Development of a New Assay for Measuring H2S Production during Alcoholic Fermentation: Application to the Evaluation of the Main Factors Impacting H2S Production by Three Saccharomycescerevisiae Wine Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Fermentation Condition
2.3. Hydrogen Sulfide Measurement
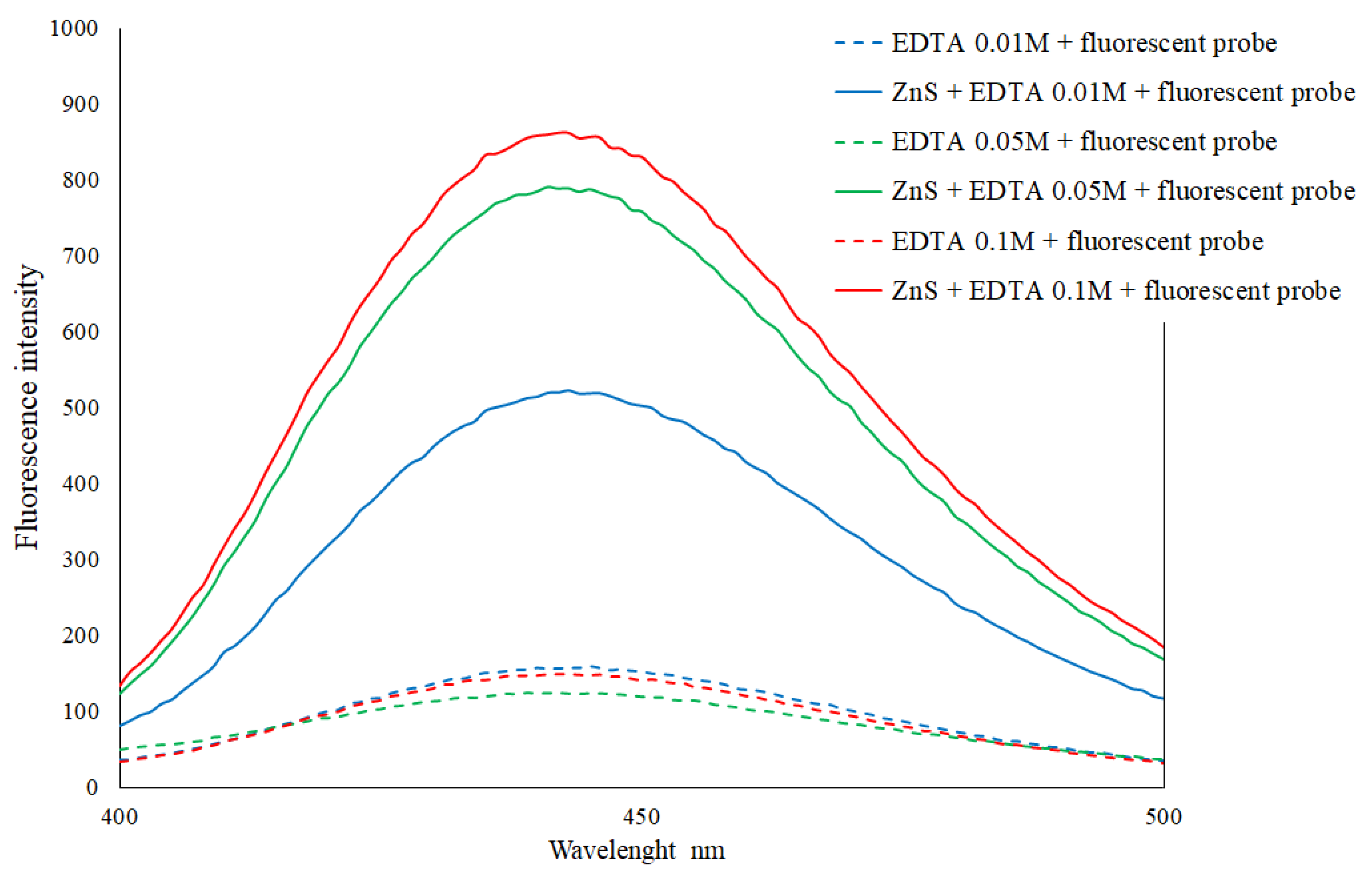
2.4. Preparation of Fluorescent Probe
2.5. Hydrogen Sulfide Detection
2.6. Experimental Design
3. Results
3.1. Development of a Colorimetric Method for the Quantification of Total Hydrogen Sulfide Production during Fermentation
3.2. Application of the Colorimetric Assay for the Assessment of Strains Response to Factors Impacting H₂S Production during Alcoholic Fermentation
4. Discussion
4.1. Development of a New Quantitative Fluorometric Method for High throughput Evaluation of H₂S Production
4.2. Evaluation of the Different Factors Influencing H₂S Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco-Luesma, E.; Sáenz-Navajas, M.P.; Valentin, D.; Ballester, J.; Rodrigues, H.; Ferreira, V. Study of the effect of H2S, MeSH and DMS on the sensory profile of wine model solutions by Rate-All-That-Apply (RATA). Food Res. Int. 2016, 87, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, S-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Thiols and related sulfur compounds. In Understanding Wine Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2016; pp. 88–98. [Google Scholar]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar] [CrossRef]
- Dittrich, H.H. Mikrobiologie des Weines, 2nd ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 1987. [Google Scholar]
- Holt, S.; Kankipati, H.; De Graeve, S.; Van Zeebroeck, G.; Foulquié-Moreno, M.R.; Lindgreen, S.; Thevelein, J.M. Major sulfonate transporter Soa1 in Saccharomyces cerevisiae and considerable substrate diversity in its fungal family. Nat. Commun. 2017, 8, 14247. [Google Scholar] [CrossRef] [Green Version]
- Cherest, H.; Davidian, J.C.; Thomas, D.; Benes, V.; Ansorge, W.; Surdin-Kerjan, Y. Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics 1997, 145, 627–635. [Google Scholar] [CrossRef]
- Hansen, J.; Johannesen, P.F. Cysteine is essential for transcriptional regulation of the sulfur assimilation genes in Saccharomyces cerevisiae. Mol. Gen. Genet. 2000, 263, 535–542. [Google Scholar] [CrossRef]
- Huang, C.W.; Walker, M.E.; Fedrizzi, B.; Gardner, R.C.; Jiranek, V. Hydrogen sulfide and its roles in Saccharomyces cerevisiae in a winemaking context. FEMS Yeast Res. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Spiropoulos, A.; Tanaka, J.; Flerianos, I. Bisson, L.F. Characterization of Hydrogen Sulfide Formation in Commercial and Natural Wine Isolates of Saccharomyces. Am. J. Enol. Vitic. 2000, 51, 233–248. [Google Scholar]
- Hansen, J.; Kielland-Brandt, M.C. Inactivation of MET10 in Brewer’s Yeast Specifically Increases SO2 Formation during Beer Production. Nat. Biotechnol. 1996, 14, 1587–1591. [Google Scholar] [CrossRef]
