Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (OFMSW) through Alkaline Hydrogen Peroxide (AHP) Pretreatment
Abstract
:1. Introduction
2. Description of the Experiments
2.1. Materials
2.2. Alkaline Hydrogen Peroxide Tests
2.3. Biomethane Potential Tests
2.4. Analytical Methods
3. Results and Discussion
3.1. Characteristics of Organic Matrices
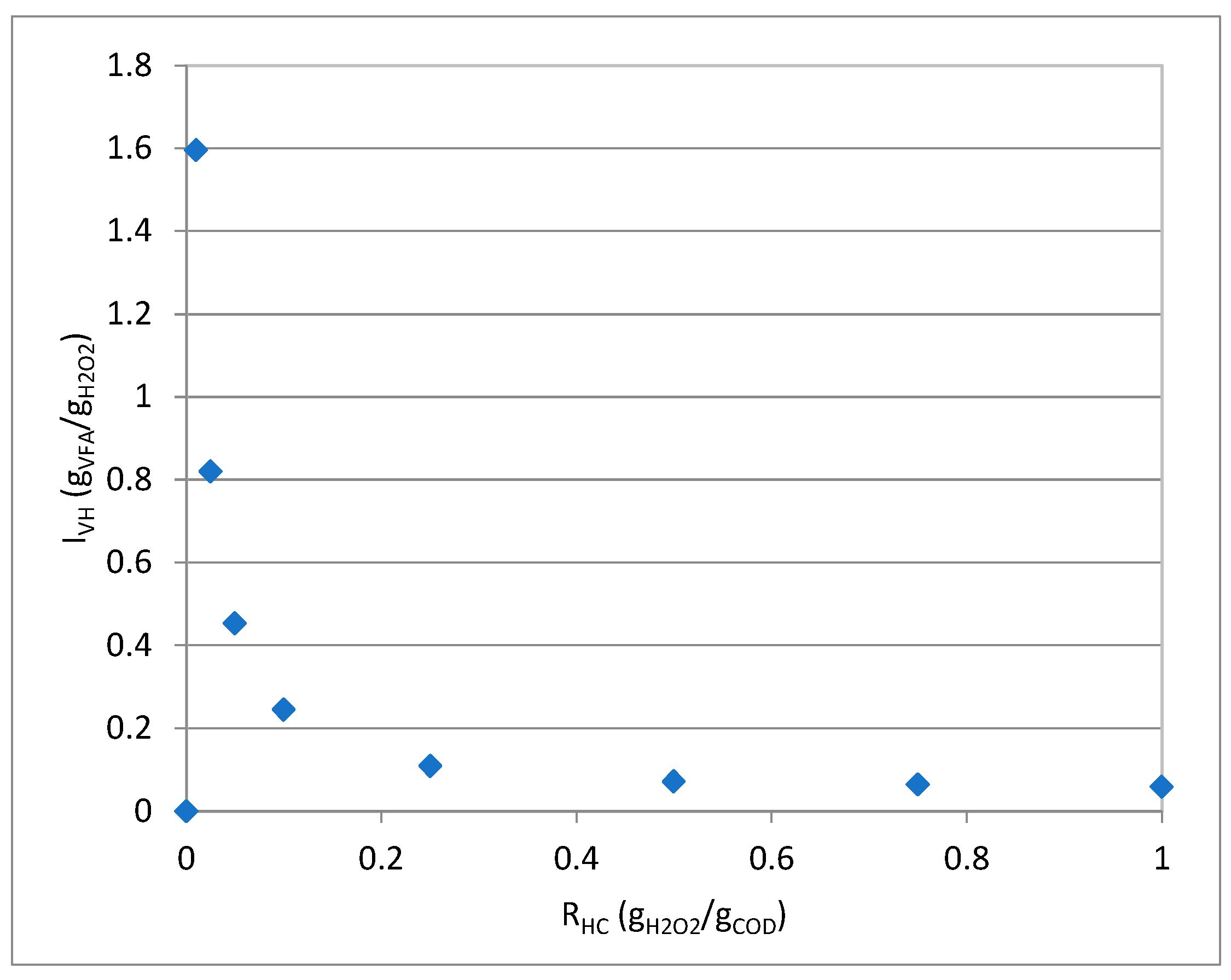
3.2. Alkaline Hydrogen Peroxide Tests
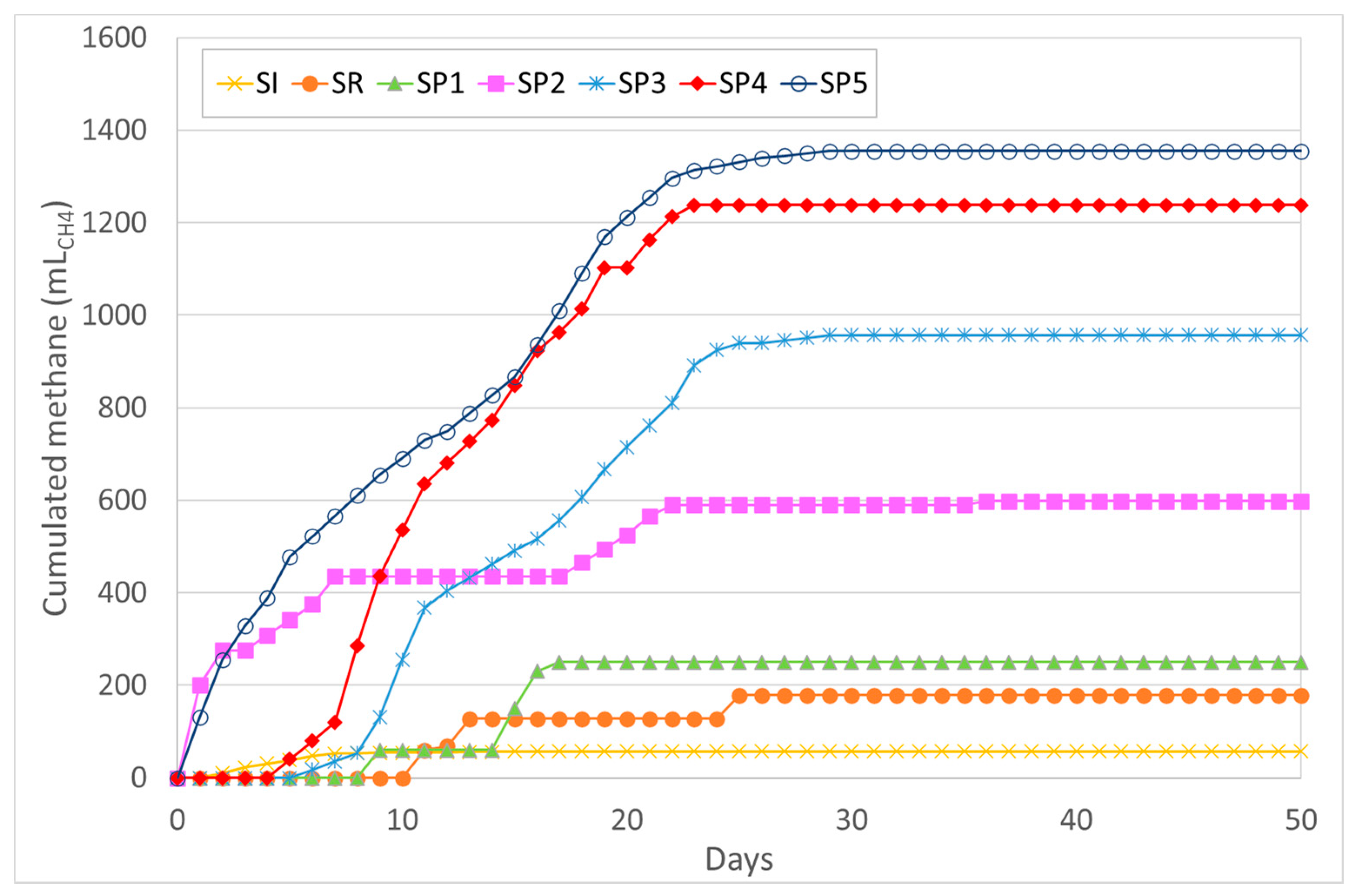
3.3. Biomethane Potential Tests
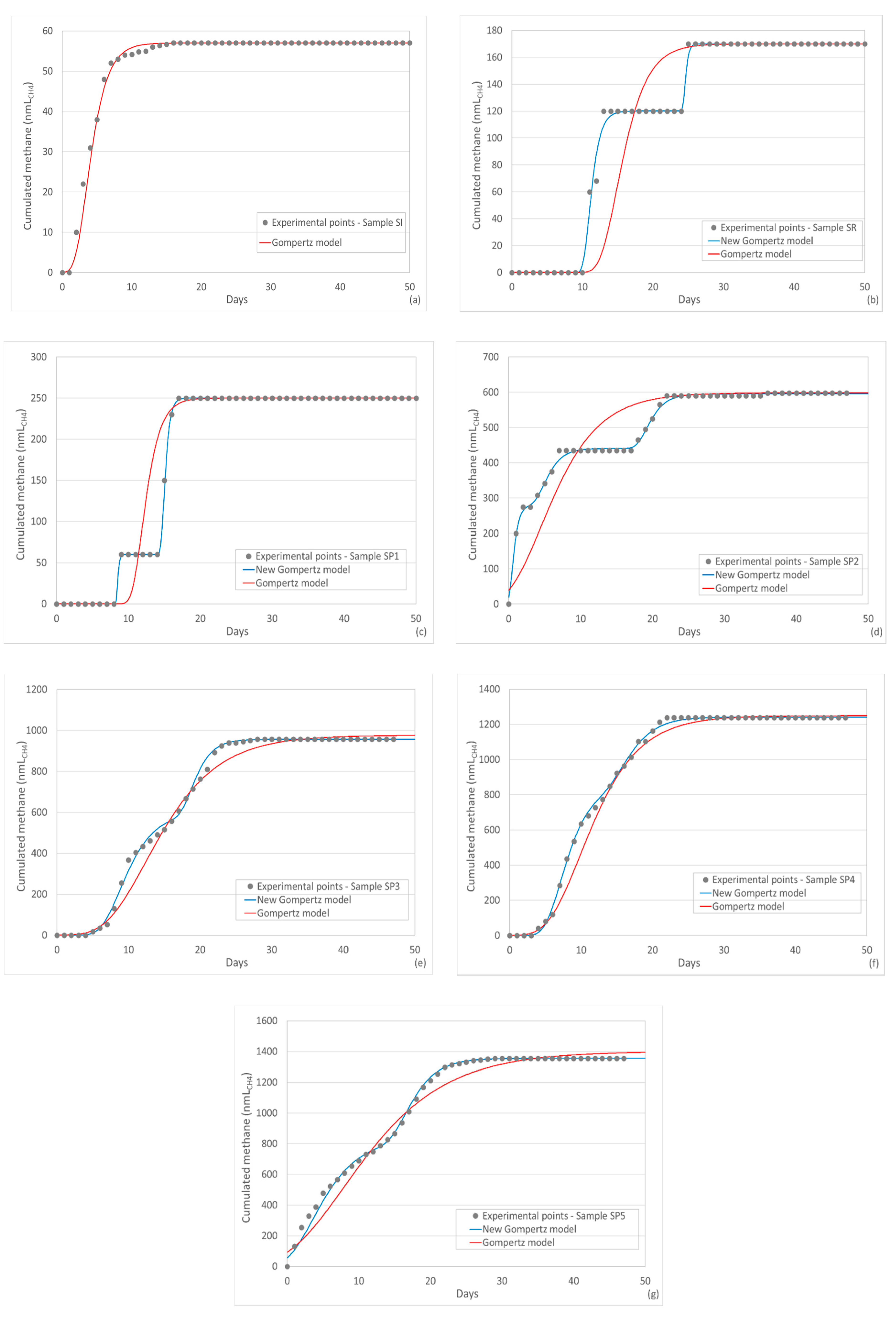
3.4. Modeling of Cumulated Methane Productions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoornweg, D.; Bhada-Tata, P. Urban development series knowledge papers. In What a Waste: A Global Review of Solid Waste Management; Urban Development & Local Government Unit, The World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Karak, T.; Bhagat, R.M.; Bhattacharyya, P. Municipal solid waste generation, composition, and management: The world scenario. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1509–1630. [Google Scholar] [CrossRef]
- Rózsenberszki, T.; Koók, L.; Hutvágner, D.; Nemestothy, N.; Bélafi-Bakó, K.; Bakonyi, P.; Kurdi, R.; Sarkady, A. Comparison of Anaerobic Degradation Processes for Bioenergy Generation from Liquid Fraction of Pressed Solid Waste. Waste Biomass Valorization 2015, 6, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Khanal, S.K. Anaerobic Biotecnology for Bioenergy Production: Principles and Applications; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustainability 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Dearman, B.; Bentham, R.H. Anaerobic digestion of food waste: Comparing leachate exchange rates in sequential batch systems digesting food waste and biosolids. Waste Manag. 2007, 27, 1792–1799. