Microstructural, Volatile Compounds, Microbiological and Organoleptical Characteristics of Low-Fat Buffalo Milk Yogurt Enriched with Whey Protein Concentrate and Ca-Caseinate during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Yogurt Starter
2.3. Yogurt Manufacture
2.4. Physicochemical Analysis
2.5. Microbiological Examination
2.6. Identification and Quantification of Volatile Compounds
2.7. Organoleptical Attributes
2.8. Water Holding Capacity
2.9. Microstructure Properties
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. Tyrosine Value
3.3. Volatile Compounds
3.3.1. Volatile Acids and Aldehyde Compounds
3.3.2. Volatile Ketone and Alcohol Compounds
3.3.3. Volatile Ester and Hydrocarbon Compounds
3.4. Microbiological Characteristics
3.5. Water Holding Capacity
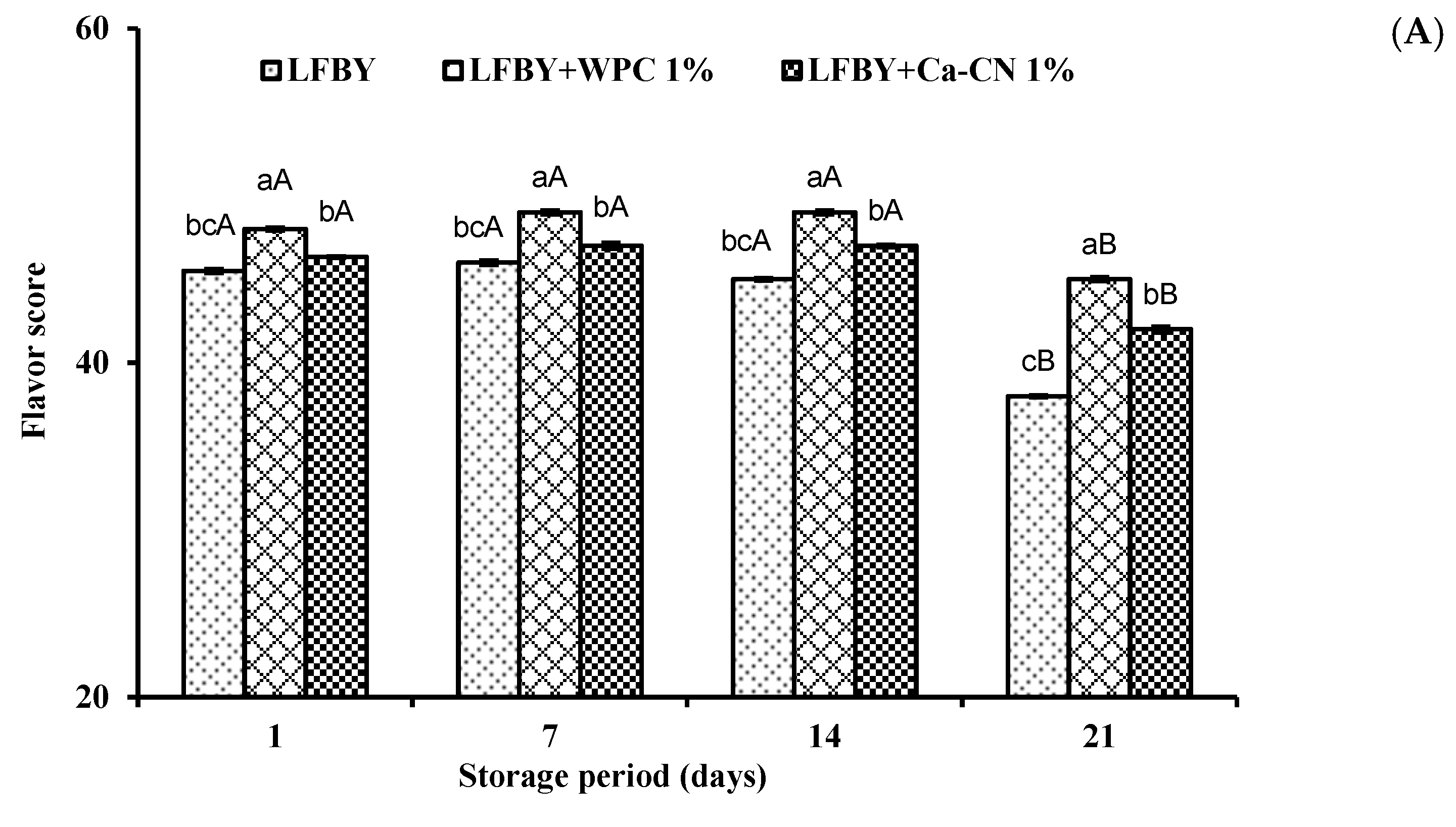
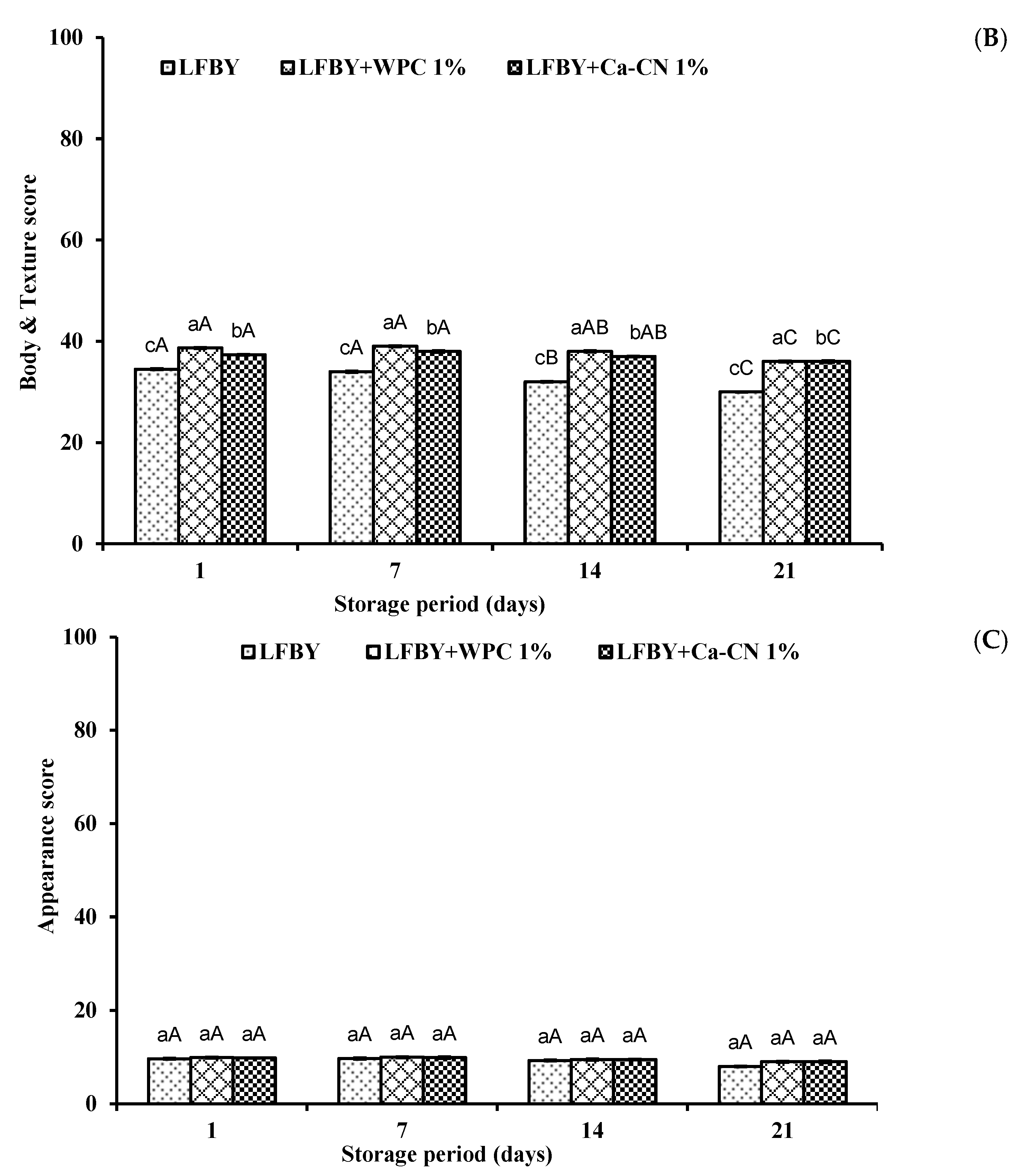
3.6. Organoleptical Attributes
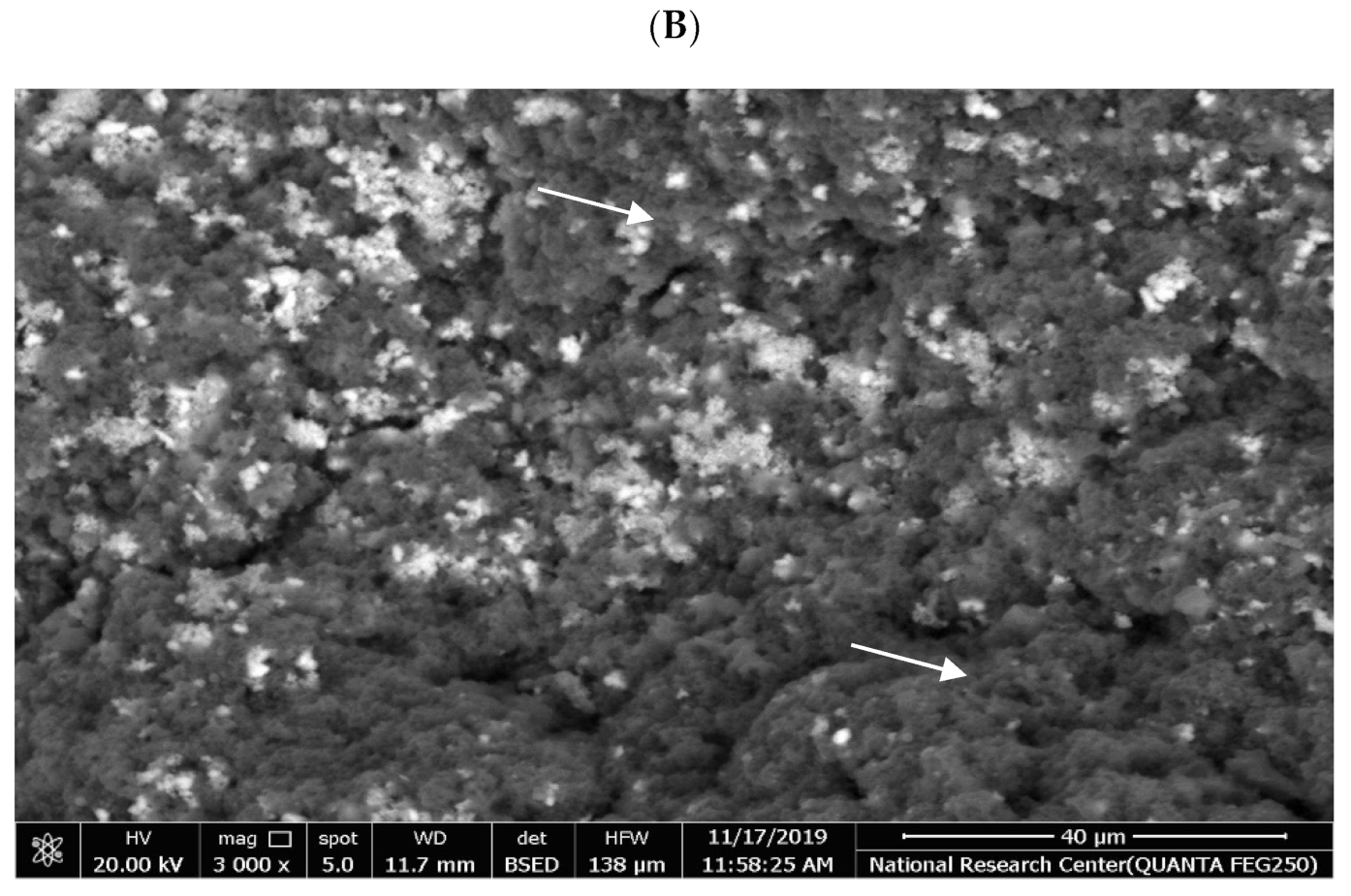
3.7. Microstructural Using Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kamal-Eldin, A.; Alhammadi, A.; Gharsallaoui, A.; Hamed, F.; Ghnimi, S. Physicochemical, rheological, and microstructural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020, 19, 26–33. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci. Technol. 2011, 91, 663. [Google Scholar] [CrossRef]
- Pelaes Vital, A.C.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, functional and rheological properties of low-fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT—Food Sci. Technol. 2015, 64, 1028–1035. [Google Scholar] [CrossRef]
- Atallah, A.A.; Morsy, O.M.; Gemiel, D.G. Characterization of functional low-fat yogurt enriched with whey protein concentrate, Ca-caseinate and spirulina. Int. J. Food Prop. 2020, 23, 1678–1691. [Google Scholar] [CrossRef]
- Boycheva, S.; Mihaylova, G.; Naydenova, N.; Dimitrov, T. Amino acid and fatty acid content of yogurt supplemented with walnut and hazelnut pieces. Trakia J. Sci. 2012, 10, 17–25. [Google Scholar]
- Sodini, I.; Remeuf, F.; Haddad, S.; Corrieu, G. The Relative Effect of Milk Base, Starter, and Process on Yogurt Texture: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 113–137. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Gee, V.L.; Hickey, D.K.; Kelly, A.L.; O’Mahony, J.A.; Fenelon, M.A. Effect of protein content on the physical stability and microstructure of a model infant formula. Int. Dairy J. 2013, 29, 53–59. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Saint-Eve, A.; Juteau, A.; Atlan, S.; Martin, N.; Souchon, I. Complex viscosity induced by protein composition variation influences the aroma release of flavored stirred yogurt. J. Agric. Food Chem. 2006, 54, 3997–4004. [Google Scholar] [CrossRef]
- Beshkova, D.; Simova, E.; Frengova, G.; Simov, Z. Production of flavour compounds by yogurt starter cultures. J. Ind. Microbiol. Biotechnol. 1998, 20, 180–186. [Google Scholar] [CrossRef]
- Ott, A.; Fay, L.B.; Chaintreau, A. Determination and Origin of the Aroma Impact Compounds of Yogurt Flavor. J. Agric. Food Chem. 1997, 45, 850–858. [Google Scholar] [CrossRef]
