Effect of Microbial Enzymes on the Changes in the Composition and Microstructure of Hydrolysates from Poultry By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biotechnological Processing of By-Product Samples
2.2. Preparation of By-Product Hydrolysates
2.3. Research of the Fermented By-Products Microstructure
2.4. Determination of the Dispersed Composition of the By-Product Hydrolysates
2.5. Determination of Free Amino Acids in By-Product Hydrolysates
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microstructure of the Fermented By-Products
3.2. Dispersed Composition
3.3. Analyses of the Free Amino Acid Composition in By-Product Hydrolysates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Human Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Ghosh, D.; Chattoraj, D.K.; Chattopadhyay, P. Studies on Changes in Microstructure and Proteolysis in Cow and Soy Milk Curd During Fermentation Using Lactic Cultures For Improving Protein Bioavailability. J. Food Sci. Technol. 2013, 50, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Kassymov, S.; Nesterenko, A.; Yunusov, E.; Radchenko, E.; Bychkova, T. Effect of Alkali-Salt Treatment of Meat By-Products on Physical, Chemical and Rheological Properties. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 3221–3224. [Google Scholar] [CrossRef]
- Kim, J.-S.; Shahidi, F.; Heu, M.-S. Tenderization of meat by salt-fermented sauce from shrimp processing by-products. Food Chem. 2005, 93, 243–249. [Google Scholar] [CrossRef]
- Paulsen Thoresenb, P.; García Álvareza, R.; Risa Vakaa, M.; Rustadb, T.; Sonea, I.; Noriega Fernándeza, E. Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2020, 64, 102377. [Google Scholar] [CrossRef]
- Arihara, K.; Yokoyamaa, I.; Ohatab, M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.C.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.B.; Llanos, S.A.V.; Heredia, J.A.; Zamuner, S.; Gamarra, F.M.C.; Farias, T.M.B.; et al. Valorization of Chicken Feet By-Product of the Poultry Industry: High Qualities of Gelatin and Biofilm from Extraction of Collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef] [Green Version]
- Mullen, A.M.; Álvarez, C.; Zeugolis, D.I.; Henchion, M.; O’Neill, E.; Drummond, L. Alternative uses for co-products: Harnessing the potential of valuable compounds from meat processing chains. Meat Sci. 2017, 132, 90–98. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of hens’ byproducts as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Vikman, Y.M.; Siipola, V.; Kanerva, H.; Šližyte, R.; Wikberg, H. Poultry By-products as a Potential Source of Nutrients. Adv. Recycl. Waste Manag. 2017, 2, 142. [Google Scholar] [CrossRef]