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Liu, X.; Mei, Z.; Ren, H.; Cao, Q.; Yan, Z. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste. Bioresour. Technol. 2017, 245, 90–97. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Otero, L.; Lema, J.M. A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour. Technol. 2010, 101, 1153–1158. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Zhang, J. Enhanced biogas production from anaerobic digestion of solid organic wastes: Current status and prospects. Bioresour. Technol. Rep. 2019, 5, 280–296. [Google Scholar] [CrossRef]
- Tezel, U.; Tandukar, M.S.; Pavlostathis, G. Anaerobic Biotreatment of Municipal Sewage Sludge. Compr. Biotechnol. 2011, 6, 447–461. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Calabrò, V. Biogas Generation through Anaerobic Digestion of Compost Leachate in Semi-Continuous Completely Stirred Tank Reactors. Processes 2019, 7, 635. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef]
- Yan, Z.; Song, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour. Technol. 2015, 177, 266–273. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Semi-continuous anaerobic co-digestion of sugar beet byproduct and pig manure: Effect of the organic loading rate (OLR) on process performance. Bioresour. Technol. 2015, 194, 283–290. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.; Sahinkaya, E.; Çalli, B. Biogas production from chicken manure: Co-digestion with spent poppy straw. Int. Biodeterior. Biodegradation 2017, 119, 205–210. [Google Scholar] [CrossRef]