- Karagül-Yüceer, Y.; Cadwallader, K.R.; Drake, M. Volatile Flavor Components of Stored Nonfat Dry Milk. J. Agric. Food Chem. 2002, 50, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mistry, V.V.; Hassan, H.N. Manufacture of nonfat yogurt from a high milk protein powder. J. Dairy Sci. 1992, 75, 947–957. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; Nout, M.J.R.; Antunes Fernandes, E.C.; Hettinga, K.A.; Vervoort, J.M.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J.; van Valenberg, H.J.F. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014, 177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. Volatile flavor compounds in yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Kaneko, D.; Igarashi, T.; Aoyama, K. Reduction of the off-flavor volatile generated by the yogurt starter culture including Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus in soymilk. J. Agric. Food Chem. 2014, 62, 1658–1663. [Google Scholar] [CrossRef]
- Isleten, M.; Karagul-Yuceer, Y. Effects of dried dairy ingredients on physical and sensory properties of nonfat yogurt. J. Dairy Sci. 2006, 89, 2865–2872. [Google Scholar] [CrossRef]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.; Deeth, H. Yogurt: Technology and Biochemistry. J. Food Prot. 1980, 43, 939–977. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, X.L.; Tian, Q.; Mao, X.Y. Effect of casein to whey protein ratios on the protein interactions and coagulation properties of low-fat yogurt. J. Dairy Sci. 2016, 99, 7768–7775. [Google Scholar] [CrossRef] [PubMed]
- Jaya, S. Microstructure analysis of dried yogurt: Effect of different drying methods. Int. J. Food Prop. 2009, 12, 469–481. [Google Scholar] [CrossRef]
- Marafon, A.P.; Sumi, A.; Granato, D.; Alcântara, M.R.; Tamime, A.Y.; Nogueira de Oliveira, M. Effects of partially replacing skimmed milk powder with dairy ingredients on rheology, sensory profiling, and microstructure of probiotic stirred-type yogurt during cold storage. J. Dairy Sci. 2011, 94, 5330–5340. [Google Scholar] [CrossRef]
- Remeuf, F.; Mohammed, S.; Sodini, I.; Tissier, J.P. Preliminary observations on the effects of milk fortification and heating on microstructure and physical properties of stirred yogurt. Int. Dairy J. 2003, 13, 773–782. [Google Scholar] [CrossRef]
- Damin, M.R.; Alcântara, M.R.; Nunes, A.P.; Oliveira, M.N. Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt. LWT—Food Sci. Technol. 2009, 42, 1744–1750. [Google Scholar] [CrossRef]
- Delikanli, B.; Ozcan, T. Improving the textural properties of yogurt fortified with milk proteins. J. Food Process. Preserv. 2017, 41, e13101. [Google Scholar] [CrossRef]
- IDF. 4A Cheese and Processed Cheese Determination of the Total Solids Content; IDF: Brussels, Belgium, 1982. [Google Scholar]
- IDF. 20A, Milk, Determination of Nitrogen Content (Kjeldahl Method) and Calculation of Crude Protein Content; IDF: Brussels, Belgium, 1986. [Google Scholar]