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT—Food Sci. Technol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Vlahova-Vangelova, D.B.; Dragoev, S.G.; Balev, D.K.; Assenova, B.K.; Amirhanov, K.J. Quality, Microstructure, and Technological Properties of Sheep Meat Marinated in Three Different Ways. J. Food Qual. 2017, 2017, 5631532. [Google Scholar] [CrossRef] [Green Version]
- Sun-Waterhouse, D.; Zhao, M.; Waterhouse, G. Protein Modification During Ingredient Preparation and Food Processing: Approaches to Improve Food Processability and Nutrition. Food Bioprocess Technol. 2014, 7, 1853–1893. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocolloids 2020, 105, 105844. [Google Scholar] [CrossRef]
- Halavach, T.N.; Kurchenko, V.P. Milk Protein Hydrolysis with Enzyme Preparation and Proteolytic Systems of Lactic Acid Bacteria; Series: Physiological, biochemical and molecular foundations of the functioning of biosystems; Belarusian State University: Minsk, Belarus, 2012; Volume 7, pp. 106–126. [Google Scholar]
- Zinina, O.; Merenkova, S.; Galimov, D. Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates. Fermentation 2021, 7, 122. [Google Scholar] [CrossRef]
- Assaad, H.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid Publication-Ready MS-Word Tables for One-Way ANOVA. In SpringerPlus; Springer: Berlin, Germany, 2014; Volume 3, p. 474. Available online: https://houssein-assaad.shinyapps.io/TableReport/ (accessed on 8 September 2021). [CrossRef] [Green Version]
- Aktas, N.; Kaya, M. The influence of marinating with weak organic acids and salts on the intramuscular connective tissue and sensory properties of beef. Eur. Food Res. Technol. 2001, 213, 88–94. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Proteinhydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry 1999, 38, 6424–16431. [Google Scholar] [CrossRef] [PubMed]
- Vorob’ev, M.M.; Sinitsyna, O.V. Degradation and assembly of β-casein micelles during proteolysis by trypsin. Int. Dairy J. 2020, 104, 104652. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Le Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Law, J.; Haandrikman, A. Proteolytic enzymes of lactic acid bacteria. Int. Dairy J. 1997, 7, 1–11. [Google Scholar] [CrossRef]
- Unsal, M.; Aktas, N. Fractionation and characterization of edible sheep tail fat. Meat Sci. 2003, 63, 235–239. [Google Scholar] [CrossRef]