- Bougrier, C.; Dognin, D.; Laroche, C.; Gonzalez, V.; Benali-Raclot, D.; Rivero, J.A.C. Anaerobic digestion of Brewery Spent Grains: Trace elements addition requirement. Bioresour. Technol. 2018, 247, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Fazzino, F.; Limonti, C.; Siciliano, A. Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Con-ductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios. Water 2021, 13, 391. [Google Scholar] [CrossRef]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Guo, J.; Ma, W.; Fang, W.; Ma, B.; Xu, X. Sewage sludge solubilization by high-pressure homogenization. Water Sci. Technol. 2013, 67, 2399–2405. [Google Scholar] [CrossRef]
- Ormaechea, P.; Castrillón, L.; Marañón, E.; Fernández-Nava, Y.; Negral, L.; Megido, L. Influence of the ultrasound pretreat-ment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017, 38, 682–686. [Google Scholar] [CrossRef]
- Rasapoor, M.; Ajabshirchi, Y.; Adl, M.; Abdi, R.; Gharibi, A. The effect of ultrasonic pretreatment on biogas generation yield from organic fraction of municipal solid waste under medium solids concentration circumstance. Energy Convers. Manag. 2016, 119, 444–452. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Sun, R.C.; Fang, J.M.; Tomkinson, J. Delignification of rye straw using hydrogen peroxide. Ind. Crop. Prod. 2000, 12, 71–83. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Amezquita Fonseca, N.A.; Andrade, R.R.; Maciel Filho, R.; Costa, A.C. Ethanol production from enzymatic hy-drolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 2011, 35, 2600–2607. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Increase of the anaerobic biodegradability of olive mill wastewaters through a pre-treatment with hydrogen peroxide in alkaline conditions. Desalination Water Treat. 2014, 55, 1735–1746. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; Limonti, C. Energetic Valorization of Wet Olive Mill Wastes through a Suitable Integrated Treatment: H2O2 with Lime and Anaerobic Digestion. Sustainability 2016, 8, 1150. [Google Scholar] [CrossRef] [Green Version]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Cao, W.; Sun, C.; Liu, R.; Yin, R.; Wu, X. Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresour. Technol. 2012, 111, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, A.; Pinet, E.; Bouix, M.; Bouchez, T.; Mansour, A.A. Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential. Waste Manag. 2012, 32, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association and Water Environment Federation: Washington, DC, USA, 1998.
- El-Gohary, F.A.; Badawy, M.I.; El-Khateeb, M.A.; El-Kalliny, A.S. Integrated treatment of olive mill wastewater (OMW) by the combination of Fenton’s reaction and anaerobic treatment. J. Hazard. Mater. 2009, 162, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Uğurlu, M.; Kula, I. Decolourization and removal of some organic compounds from olive mill wastewater by advanced oxidation processes and lime treatment. Environ. Sci. Pollut. Res. 2007, 14, 319–325. [Google Scholar] [CrossRef]
- Iboukhoulef, H.; Amrane, A.; Kadi, H. Microwave-enhanced Fenton-like system, Cu(II)/H2O2, for olive mill wastewater treatment. Environ. Technol. 2013, 34, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Chamarro, E.; Marco, A.; Esplugas, S. Use of Fenton reagent to improve organic chemical biodegradability. Wat. Res. 2001, 35, 1047–1051. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Grassi, M.; Belgiorno, V. Pre-treatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep. Purif. Technol. 2008, 63, 648–653. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Hu, S.; Hand, D.W.; Green, S.A. A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Res. 1999, 33, 2315–2328. [Google Scholar] [CrossRef]