- IDF. 152A. Milk and Milk Products: Determination of Fat Content; General Guidance on the Use of Butyrometric Methods; IDF: Brussels, Belgium, 1997. [Google Scholar]
- Hull, M.E. Studies on milk proteins. 2. Colonmetric determination of the partial hydrolysis of the proteins in milk. J. Dairy Sci. 1947, 30, 881–884. [Google Scholar] [CrossRef]
- Ryan, M.P.; Rea, M.C.; Hill, C.; Ross, R.P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 1996, 62, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehr, H.M.; Frank, J.F. Standard Methods for the Examination of Dairy Products; American Public Health Association: Washington, DC, USA, 2004. [Google Scholar]
- IDF. International IDF Standard 94B: Milk & Milk Products. Enumeration of Yeasts & Moulds. Colony Count Technique at 25 Degrees C; IDF: Brussels, Belgium, 1990. [Google Scholar]
- Ning, L.; Fu-Ping, Z.; Hai-Tao, C.; Si-Yuan, L.; Chen, G.; Zhen-Yang, S.; Bao-Guo, S. Identification of volatile components in Chinese Sinkiang fermented camel milk using SAFE, SDE, and HS-SPME-GC/MS. Food Chem. 2011, 129, 1242–1252. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehanna, N.; Saleh, T.M.; Mehanna, A.; El-Asfory, S. The quality of low-calorie buffalo zabady. Egypt. J. Dairy Sci. 2000, 28, 59–71. [Google Scholar]
- Guzmán-González, M.; Morais, F.; Ramos, M.; Amigo, L. Influence of skimmed milk concentrate replacement by dry dairy products in a low fat set-type yoghurt model system. I: Use of whey protein concentrates, milk protein concentrates and skimmed milk powder. J. Sci. Food Agric. 1999, 79, 1117–1122. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill College: New York, NY, USA, 1997. [Google Scholar]
- Lee, W.J.; Lucey, J.A. Formation and physical properties of yogurt. Asian-Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Asperger, H. Applicability of analytical methods for the assessment of yogurt quality. Dairy Sci. Abstr. 1977, 39, 73. [Google Scholar]
- Slocum, S.A.; Jasinski, E.M.; Kilara, A. Processing Variables Affecting Proteolysis in Yogurt during Incubation. J. Dairy Sci. 1988, 71, 596–603. [Google Scholar] [CrossRef]
- Chandan, R.C.; Argyle, P.J.; Mathison, G.E. Action of Lactobacillus bulgaricus proteinase preparations on milk proteins. J. Dairy Sci. 1982, 65, 1408–1413. [Google Scholar] [CrossRef]
- Sindayikengera, S.; Xia, W.S. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J. Zhejiang Univ. Sci. B 2006, 7, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanier, A.M.; Shahidi, F.; Parliment, T.H.; Mussinan, C.; Ho, C.T.; Contis, E.T. Food Flavors and Chemistry: Advances of the New Millennium; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Ongol, M.P.; Sawatari, Y.; Ebina, Y.; Sone, T.; Tanaka, M.; Tomita, F.; Yokota, A.; Asano, K. Yoghurt fermented by Lactobacillus delbrueckii subsp. bulgaricus H+-ATPase-defective mutants exhibits enhanced viability of Bifidobacterium breve during storage. Int. J. Food Microbiol. 2007, 116, 358–366. [Google Scholar] [CrossRef]