- Cordente, A.G.; Heinrich, A.; Pretorius, I.S.; Swiegers, J.H. Isolation of sulfite reductase variants of a commercial wine yeast with significantly reduced hydrogen sulfide production. FEMS Yeast Res. 2009, 9, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Linderholm, A.; Dietzel, K.; Hirst, M.; Bisson, L.F. Identification of MET10-932 and characterization as an allele reducing hydrogen sulfide formation in wine strains of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2010, 76, 7699–7707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, H.; Edwards, C.G. Hydrogen sulphide production by Saccharomyces cerevisiae UCD 522 in a synthetic grape juice medium deficient of thiamin (vitamin B1) and/or pyridoxine (vitamin B6). Lett. Appl. Microbiol. 2019, 69, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Bohlscheid, J.C.; Fellman, J.K.; Wang, X.D.; Ansen, D.; Edwards, C.G. The influence of nitrogen and biotin interactions on the performance of Saccharomyces in alcoholic fermentations. J. Appl. Microbiol. 2007, 102, 390–400. [Google Scholar] [CrossRef]
- Wang, X.D.; Bohlscheid, J.C.; Edwards, C.G. Fermentative activity and production of volatile compounds by Saccharomyces grown in synthetic grape juice media deficient in assimilable nitrogen and/or pantothenic acid. J. Appl. Microbiol. 2003, 94, 349–359. [Google Scholar] [CrossRef]
- Lee, T.A.; Jorgensen, P.; Bognar, A.L.; Peyraud, C.; Thomas, D.; Tyers, M. Dissection of Combinatorial Control by the Met4 Transcriptional Complex. Mol. Biol. Cell 2010, 21, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Noble, J.; Sanchez, I.; Blondin, B. Identification of new Saccharomyces cerevisiae variants of the MET2 and SKP2 genes controlling the sulfur assimilation pathway and the production of undesirable sulfur compounds during alcoholic fermentation. Microb. Cell Factories 2015, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Ferreira, A.; Barbosa, C.; Falco, V.; Leão, C.; Mendes-Faia, A. The production of hydrogen sulphide and other aroma compounds by wine strains of Saccharomyces cerevisiae in synthetic media with different nitrogen concentrations. J. Ind. Microbiol. Biotechnol. 2009, 36, 571–583. [Google Scholar] [CrossRef]
- Patrignani, F.; Chinnici, F.; Serrazanetti, I.D.; Vernocchi, P.; Ndagijimana, M.; Riponi, C.; Lanciotti, R. Production of volatile and sulfur compounds by 10 Saccharomyces cerevisiae strains inoculated in trebbiano must. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Giudici, P.; Kunkee, R.E. The Effect of Nitrogen Deficiency and Sulfur-Containing Amino Acids on the Reduction of Sulfate to Hydrogen Sulfide by Wine Yeasts. Am. J. Enol. Vitic. 1994, 45, 107–112. [Google Scholar]
- Ugliano, M.; Kolouchova, R.; Henschke, P.A. Occurrence of hydrogen sulfide in wine and in fermentation: Influence of yeast strain and supplementation of yeast available nitrogen. J. Ind. Microbiol. Biotechnol. 2011, 38, 423–429. [Google Scholar] [CrossRef]