- De Rosa, S.; Siciliano, A. A catalytic oxidation process of olive oil mill wastewaters using hydrogen peroxide and copper. Desalination Water Treat. 2010, 23, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Xie, S.; Frost, J.P.; Lawlor, P.G.; Wu, G.; Zhan, X. Effects of thermo-chemical pre-treatment of grass silage on methane pro-duction by anaerobic digestion. Biores. Technol. 2011, 102, 8748–8755. [Google Scholar] [CrossRef] [PubMed]
- Rinzema, A.; van Lier, J.; Lettinga, G. Sodium inhibition of acetoclastic methanogens in granular sludge from a UASB reactor. Enzym. Microb. Technol. 1988, 10, 24–32. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef]
- Yilmaz, T.; Erdirencelebi, D.; Berktay, A. Effect of COD/SO ratio on anaerobic treatment of landfill leachate during the start-up period. Environ. Technol. 2012, 33, 313–320. [Google Scholar] [CrossRef]
- Albuquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertilizer potential of digestates from farm and agroindustrial residues. Biomass Bioener. 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Kaur, H.; Kommalapati, R.R. Optimizing anaerobic co digestion of goat manure and cotton gin trash using biochemical me-thane potential (BMP) test and mathematical modeling. SN Appl. Sci. 2021, 3, 724. [Google Scholar] [CrossRef]
- Lay, J.-J.; Li, Y.-Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]



| pH | Cond mS/cm | COD g/L | TS g/L | VS g/L | VFA gCH3COOH/L | ALK gCaCO3/L | N-NH4+ g/L | TKN gN/L | P-PO43− mg/L | SO42− mg/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum | 7.8 ± 0.1 | 23.6 ± 0.2 | 12.5 ± 0.6 | 21.6 ± 0.7 | 10.2 ± 0.4 | 0.91 ± 0.06 | 13.6 ± 0.7 | 1.415 ± 0.06 | 1.61 ± 0.05 | 215.1 ± 0.03 | 542.7 ± 21 |
| OFMSW | 5.3 ± 0.1 | 2.29 ± 0.1 | 254.4 ± 4.1 | 191.1 ± 1.6 | 178.1 ± 1.8 | 3.1 ± 0.02 | 15.98 ± 0.5 | 0.159 ± 0.007 | 2.22 ± 0.06 | 265.7 ± 12.1 | 192.1 ± 9.1 |
| Sample | SI | SR | SP1 | SP2 | SP3 | SP4 | SP5 |