- Pereda, J.; Jaramillo, D.P.; Quevedo, J.M.; Ferragut, V.; Guamis, B.; Trujillo, A.J. Characterization of volatile compounds in ultra-high-pressure homogenized milk. Int. Dairy J. 2008, 18, 826–834. [Google Scholar] [CrossRef]
- Pogačić, T.; Maillard, M.-B.; Leclerc, A.; Hervé, C.; Chuat, V.; Valence, F.; Thierry, A. Lactobacillus and Leuconostoc volatilomes in cheese conditions. Appl. Microbiol. Biotechnol. 2016, 100, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Díaz, M.P.; Tejero, J.M.; Mendoza-Garcia, P.G.; García, H.S.; Salgado-Cervantes, M.A. Spray Drying Yogurt Incorporating Hydrocolloids: Structural Analysis, Acetaldehyde Content, Viable Bacteria, and Rheological Properties. Food Bioprocess Technol. 2012, 5, 560–567. [Google Scholar] [CrossRef]
- Routray, W.; Mishra, H.N. Scientific and Technical Aspects of Yogurt Aroma and Taste: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Gaafar, A.M. Volatile flavour compounds of yoghurt. Int. J. Food Sci. Technol. 1992, 27, 87–91. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Siek, T.J.; Albin, I.A.; Sather, L.A.; Lindsay, R.C. Taste thresholds of butter volatiles in deodorized butteroil medium. J. Food Sci. 1969, 34, 265-265. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt: Science and Technology, 3rd ed.; CRC Press: Cambridge, UK, 2007. [Google Scholar]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC/Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Hruškar, M.; Vahčić, N.; Ritz, M. Aroma profiles and sensory evaluation of yogurt during storage. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 1995, 45, 175–190. [Google Scholar]
- Chaves, A.C.S.D.; Fernandez, M.; Lerayer, A.L.S.; Mierau, I.; Kleerebezem, M.; Hugenholtz, J. Metabolic Engineering of Acetaldehyde Production by Streptococcus thermophilus. Appl. Environ. Microbiol. 2002, 68, 5656–5662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, V.M.; Cole, W.M. Threonine aldolase and alcohol dehydogenase activities in Lactobacillus bulgaricus and Latobacillus acidophilus and their contribution to flavour production in fermented milks. J. Dairy Res. 1983, 50, 375–379. [Google Scholar] [CrossRef]
- Raya, R.; Manca de Nadra, M.; de Ruiz Holgado, A.P.; Oliver, G. Acetaldehyde metabolismm in lactic acid bacteria. Milchwissenschaft 1986, 41, 397–399. [Google Scholar]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624. [Google Scholar] [CrossRef] [Green Version]
- Beshkova, D.M.; Simova, E.D.; Frengova, G.I.; Simov, Z.I.; Dimitrov, Z.P. Production of volatile aroma compounds by kefir starter cultures. Int. Dairy J. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Nieto-Arribas, P.; Seseña, S.; Poveda, J.M.; Chicón, R.; Cabezas, L.; Palop, L. Enterococcus populations in artisanal Manchego cheese: Biodiversity, technological and safety aspects. Food Microbiol. 2011, 28, 891–899. [Google Scholar] [CrossRef]
- De Vos, W.M.; Hugenholtz, J. Engineering metabolic highways in Lactococci and other lactic acid bacteria. Trends Biotechnol. 2004, 22, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S. Effect of volatile flavor compound on yogurt during refrigerated storage. Korean J. Food Sci. Technol. 1995, 27, 939–943. [Google Scholar]