- Seong, P.N.; Kang, G.H.; Park, K.M.; Cho, S.H.; Kang, S.M.; Park, B.Y. Characterization of Hanwoo Bovine By-products by Means of Yield, Physicochemical and Nutritional Compositions. Korean J. Food Sci. Anim. Resour. 2014, 34, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]



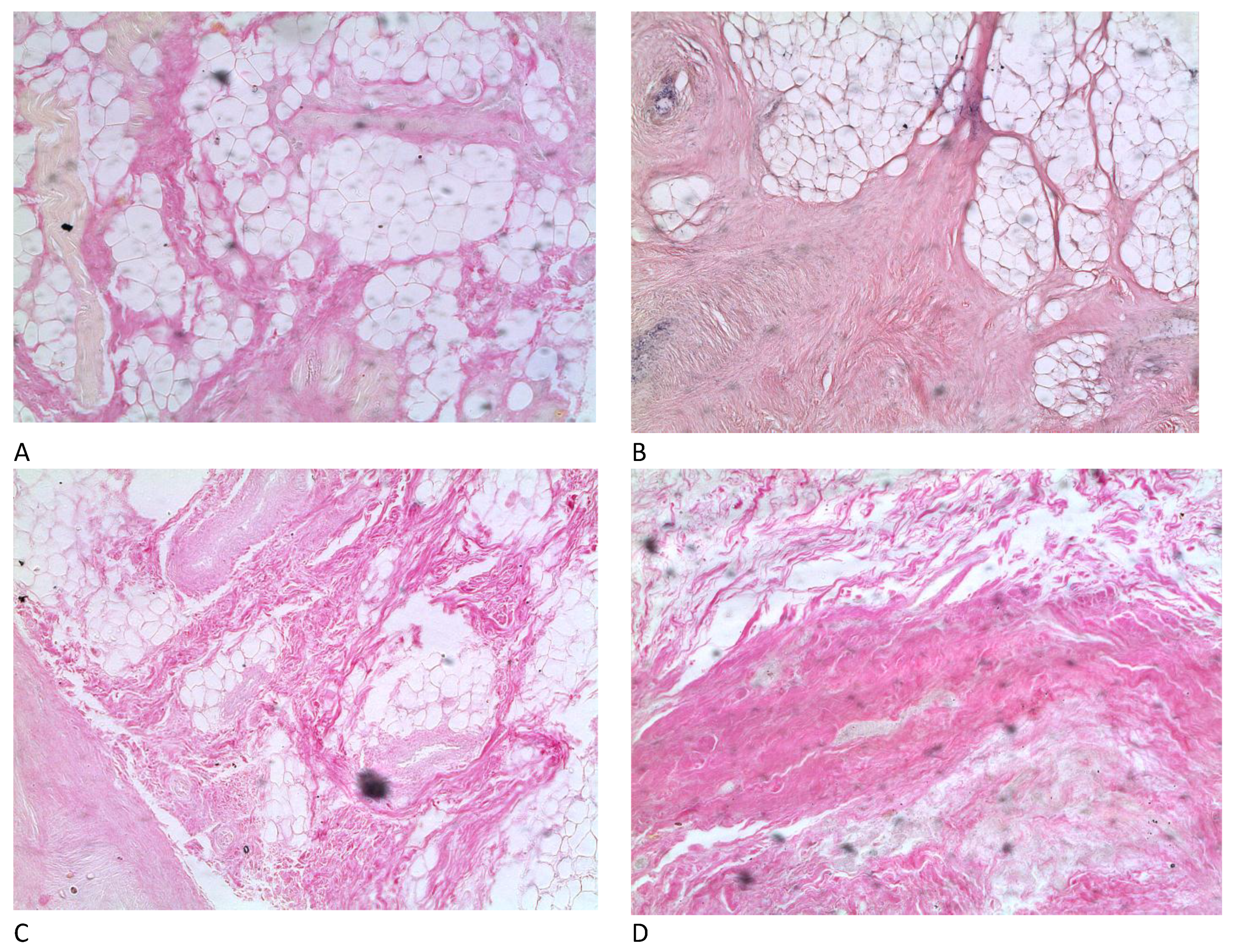




| Indicators | Control Sample in the Start Time | Fermented Control Sample | Fermented Test Sample with BLC | Fermented Test Sample with Propionix LCSC |
|---|---|---|---|---|
| hen gizzards | ||||
| Specific area of connective tissue (%) | 61.43 ± 1.922 a | 37.91 ± 1.259 b | 33.80 ± 1.981 c | 24.99 ± 0.266 d |
| Average thickness of collagen fibers (microns) | 15.36 ± 3.056 a | 10.37 ± 2.071 b | 6.51 ± 0.958 c | 6.65 ± 0.953 c |
| Average thickness of myocytes (microns) | 15.59 ± 1.856 a | 11.18 ± 1.764 b | 4.54 ± 1.055 c | 5.53 ± 0.855 c |
| hen combs | ||||
| Specific area of connective tissue (%) | 59.22 ± 3.067 a | 54.85 ± 1.439 a | 51.58 ± 3.239 b | 43.77 ± 1.742 c |
| Average thickness of collagen fibers (microns) | 19.90 ± 3.006 a | 6.58 ± 0.9615 b | 4.49 ± 1.267 c | 7.18 ± 2.492 b |
| Indicator | Control Sample in the Start Time | Hydrolyzed Control Sample | Hydrolyzed Test Sample with Propionix LCSC | Hydrolyzed Test Sample with BLC |
|---|---|---|---|---|
| hen gizzards | ||||
| MN (micron) | 11.83 ± 0.85 a | 6.60 ± 0.62 b | 4.69 ± 0.35 c | 2.73 ± 0.24 d |
| hen combs | ||||
| MN (micron) | 1.93 ± 0.11 a | 1.91 ± 0.08 a | 1.28 ± 0.07 b | 1.53 ± 0.08 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merenkova, S.; Zinina, O.; Lykasova, I.; Kuznetsov, A.; Shnyakina, T. Effect of Microbial Enzymes on the Changes in the Composition and Microstructure of Hydrolysates from Poultry By-Products. Fermentation 2021, 7, 190. https://doi.org/10.3390/fermentation7030190
Merenkova S, Zinina O, Lykasova I, Kuznetsov A, Shnyakina T. Effect of Microbial Enzymes on the Changes in the Composition and Microstructure of Hydrolysates from Poultry By-Products. Fermentation. 2021; 7(3):190. https://doi.org/10.3390/fermentation7030190
Chicago/Turabian StyleMerenkova, Svetlana, Oksana Zinina, Irina Lykasova, Alexander Kuznetsov, and Tatyana Shnyakina. 2021. "Effect of Microbial Enzymes on the Changes in the Composition and Microstructure of Hydrolysates from Poultry By-Products" Fermentation 7, no. 3: 190. https://doi.org/10.3390/fermentation7030190
APA StyleMerenkova, S., Zinina, O., Lykasova, I., Kuznetsov, A., & Shnyakina, T. (2021). Effect of Microbial Enzymes on the Changes in the Composition and Microstructure of Hydrolysates from Poultry By-Products. Fermentation, 7(3), 190. https://doi.org/10.3390/fermentation7030190