- Kumar, G.R.; Ramakrishnan, V.; Bisson, L.F. Survey of hydrogen sulfide production in wine strains of Saccharomyces cerevisiae Am. J. Enol. Vitic. 2010, 61, 365–371. [Google Scholar]
- Morgan, S.C.; Haggerty, J.J.; Johnston, B.; Jiranek, V.; Durall, D.M. Response to sulfur dioxide addition by two commercial Saccharomyces cerevisiae strains. Fermentation 2019, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Scafa, N.; Xu, L.-P.; Zhou, S.; Al-Ghanem, K.A.; Mahboob, S.; Fugetsu, B.; Zhang, X. Electrochemical hydrogen sulfide biosensors. Analyst 2016, 141, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compouds in wine. Basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef]
- Catalan, L.J.J.; Liang, V.; Jia, C.Q. Comparison of various detection limit estimates for volatile sulphur compounds by gas chromatography with pulsed flame photometric detection. J. Chromatogr. A 2006, 1136, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, D.; Kürbel, H.; MacNamara, K.; Grossmann, M. Headspace GC-SCD monitoring of low volatile sulfur compounds during fermentation and in wine. Analusis 1998, 26, 142–144. [Google Scholar] [CrossRef] [Green Version]
- Duan, N.; Yang, S.; Tian, H.; Sun, B. The recent advance of organic fluorescent probe rapid detection for common substances in beverages. Food Chem. 2021, 358, 129839. [Google Scholar] [CrossRef]
- Novo, M.; Bigey, F.; Beyne, E.; Galeote, V.; Gavory, F.; Mallet, S.; Cambon, B.; Legras, J.-L.; Wincker, P.; Casaregola, S.; et al. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc. Natl. Acad. Sci. USA 2009, 106, 16333–16338. [Google Scholar] [CrossRef] [Green Version]
- Marsit, S.; Mena, A.; Bigey, F.; Sauvage, F.-X.; Couloux, A.; Guy, J.; Legras, J.-L.; Barrio, E.; Dequin, S.; Galeote, V. Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Mol. Biol. Evol. 2015, 32, 1695–1707. [Google Scholar] [CrossRef] [Green Version]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of Alcoholic Fermentation Kinetics: Its Variability and Significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Wang, H.; Wang, J.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. Highly selective and rapidly responsive fluorescent probe for hydrogen sulfide detection in wine. Food Chem. 2018, 257, 150–154. [Google Scholar] [CrossRef] [PubMed]
- ICH. Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2014. [Google Scholar]
- Ferreira-Lima, N.; Vallverdú-Queralt, A.; Meudec, E.; Mazauric, J.P.; Sommerer, N.; Bordignon-Luiz, M.T.; Cheynier, V.; le Guernevé, C. Synthesis, Identification, and Structure Elucidation of Adducts Formed by Reactions of Hydroxycinnamic Acids with Glutathione or Cysteinylglycine. J. Nat. Prod. 2016, 79, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, B. AlgDesign: Algorithmic Experimental Design. 2011. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.304.838&rep=rep1&type=pdf (accessed on 4 February 2021).