- Gursel, A.; Gursoy, A.; Anli, E.A.K.; Budak, S.O.; Aydemir, S.; Durlu-Ozkaya, F. Role of milk protein–based products in some quality attributes of goat milk yogurt. J. Dairy Sci. 2016, 99, 2694–2703. [Google Scholar] [CrossRef] [Green Version]
- Guler, M.O.; Stupp, S.I. A Self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc. 2007, 129, 12082–12083. [Google Scholar] [CrossRef]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Conte, F. Solid-phase microextraction and gas chromatography mass spectrometry analysis of dairy product volatiles for the determination of shelf-life. Int. Dairy J. 2008, 18, 819–825. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Mccomas, K.A., Jr.; Gilliland, S.E. Growth of probiotic and traditional yogurt cultures in milk supplemented with whey protein hydrolysate. J. Food Sci. 2003, 68, 2090–2095. [Google Scholar] [CrossRef]
- Hashim, M.A.; Nadtochii, L.A.; Muradova, M.B.; Proskura, A.V.; Alsaleem, K.A.; Hammam, A.R.A. Nonfat yogurt fortified with whey protein isolate: Physicochemical, rheological, and microstructural properties. Foods 2021, 10, 1762. [Google Scholar] [CrossRef]
- Ott, A.; Hugi, A.; Baumgartner, M.; Chaintreau, A. Sensory investigation of yogurt flavor perception: Mutual influence of volatiles and acidity. J. Agric. Food Chem. 2000, 48, 441–450. [Google Scholar] [CrossRef]
- Matumoto-Pintro, P.T.; Rabiey, L.; Robitaille, G.; Britten, M. Use of modified whey protein in yoghurt formulations. Int. Dairy J. 2011, 21, 21–26. [Google Scholar] [CrossRef]
- Puvanenthiran, A.; Williams, R.P.W.; Augustin, M.A. Structure and visco-elastic properties of set yoghurt with altered casein to whey protein ratios. Int. Dairy J. 2002, 12, 383–391. [Google Scholar] [CrossRef]
- Krzeminski, A.; Großhable, K.; Hinrichs, J. Structural properties of stirred yoghurt as influenced by whey proteins. LWT—Food Sci. Technol. 2011, 44, 2134–2140. [Google Scholar] [CrossRef]
- Singh, B.R.; Myhr, K. Cadmium uptake by barley as affected by Cd sources and pH levels. Geoderma 1998, 84, 185–194. [Google Scholar] [CrossRef]
- Smits, P.; Brouwershaven, J.H.V. Heat-induced association of β-lactoglobulin and casein mi-celles. J. Dairy Res. 1980, 47, 313–325. [Google Scholar] [CrossRef]
- Haque, Z.; Kinsella, J.E. Interaction between heated κ-casein and β-lactoglobulin: Predominance of hydrophobic interactions in the initial stages of complex formation. J. Dairy Res. 1988, 55, 67–78. [Google Scholar] [CrossRef]





| Parameters | Treatment | Storage Period (Day) | |||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | ||
| Total solid (%) | LFBY | 13.03 ± 0.16 bA | 13.04 ± 0.10 cA | 13.05 ± 0.20 cA | 13.03 ± 0.17 cA |
| LFBY + 1% WPC | 13.97 ± 0.37 aB | 13.98 ± 0.29 aAB | 14.00 ± 0.32 aA | 14.00 ± 0.33 aA | |
| LFBY + 1% Ca-CN | 13.97 ± 0.27 aA | 13.96 ± 0.33 bA | 13.97 ± 0.32 bA | 13.98 ± 0.28 bA | |
| Protein (%) | LFBY | 3.98 ± 0.05 cAB | 3.98 ± 0.03 bAB | 3.99 ± 0.04 cA | 3.97 ± 0.04 cB |