|---|---|---|---|---|---|---|---|
| pH | 7.2 ± 0.1 | 6.85 ± 0.1 | 7.4 ± 0.2 | 7.56 ± 0.1 | 7.61 ± 0.1 | 7.43 ± 0.1 | 7.60 ± 0.1 |
| Cond (mS/cm) | 14.2 ± 0.11 | 14.35 ± 0.09 | 17.26 ± 0.07 | 18.28 ± 0.10 | 18.00 ± 0.08 | 17.78 ± 0.04 | 19.08 ± 0.09 |
| COD (g/L) | 7.34 ± 0.21 | 48.20 ± 0.92 | 45.08 ± 0.86 | 41.83 ± 1.4 | 42.52 ± 0.45 | 38.32 ± 1.23 | 33.90 ± 0.35 |
| TS (g/L) | 13.1 ± 0.34 | 43.54 ± 1,4 | 41.16 ± 1.7 | 37.92 ± 0.63 | 38.44 ± 1.21 | 35.32 ± 0.46 | 32.50 ± 0.98 |
| VS (g/L) | 6.22 ± 0.23 | 34.61 ± 1.07 | 32.78 ± 0.65 | 29.76 ± 0.24 | 30.27 ± 1.43 | 27.3 ± 0.76 | 24.16 ± 1.10 |
| VFA (gCH3COOH/L) | 0.49 ± 0.02 | 1.14 ± 0.04 | 2.07 ± 0.08 | 2.17 ± 0.03 | 2.22 ± 0.02 | 2.54 ± 0.05 | 3.65 ± 0.07 |
| ALK (gCaCO3/L) | 7.99 ± 0.35 | 10.71 ± 0.45 | 13.24 ± 0.56 | 13.17 ± 0.27 | 13.21 ± 0.49 | 13.26 ± 0.61 | 13.31 ± 0.38 |
| N-NH4+ (g/L) | 0.856 ± 0.023 | 0.874 ± 0.011 | 0.904 ± 0.029 | 0.903 ± 0.034 | 0.899 ± 0.025 | 0.895 ± 0.040 | 0.890 ± 0.038 |
| TKN (gN/L) | 0.954 ± 0.041 | 1.323 ± 0.021 | 1.705 ± 0.012 | 1.539 ± 0.056 | 1.513 ± 0.067 | 1.437 ± 0.034 | 1.406 ± 0.052 |
| P-PO43− (mg/L) | 129.6 ± 7.4 | 170.6 ± 10.8 | 303.7 ± 15.1 | 358.8 ± 7.8 | 348.6 ± 12.8 | 333.15 ± 14.5 | 353.9 ± 12.9 |
| SO42− (mg/L) | 302.6±9.76 | 356.3±7.89 | 355.9±10.54 | 352.8±4.89 | 353.6±12.71 | 349.8±5.26 | 345.3±15.15 |
| VFA/ALK (gCH3COOH/gCaCO3) | 0.061 | 0.106 | 0.156 | 0.165 | 0.168 | 0.191 | 0.274 |
| COD/TKN (g/g) | 7.69 | 36.43 | 26.44 | 27.18 | 28.10 | 26.67 | 24.11 |
| Sample | SI | SR | SP1 | SP2 | SP3 | SP4 | SP5 |
|---|---|---|---|---|---|---|---|
| IMS (mLCH4/gTS) | 29.32 | 37.06 | 59.10 | 159.72 | 250.13 | 369.11 | 463.72 |
| YMC (LCH4/gCODremoved) | 0.138 | 0.059 | 0.064 | 0.149 | 0.242 | 0.281 | 0.314 |
| Sample | SI | SR | SP1 | SP2 | SP3 | SP4 | SP5 |
|---|---|---|---|---|---|---|---|