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Alvarez, M.T.; Crespo, C.; Mattiasson, B. Precipitation of Zn(II), Cu(II) and Pb(II) at bench-scale using biogenic hydrogen sulfide from the utilization of volatile fatty acids. Chemosphere 2007, 66, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.J.A.; Gray, R.S.; Group, O.M. The Origin and Control of Hydrogen Sulfide During. Am. J. Enol. Vitic. 1979, 30, 187–197. [Google Scholar]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Regulation of hydrogen sulfide liberation in wine-producing Saccharomyces cerevisiae strains by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Gibney, P.; Cheng, L.; Liu, S.; Peck, G. Yeast Assimilable Nitrogen Concentrations Influence Yeast Gene Expression and Hydrogen Sulfide Production During Cider Fermentation. Front. Microbiol. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Ugliano, M.; Fedrizzi, B.; Siebert, T.; Travis, B.; Magno, F.; Versini, G.; Henschke, P.A. Effect of nitrogen supplementation and saccharomyces species on hydrogen sulfide and other volatile sulfur compounds in Shiraz fermentation and wine. J. Agric. Food Chem. 2009, 57, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortín, J.E.; Querol, A.; Puig, S.; Barrio, E. Molecular characterization of a chromosomal rearrangement involved in the adaptie evolution of yeast strains. Genome Res. 2002, 12, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, A.; Durand, C.; Loira, N.; Durrens, P.; Sherman, D.J.; Marullo, P. QTL Dissection of Lag Phase in Wine Fermentation Reveals a New Translocation Responsible for Saccharomyces cerevisiae Adaptation to Sulfite. PLoS ONE 2014, 9, e86298. [Google Scholar] [CrossRef] [Green Version]
- Nadai, C.; Treu, L.; Campanaro, S.; Giacomini, A.; Corich, V. Different mechanisms of resistance modulate sulfite tolerance in wine yeasts. Appl. Microbiol. Biotechnol. 2016, 100, 797–813. [Google Scholar] [CrossRef]
- García-Ríos, E.; Guillamón, J.M. Sulfur dioxide resistance in Saccharomyces cerevisiae: Beyond SSU1. Microb. Cell 2019, 6, 509–523. [Google Scholar] [CrossRef]
- Huang, C.; Roncoroni, M.; Gardner, R.C. MET2 affects production of hydrogen sulfide during wine fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 7125–7135. [Google Scholar] [CrossRef]
- Tezuka, H.; Mori, T.; Okumura, Y.; Kitabatake, K.; Tsumura, Y. Cloning of a Gene Suppressing Hydrogen Sulfide Production by Saccharomyces cerevisiae and Its Expression in a Brewing Yeast. J. Am. Soc. Brew. Chem. 1992, 50, 130–133. [Google Scholar] [CrossRef]
- Peter, J.; de Chiara, M.; Friedrich, A.; Yue, J.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef]