| LFBY + 1% WPC | 4.41 ± 0.10 bA | 4.42 ± 0.08 aA | 4.42 ± 0.10 bA | 4.41 ± 0.09 bA | |
| LFBY + 1% Ca-CN | 4.44 ± 0.08 aA | 4.43 ± 0.07 aA | 4.44 ± 0.08 aA | 4.44 ± 0.06 aA | |
| Fat (%) | LFBY | 1.47 ± 0.03 aAB | 1.48 ± 0.02 aA | 1.47 ± 0.03 aAB | 1.46 ± 0.02 aB |
| LFBY + 1% WPC | 1.46 ± 0.05 aA | 1.46 ± 0.03 bA | 1.47 ± 0.02 aA | 1.47 ± 0.01 aA | |
| LFBY + 1% Ca-CN | 1.47 ± 0.03 aA | 1.47 ± 0.02 a bA | 1.46 ± 0.02 aA | 1.46 ± 0.00 aA | |
| Titratable acidity * (%) | LFBY | 0.71 ± 0.01 aA | 0.85 ± 0.02 aB | 0.94 ± 0.00 aC | 1.02 ± 0.03 aD |
| LFBY + 1% WPC | 0.72 ± 0.00 aA | 0.76 ± 0.01 bB | 0.85 ± 0.01 bC | 0.93 ± 0.02 bD | |
| LFBY + 1% Ca-CN | 0.70 ± 0.01 aA | 0.78 ± 0.02 bB | 0.86 ± 0.02 bC | 0.95 ± 0.03 bD | |
| pH value | LFBY | 4.54 ± 0.16 aA | 4.00 ± 0.15 a bB | 3.69 ± 0.13 bC | 3.21 ± 0.18 bD |
| LFBY + 1% WPC | 4.55 ± 0.21 aA | 4.28 ± 0.12 aAB | 4.09 ± 0.21 aB | 3.97 ± 0.15 aB | |
| LFBY + 1% Ca-CN | 4.54 ± 0.16 aA | 4.26 ± 0.15 aAB | 4.10 ± 0.13 aB | 3.92 ± 0.18 aB | |
| Treatments | Storage Periods (Days) | |||
|---|---|---|---|---|
| 1 | 7 | 14 | 21 | |
| LFBY | 0.212 ± 0.01 bD | 0.307 ± 0.00 cC | 0.335 ± 0.00 cA | 0.300 ± 0.00 cB |
| LFBY + 1% WPC | 0.209 ± 0.00 bD | 0.322 ± 0.00 bC | 0.363 ± 0.01 bA | 0.354 ± 0.00 bB |
| LFBY + 1% Ca-CN | 0.287 ± 0.01 aD | 0.358 ± 0.01 aC | 0.387 ± 0.00 aA | 0.379 ± 0.01 aB |
| Volatile Compounds | RT 1 (min) | Storage (Days) | Treatments | Method 2 | RI 3 | Formula | ||
|---|---|---|---|---|---|---|---|---|
| LFBY | LFBY + 1% WPC | LFBY + 1% Ca-CN | ||||||
| Acid compounds (mg 100 g−1) | ||||||||
| Acetic acid | 3.56 | 1 | 6.22 ± 0.01 cB | 7.99 ± 0.09 aB | 7.30 ± 0.05 bB | MS 4, STD 5 | STD | C2H4O2 |
| 21 | 16.23 ± 0.00 cA | 20.18 ± 0.08 aA | 18.10 ± 0.21 bA | |||||
| Butanoic acid | 5.57 | 1 | 20.89 ± 0.02 aA | 20.94 ± 0.11 aA | 20.90 ± 0.11 aA | MS, RI | 790.66 | C4H8O2 |
| 21 | ND 6 | ND | ND | |||||
| Pentanoic acid | 9.14 | 1 | 0.97 ± 0.03 aA | 0.99 ± 0.01 aA | 0.98 ± 0.01 aA | MS, RI | 868.78 | C5H10O2 |
| 21 | ND | ND | ND | |||||
| Hexanoic acid | 14.18 | 1 | 5.02 ± 0.04 aB | 4.99 ± 0.06 aB | 5.00 ± 0.07 aB | MS, RI | 999.24 | C6H12O2 |
| 21 | 9.23 ± 0.08 cA | 11.12 ± 0.07 aA | 10.11 ± 0.09 bA | |||||
| Heptanoic acid | 17.12 | 1 | 0.21 ± 0.07 aB | 0.24 ± 0.00 aB | 0.24 ± 0.01 aB | MS, RI | 1071.70 | C7H14O2 |
| 21 | 1.16 ± 0.05 cA | 2.89 ± 0.04 aA | 1.98 ± 0.04 bA | |||||
| Benzoic acid | 19.61 | 1 | 1.02 ± 0.01 aA | 1.07 ± 0.00 aA | 1.02 ± 0.01 aA | MS, RI | 1159.11 | C7H6O2 |
| 21 | ND | ND | ND | |||||
| Octanoic acid | 19.98 | 1 | 1.94 ± 0.07 aB | 2.05 ± 0.08 aB | 1.89 ± 0.07 aB | MS, RI | 1177.38 | C8H16O2 |
| 21 | 2.38 ± 0.06 aA | 2.41 ± 0.04 aA | 2.40 ± 0.06 aA | |||||
| n-Decanoic acid | 24.29 | 1 | 0.35 ± 0.01 aB | 0.32 ± 0.01 aB | 0.40 ± 0.01 aB | MS, RI | 1341.01 | C10H20O2 |
| 21 | 1.98 ± 0.04 aA | 2.01 ± 0.07 aA | 1.97 ± 0.00 aA | |||||
| Aldehyde compounds (mg 100 g−1) | ||||||||
| Acetaldehyde | 1.38 | 1 | 20.12 ± 0.03 bA | 21.83 ± 0.20 aA | 20.08 ± 0.21 bA | MS, STD | STD | C2H4O |
