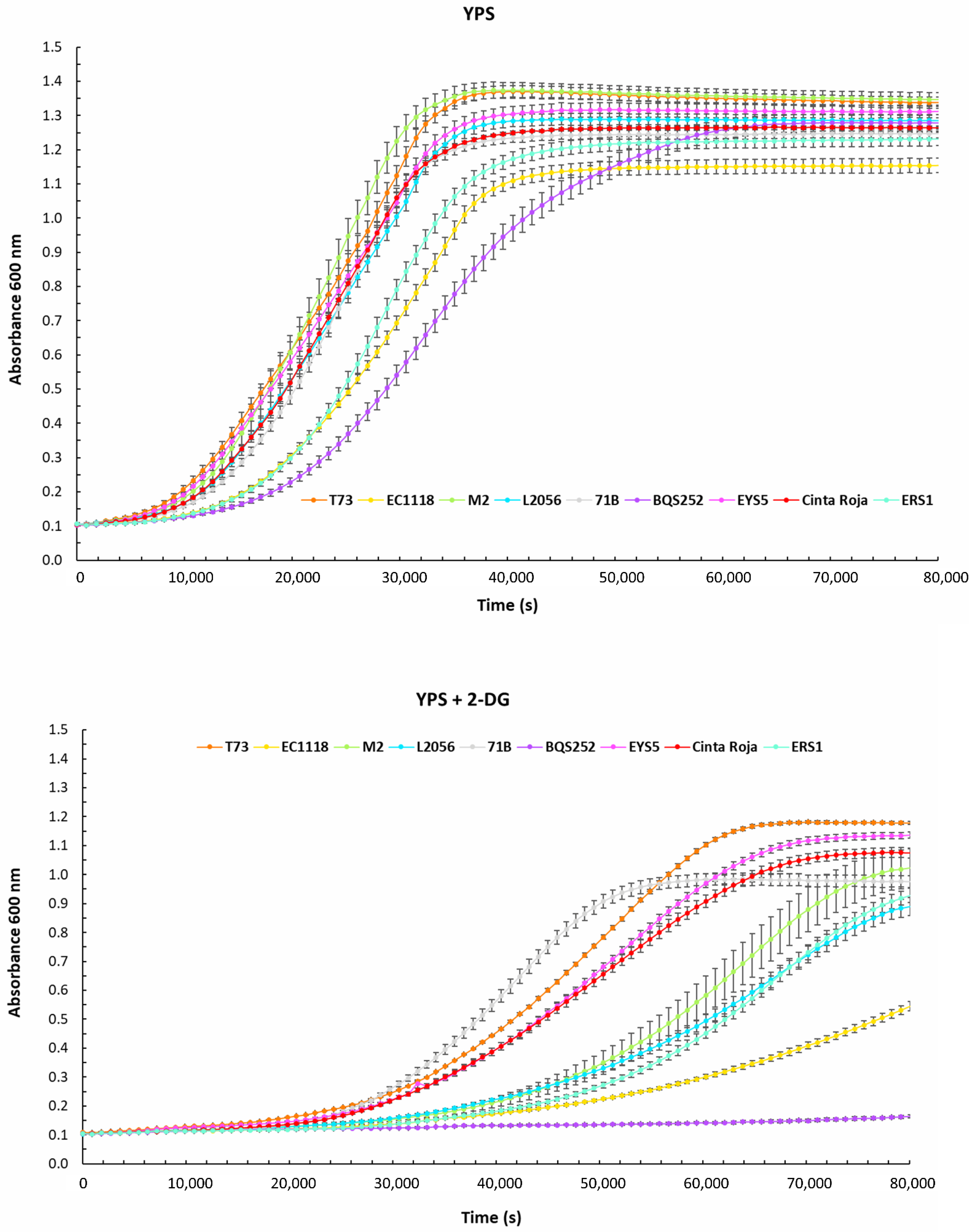


| YPD | YPD + Antimycin A | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vmax | ODmax | Lag Time | Vmax | ODmax | Lag Time | Ratios | |||||||||
| Av | SD | Av | SD | Av | SD | Av | SD | Av | SD | Av | SD | Vmax MaxOD Lag | |||
| T73 | 0.219 | 0.046 | 1.150 | 0.024 | 11,710 | 269 | 0.259 | 0.009 | 1.060 | 0.057 | 15,720 | 442 | 1.18 | 0.82 | 1.34 |
| EC1118 | 0.184 | 0.007 | 0.989 | 0.016 | 17,705 | 417 | 0.218 | 0.007 | 1.112 | 0.024 | 16,570 | 438 | 1.18 | 1.12 | 0.94 |
| M2 | 0.330 | 0.009 | 1.077 | 0.008 | 14,807 | 247 | 0.287 | 0.024 | 1.017 | 0.064 | 15,413 | 1592 | 0.87 | 0.94 | 1.04 |
| L2056 | 0.293 | 0.040 | 1.084 | 0.022 | 14,597 | 1251 | 0.239 | 0.000 | 1.124 | 0.011 | 14,365 | 375 | 0.81 | 1.04 | 0.98 |
| 71B | 0.328 | 0.055 | 1.114 | 0.010 | 14,337 | 1070 | 0.254 | 0.005 | 0.940 | 0.005 | 16,920 | 57 | 0.77 | 0.84 | 1.18 |
| BQS252 | 0.175 | 0.031 | 0.930 | 0.018 | 21,755 | 375 | 0.141 | 0.003 | 0.902 | 0.003 | 22,670 | 693 | 0.81 | 0.97 | 1.04 |
| EYS5 | 0.275 | 0.010 | 1.092 | 0.038 | 14,390 | 1067 | 0.240 | 0.010 | 0.998 | 0.038 | 15,950 | 622 | 0.88 | 0.91 | 1.11 |
| ERS1 | 0.236 | 0.001 | 1.061 | 0.029 | 17,967 | 724 | 0.214 | 0.005 | 0.972 | 0.012 | 19,887 | 351 | 0.91 | 0.92 | 1.11 |
| Cinta Roja | 0.224 | 0.010 | 1.014 | 0.025 | 14,227 | 547 | 0.219 | 0.005 | 0.952 | 0.006 | 15,185 | 290 | 0.98 | 0.94 | 1.07 |
| YPs | YPS + 2DG | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vmax | Max OD | Lag Time | Vmax | Max OD | Lag Time | Ratios | |||||||||
| Av | SD | Av | SD | Av | SD | Av | SD | Av | SD | Av | SD | Vmax MaxOD Lag | |||
| T73 | 0.24 | 0.0001 | 1.19 | 0.0071 | 9667 | 400 | 0.13 | 0.0001 | 1.02 | 0.0218 | 25,203 | 410 | 0.70 | 0.87 | 1.92 |
| EC1118 | 0.19 | 0.0034 | 1.04 | 0.0297 | 15,840 | 57 | 0.06 | 0.0037 | 0.48 | 0.0232 | 47,137 | 621 | 0.32 | 0.46 | 2.98 |