| pH | 7.9 ± 0.1 | 6.34 ± 0.1 | 7.22 ± 0.1 | 7.12 ± 0.1 | 7.53 ± 0.1 | 7.55 ± 0.1 | 7.61 ± 0.1 |
| Cond (mS/cm) | 14.82 ± 0.15 | 14.75 ± 0.11 | 18.12 ± 0.04 | 18.75 ± 0.06 | 19.34 ± 0.10 | 18.34 ± 0.05 | 19.52 ± 0.12 |
| COD (g/L) | 4.60 ± 0.14 | 29.01 ± 0.11 | 19.22 ± 0.75 | 15.13 ± 0.67 | 16.23 ± 0.59 | 9.04 ± 0.41 | 5.21 ± 0.14 |
| TS (g/L) | 9.57 ± 0.27 | 27.54 ± 0.95 | 21.01 ± 1.0 | 18.97 ± 0.52 | 18.94 ± 0.71 | 13.82 ± 0.58 | 12.00 ± 0.56 |
| VS (g/L) | 3.68 ± 0.11 | 19.54 ± 0.76 | 13.23 ± 0.52 | 11.01 ± 0.50 | 11.26 ± 0.39 | 6.08 ± 0.29 | 3.67 ± 0.18 |
| VFA (gCH3COOH/L) | 0.40 ± 0.01 | 6.60 ± 0.09 | 0.82 ± 0.03 | 1.23 ± 0.05 | 0.95 ± 0.03 | 1.04 ± 0.04 | 1.02 ± 0.05 |
| ALK (gCaCO3/L) | 7.12 ± 0.24 | 8.96 ± 0.27 | 12.46 ± 0.35 | 11.56 ± 0.39 | 10.60 ± 0.65 | 11.5 ± 0.42 | 11.21 ± 0.47 |
| N-NH4+ (g/L) | 0.914 ± 0.041 | 0.918 ± 0.037 | 0.978 ± 0.026 | 0.994 ± 0.025 | 1.011 ± 0.03 | 1.108 ± 0.036 | 1.204 ± 0.040 |
| TKN (g/L) | 0.947 ± 0.026 | 1.30 ± 0.043 | 1.678 ± 0.029 | 1.426 ± 0.032 | 1.407 ± 0.045 | 1.387 ± 0.051 | 1.325 ± 0.044 |
| P-PO43- (mg/L) | 139 ± 3.49 | 158.1 ± 9.8 | 301.5 ± 15.3 | 284.8 ± 16.0 | 295.0 ± 13.8 | 271.8 ± 12.5 | 205.38 ± 9.44 |
| SO42- (mg/L) | 347.6 ± 11.9 | 378.9 ± 14.6 | 394.6 ± 11.4 | 375.3 ± 15.7 | 398.2 ± 14.7 | 394.4 ± 17.6 | 395.8 ± 11.8 |
| VFA/ALK (gCH3COOH/gCaCO3) | 0.056 | 0.736 | 0.066 | 0.106 | 0.089 | 0.090 | 0.091 |
| COD/TKN (g/g) | 4.857 | 22.38 | 11.45 | 10.61 | 11.53 | 6.51 | 3.93 |
| SI | SR | SP1 | SP2 | SP3 | SP4 | SP5 |
|---|---|---|---|---|---|---|
| Pm | Pm | Pm | Pm | Pm | Pm | Pm |
| 57 | 170 | 250 | 598.5 | 976.5 | 1249 | 1401 |
| Rm | Rm | Rm | Rm | Rm | Rm | Rm |
| 12.5 | 26.2 | 62.5 | 48.7 | 64.1 | 100.9 | 65.2 |
| λ | λ | λ | λ | λ | λ | λ |
| 1.8 | 12.5 | 10.5 | 10−5 | 6.8 | 5.3 | 10−5 |
| R2 | R2 | R2 | R2 | R2 | R2 | R2 |