| 21 | ND | ND | ND | |||||
| Pentanal | 3.25 | 1 | 2.30 ± 0.04 bA | 3.99 ± 0.07 aA | 2.07 ± 0.05 cA | MS, RI | 664.98 | C5H10O |
| 21 | ND | ND | ND | |||||
| Hexanal | 6.45 | 1 | 1.31 ± 0.06 cA | 3.08 ± 0.01 aA | 2.41 ± 0.09 bA | MS, RI | 798.84 | C6H12O |
| 21 | 0.85 ± 0.07 aB | 0.91 ± 0.01 aB | 0.89 ± 0.01 aB | |||||
| Heptanal | 9.98 | 1 | 0.33 ± 0.07 aB | 0.41 ± 0.07 aB | 0.39 ± 0.02 aB | MS, RI | 889.78 | C7H14O |
| 21 | 1.61 ± 0.01 cA | 3.69 ± 0.01 aA | 2.44 ± 0.03 bA | |||||
| Benzaldehyde | 12.47 | 1 | 0.40 ± 0.00 cA | 1.98 ± 0.03 aA | 1.20 ± 0.00 bA | MS, RI | 977.14 | C7H6O |
| 21 | ND | ND | ND | |||||
| 2-Methylundecanal | 16.97 | 1 | 3.12 ± 0.04 cA | 5.45 ± 0.08 aA | 4.02 ± 0.08 bA | MS, RI | 1085.05 | C12H24O |
| 21 | ND | ND | ND | |||||
| Nonanal | 17.86 | 1 | 0.81 ± 0.04 aA | 0.86 ± 0.00 aA | 0.87 ± 0.04 aA | MS, RI | 1114.01 | C9H18O |
| 21 | 0.21 ± 0.01 aB | 0.20 ± 0.05 aB | 0.20 ± 0.03 aB | |||||
| Volatile Compounds | RT 1 (min) | Storage (Days) | Treatments | Method 2 | RI 3 | Formula | ||
|---|---|---|---|---|---|---|---|---|
| LFBY | LFBY + 1% WPC | LFBY + 1% Ca-CN | ||||||
| Ketone compounds (mg 100 g−1) | ||||||||
| 2,3-Butanedione | 1.99 | 1 | ND 4 | ND | ND | MS 5, STD 6 | STD | C4H6O2 |
| 21 | 10.23 ± 0.09 cA | 13.36 ± 0.31 aA | 12.19 ± 0.04 bA | |||||
| 2-Pentanone | 3.05 | 1 | 1.26 ± 0.04 cA | 3.29 ± 0.08 aA | 2.25 ± 0.07 bA | MS, RI | 657.44 | C5H10O |
| 21 | 0.22 ± 0.02 cB | 1.29 ± 0.03 aB | 0.71 ± 0.01 bB | |||||
| 3-Methyl-2-butanone | 3.08 | 1 | 1.99 ± 0.06 aA | 2.04 ± 0.04 aA | 2.00 ± 0.00 aA | MS, RI | 659.01 | C5H10O |
| 21 | ND | ND | ND | |||||
| Acetoin | 4.01 | 1 | 3.51 ± 0.07 cA | 5.52 ± 0.06 aA | 4.45 ± 0.08 bA | MS, RI | 700.04 | C4H8O2 |
| 21 | ND | ND | ND | |||||
| 4-Methyl-2-hexanone | 9.45 | 1 | 1.21 ± 0.00 cA | 2.69 ± 0.04 aA | 1.90 ± 0.03 bA | MS, RI | 899.98 | C7H14O |
| 21 | 0.23 ± 0.02 cB | 1.48 ± 0.06 aB | 0.98 ± 0.01 bB | |||||
| 2-Heptanone | 10.05 | 1 | 18.22 ± 0.21 cB | 20.03 ± 0.21 aB | 19.19 ± 0.22 bB | MS, RI | 912.07 | C7H14O |
| 21 | 25.23 ± 0.35 cA | 29.32 ± 0.32 aA | 27.19 ± 0.27 bA | |||||
| Acetophenone | 15.84 | 1 | 0.29 ± 0.04 aA | 0.30 ± 0.01 aA | 0.28 ± 0.03 aA | MS, RI | 1057.77 | C8H8O |
| 21 | ND | ND | ND | |||||
| 2-Nonanone | 17.04 | 1 | 7.01 ± 0.08 aB | 6.99 ± 0.08 aB | 6.97 ± 0.09 aB | MS, RI | 1108.49 | C9H18O |
| 21 | 10.02 ± 0.07 aA | 10.07 ± 0.11 aA | 10.02 ± 0.12 aA | |||||
| 2-Undecanone | 22.87 | 1 | 0.91 ± 0.01 aA | 0.91 ± 0.03 aA | 0.94 ± 0.03 aA | MS, RI | 1321.02 | C11H22O |
| 21 | 0.32 ± 0.01 aB | 0.30 ± 0.02 aB | 0.29 ± 0.02 aB | |||||
| Alcohol compounds (mg 100 g−1) | ||||||||
| Ethanol | 1.28 | 1 | 4.23 ± 0.07 bB | 5.98 ± 0.09 aB | 5.19 ± 0.21 bB | MS, STD | STD | C2H6O |
| 21 | 5.01 ± 0.09 bA | 6.98 ± 0.11 aA | 6.03 ± 0.11 bA | |||||
| 3-Methyl-2-pentanol | 4.45 | 1 | ND | ND | ND | MS, RI | 777.2 | C6H14O |
| 21 | 0.32 ± 0.00 bA | 0.89 ± 0.03 aA | 0.30 ± 0.01 bA | |||||
| 1-Hexanol | 9.65 | 1 | 0.81 ± 0.03 bB | 1.52 ± 0.04 aB | 1.50 ± 0.07 aB | MS, RI | 868.98 | C6H14O |