| M2 | 0.26 | 0.0076 | 1.25 | 0.0289 | 10,993 | 824 | 0.12 | 0.0078 | 0.91 | 0.0812 | 39,020 | 2365 | 0.46 | 0.73 | 3.55 |
| L2056 | 0.22 | 0.0067 | 1.17 | 0.0202 | 11,870 | 531 | 0.10 | 0.0040 | 0.79 | 0.0373 | 37,667 | 883 | 0.42 | 0.67 | 3.17 |
| 71B | 0.23 | 0.0039 | 1.13 | 0.0161 | 12,597 | 367 | 0.14 | 0.0016 | 0.87 | 0.0246 | 26,827 | 300 | 0.62 | 0.77 | 2.13 |
| BQS252 | 0.17 | 0.0026 | 1.16 | 0.0297 | 18,433 | 717 | 0.00 | 0.00 | 0.00 | ||||||
| EYS5 | 0.22 | 0.0020 | 1.20 | 0.0250 | 10,342 | 346 | 0.16 | 0.0378 | 1.02 | 0.0135 | 28,543 | 526 | 0.76 | 0.85 | 2.76 |
| ERS1 | 0.22 | 0.0017 | 1.11 | 0.0242 | 16,057 | 487 | 0.11 | 0.0058 | 0.83 | 0.0280 | 43,797 | 922 | 0.49 | 0.75 | 2.73 |
| Cinta Roja | 0.21 | 0.0022 | 1.15 | 0.0038 | 11,697 | 175 | 0.10 | 0.0030 | 0.96 | 0.0205 | 28,877 | 437 | 0.48 | 0.83 | 2.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Pinós, A.; Garrigós, V.; Matallana, E.; Aranda, A. Mechanisms of Metabolic Adaptation in Wine Yeasts: Role of Gln3 Transcription Factor. Fermentation 2021, 7, 181. https://doi.org/10.3390/fermentation7030181
Ferrer-Pinós A, Garrigós V, Matallana E, Aranda A. Mechanisms of Metabolic Adaptation in Wine Yeasts: Role of Gln3 Transcription Factor. Fermentation. 2021; 7(3):181. https://doi.org/10.3390/fermentation7030181
Chicago/Turabian StyleFerrer-Pinós, Aroa, Víctor Garrigós, Emilia Matallana, and Agustín Aranda. 2021. "Mechanisms of Metabolic Adaptation in Wine Yeasts: Role of Gln3 Transcription Factor" Fermentation 7, no. 3: 181. https://doi.org/10.3390/fermentation7030181
APA StyleFerrer-Pinós, A., Garrigós, V., Matallana, E., & Aranda, A. (2021). Mechanisms of Metabolic Adaptation in Wine Yeasts: Role of Gln3 Transcription Factor. Fermentation, 7(3), 181. https://doi.org/10.3390/fermentation7030181