- Legras, J.-L.; Galeote, V.; Bigey, F.; Camarasa, C.; Marsit, S.; Nidelet, T.; Sanchez, I.; Couloux, A.; Guy, J.; Franco-Duarte, R.; et al. Adaptation of S. cerevisiae to fermented food environments reveals remarkable genome plasticity and the footprints of domestication. Mol. Biol. Evol. 2018, 35, 1712–1727. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-F.; Han, P.-J.; Wang, Q.-M.; Liu, W.-Q.; Shi, J.-Y.; Li, K.; Zhang, X.-L.; Bai, F.-Y. The origin and adaptive evolution of domesticated populations of yeast from Far East Asia. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Method | Characteristics | References |
|---|---|---|
| Lead Acetate Paper Strips | Sensitive, high throughput, qualitative | [22] |
| Electrochemical: H2S Sensors | Rapid, highly sensitive, simple, real-time, quantitative, one-by-one measurement * | [26] |
| GC-MS or GC Coupled with Sulfur Detectors (FPD, PFPD, SCD) | Real-time, quantitative, only headspace H2S content, one-by-one measurement * | [2,27,28,29] |
| Fluorescent Probes | Sensitive, easy to use, suitable for high throughput analyses, cannot be used directly on a gas (as H2S) | [30] |
| Response: H2S | ||||||
|---|---|---|---|---|---|---|
| Df | Sum Sq | Mean Sq | F value | Pr (>F) | ||
| strain | 2 | 4,829,394 | 2,414,697 | 108.4715 | < 2.2 × 10−16 | *** |
| N | 2 | 1,610,762 | 805,381 | 36.1788 | 2.24 × 10−10 | *** |
| SO2 | 2 | 293,462 | 146,731 | 6.5914 | 0.002917 | ** |
| strain:N | 4 | 1,873,705 | 468,426 | 21.0424 | 3.79 × 10−10 | *** |
| strain:SO2 | 4 | 252,459 | 63,115 | 2.8352 | 0.03415 | * |
| Residuals | 49 | 1,090,795 | 22,261 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Guidi, I.; Farines, V.; Legras, J.-L.; Blondin, B. Development of a New Assay for Measuring H2S Production during Alcoholic Fermentation: Application to the Evaluation of the Main Factors Impacting H2S Production by Three Saccharomycescerevisiae Wine Strains. Fermentation 2021, 7, 213. https://doi.org/10.3390/fermentation7040213
De Guidi I, Farines V, Legras J-L, Blondin B. Development of a New Assay for Measuring H2S Production during Alcoholic Fermentation: Application to the Evaluation of the Main Factors Impacting H2S Production by Three Saccharomycescerevisiae Wine Strains. Fermentation. 2021; 7(4):213. https://doi.org/10.3390/fermentation7040213
Chicago/Turabian StyleDe Guidi, Irene, Vincent Farines, Jean-Luc Legras, and Bruno Blondin. 2021. "Development of a New Assay for Measuring H2S Production during Alcoholic Fermentation: Application to the Evaluation of the Main Factors Impacting H2S Production by Three Saccharomycescerevisiae Wine Strains" Fermentation 7, no. 4: 213. https://doi.org/10.3390/fermentation7040213
APA StyleDe Guidi, I., Farines, V., Legras, J.-L., & Blondin, B. (2021). Development of a New Assay for Measuring H2S Production during Alcoholic Fermentation: Application to the Evaluation of the Main Factors Impacting H2S Production by Three Saccharomycescerevisiae Wine Strains. Fermentation, 7(4), 213. https://doi.org/10.3390/fermentation7040213