| 21 | 1.32 ± 0.07 bA | 2.34 ± 0.06 aA | 2.30 ± 0.04 aA | |||||
| 1-Heptanol | 13.35 | 1 | 1.00 ± 0.02 bA | 1.72 ± 0.08 aA | 1.71 ± 0.07 aA | MS, RI | 988.45 | C7H16O |
| 21 | 0.22 ± 0.02 bB | 0.95 ± 0.03 aB | 0.91 ± 0.01 aB | |||||
| 2-Nonanol | 17.74 | 1 | 0.31 ± 0.04 aB | 0.33 ± 0.07 aB | 0.30 ± 0.02 aB | MS, RI | 1152.04 | C9H20O |
| 21 | 1.02 ± 0.07 aA | 0.97 ± 0.02 aA | 1.00 ± 0.04 aA | |||||
| Volatile Compounds | RT 1 (min) | Storage (Days) | Treatments | Method 2 | RI 3 | Formula | ||
|---|---|---|---|---|---|---|---|---|
| LFBY | LFBY + 1% WPC | LFBY + 1% Ca-CN | ||||||
| Ester compounds (mg 100 g−1) | ||||||||
| Formic acid ethenyl ester | 1.55 | 1 | ND 4 | ND | ND | MS 5, STD 6 | STD | C3H4O2 |
| 21 | 8.54 ± 0.09 cA | 11.03 ± 0.11 aA | 9.31 ± 0.08 bA | |||||
| Acetic acid ethenyl ester | 1.99 | 1 | 8.02 ± 0.11 aA | 7.98 ± 0.01 aA | 7.97 ± 0.05 aA | MS, STD | STD | C4H6O2 |
| 21 | 0.78 ± 0.03 aB | 0.77 ± 0.04 aB | 0.81 ± 0.02 aB | |||||
| δ-Nonalactone | 29.11 | 1 | 0.32 ± 0.04 aA | 0.30 ± 0.02 aA | 0.29 ± 0.04 aA | MS, RI | 1489.59 | C9H16O2 |
| 21 | ND | ND | ND | |||||
| Hydrocarbon compounds (mg 100 g−1) | ||||||||
| Heptane | 3.32 | 1 | ND | ND | ND | MS, RI | 687.88 | C7H15Cl |
| 21 | ND | ND | ND | |||||
| Toluene | 5.55 | 1 | 0.45 ± 0.05 aA | 0.39 ± 0.00 aA | 0.41 ± 0.04 aA | MS, RI | 789.02 | C7H8 |
| 21 | ND | ND | ND | |||||
| o-Xylene | 9.39 | 1 | 0.31 ± 0.02 aA | 0.34 ± 0.00 aA | 0.30 ± 0.03 aA | MS, RI | 899.22 | C8H10 |
| 21 | ND | ND | ND | |||||
| p-Xylene | 9.41 | 1 | 0.27 ± 0.06 aB | 0.25 ± 0.01 aB | 0.29 ± 0.01 aB | MS, RI | 899.25 | C8H10 |
| 21 | 0.71 ± 0.07 aA | 0.85 ± 0.00 aA | 0.88 ± 0.00 aA | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Mohamed, A.; Gemiel, D.G.; Atallah, A.A. Microstructural, Volatile Compounds, Microbiological and Organoleptical Characteristics of Low-Fat Buffalo Milk Yogurt Enriched with Whey Protein Concentrate and Ca-Caseinate during Cold Storage. Fermentation 2021, 7, 250. https://doi.org/10.3390/fermentation7040250
Barakat H, Mohamed A, Gemiel DG, Atallah AA. Microstructural, Volatile Compounds, Microbiological and Organoleptical Characteristics of Low-Fat Buffalo Milk Yogurt Enriched with Whey Protein Concentrate and Ca-Caseinate during Cold Storage. Fermentation. 2021; 7(4):250. https://doi.org/10.3390/fermentation7040250
Chicago/Turabian StyleBarakat, Hassan, Ahmed Mohamed, Dalia G. Gemiel, and Atallah A. Atallah. 2021. "Microstructural, Volatile Compounds, Microbiological and Organoleptical Characteristics of Low-Fat Buffalo Milk Yogurt Enriched with Whey Protein Concentrate and Ca-Caseinate during Cold Storage" Fermentation 7, no. 4: 250. https://doi.org/10.3390/fermentation7040250
APA StyleBarakat, H., Mohamed, A., Gemiel, D. G., & Atallah, A. A. (2021). Microstructural, Volatile Compounds, Microbiological and Organoleptical Characteristics of Low-Fat Buffalo Milk Yogurt Enriched with Whey Protein Concentrate and Ca-Caseinate during Cold Storage. Fermentation, 7(4), 250. https://doi.org/10.3390/fermentation7040250