| 0.990 | 0.848 | 0.907 | 0.855 | 0.986 | 0.990 | 0.980 |
| SR | SP1 | SP2 | SP3 | SP4 | SP5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | n | n | |||||||
| 2 | 2 | 3 | 2 | 2 | 2 | |||||||
| Pm1 | Pm2 | Pm1 | Pm2 | Pm1 | Pm2 | Pm3 | Pm1 | Pm2 | Pm1 | Pm2 | Pm1 | Pm2 |
| 120 | 50 | 60.1 | 190 | 279.5 | 161.2 | 154.6 | 601.2 | 355.4 | 853.6 | 388 | 833.4 | 521.6 |
| Rm1 | Rm2 | Rm1 | Rm2 | Rm1 | Rm2 | Rm3 | Rm1 | Rm2 | Rm1 | Rm2 | Rm1 | Rm2 |
| 5.9 | 59.5 | 131 | 152 | 198.5 | 41.9 | 38.3 | 81.5 | 81.2 | 135.3 | 55.9 | 85.6 | 79.5 |
| λ1 | λ2 | λ1 | λ2 | λ1 | λ2 | λ3 | λ1 | λ2 | λ1 | λ2 | λ1 | λ2 |
| 10.1 | 24.2 | 8.3 | 14.5 | 10−5 | 3.5 | 17.7 | 6.1 | 17.2 | 4.9 | 13.5 | 10−5 | 14.2 |
| R2 | R2 | R2 | R2 | R2 | R2 | |||||||
| 0.995 | 0.999 | 0.997 | 0.998 | 0.999 | 0.997 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Limonti, C.; Curcio, G.M. Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (OFMSW) through Alkaline Hydrogen Peroxide (AHP) Pretreatment. Fermentation 2021, 7, 197. https://doi.org/10.3390/fermentation7030197
Siciliano A, Limonti C, Curcio GM. Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (OFMSW) through Alkaline Hydrogen Peroxide (AHP) Pretreatment. Fermentation. 2021; 7(3):197. https://doi.org/10.3390/fermentation7030197
Chicago/Turabian StyleSiciliano, Alessio, Carlo Limonti, and Giulia Maria Curcio. 2021. "Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (OFMSW) through Alkaline Hydrogen Peroxide (AHP) Pretreatment" Fermentation 7, no. 3: 197. https://doi.org/10.3390/fermentation7030197
APA StyleSiciliano, A., Limonti, C., & Curcio, G. M. (2021). Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (OFMSW) through Alkaline Hydrogen Peroxide (AHP) Pretreatment. Fermentation, 7(3), 197. https://doi.org/10.3390/fermentation7030197








