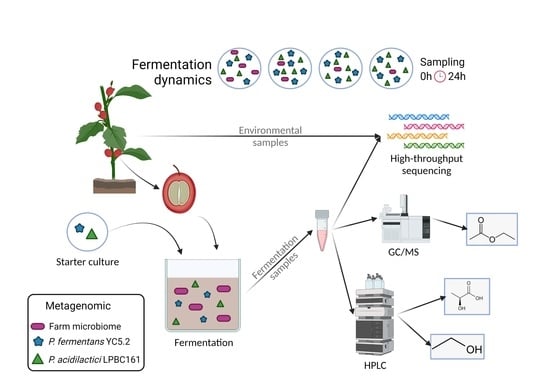
Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation
Abstract
:1. Introduction
2. Material and Methods
2.1. Cultivation of Lactic Acid Bacteria and Yeast
2.2. Area of Study and Sampling Procedure
2.3. Microbial Community Analysis by High-Throughput Sequencing
2.4. HPLC Analysis of Fermenting Coffee Pulp
2.5. GC/MS Analysis of Coffee Pulp and Beans
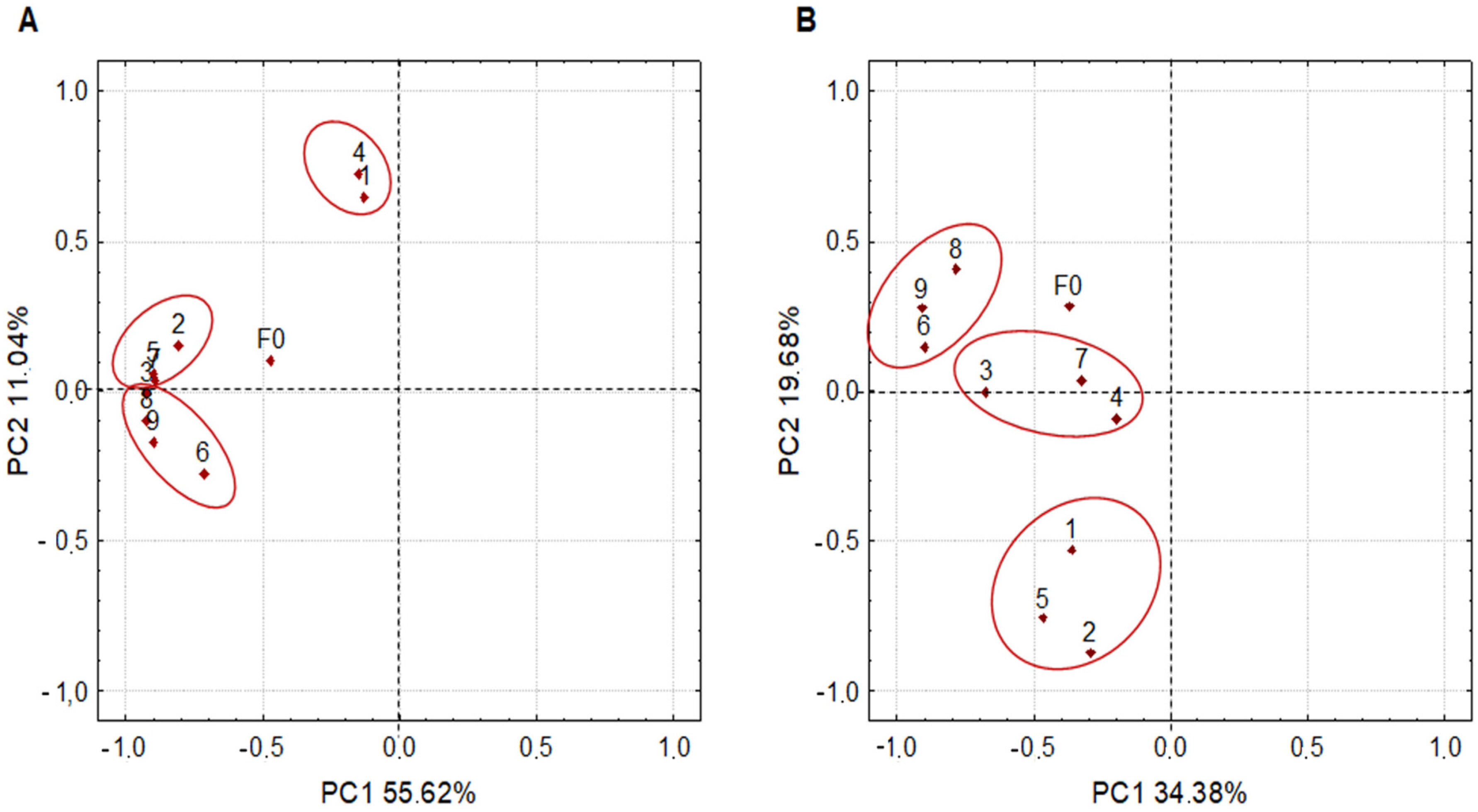
2.6. Statistical Analysis
3. Results and Discussion
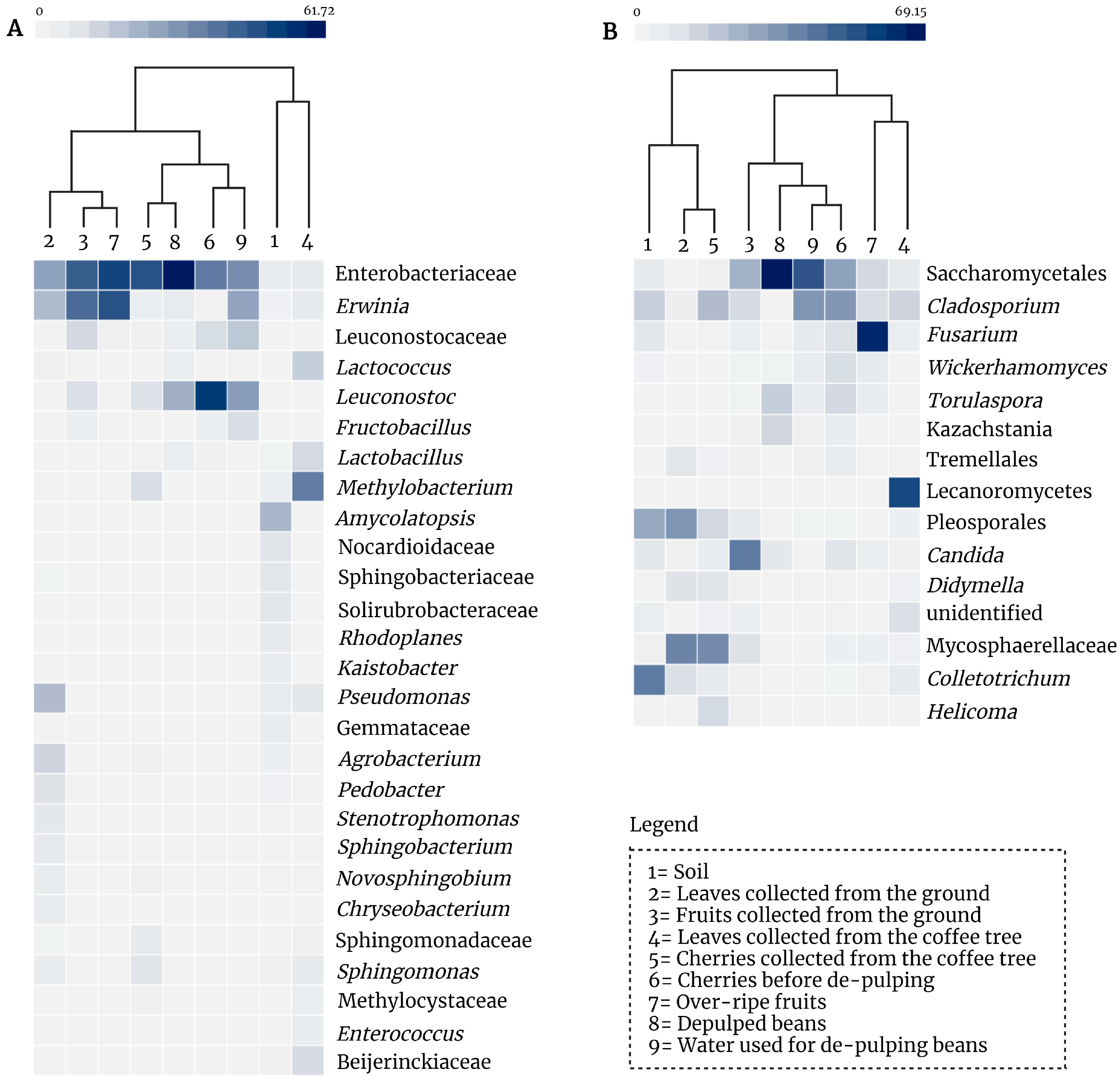
3.1. Farm Microbiome
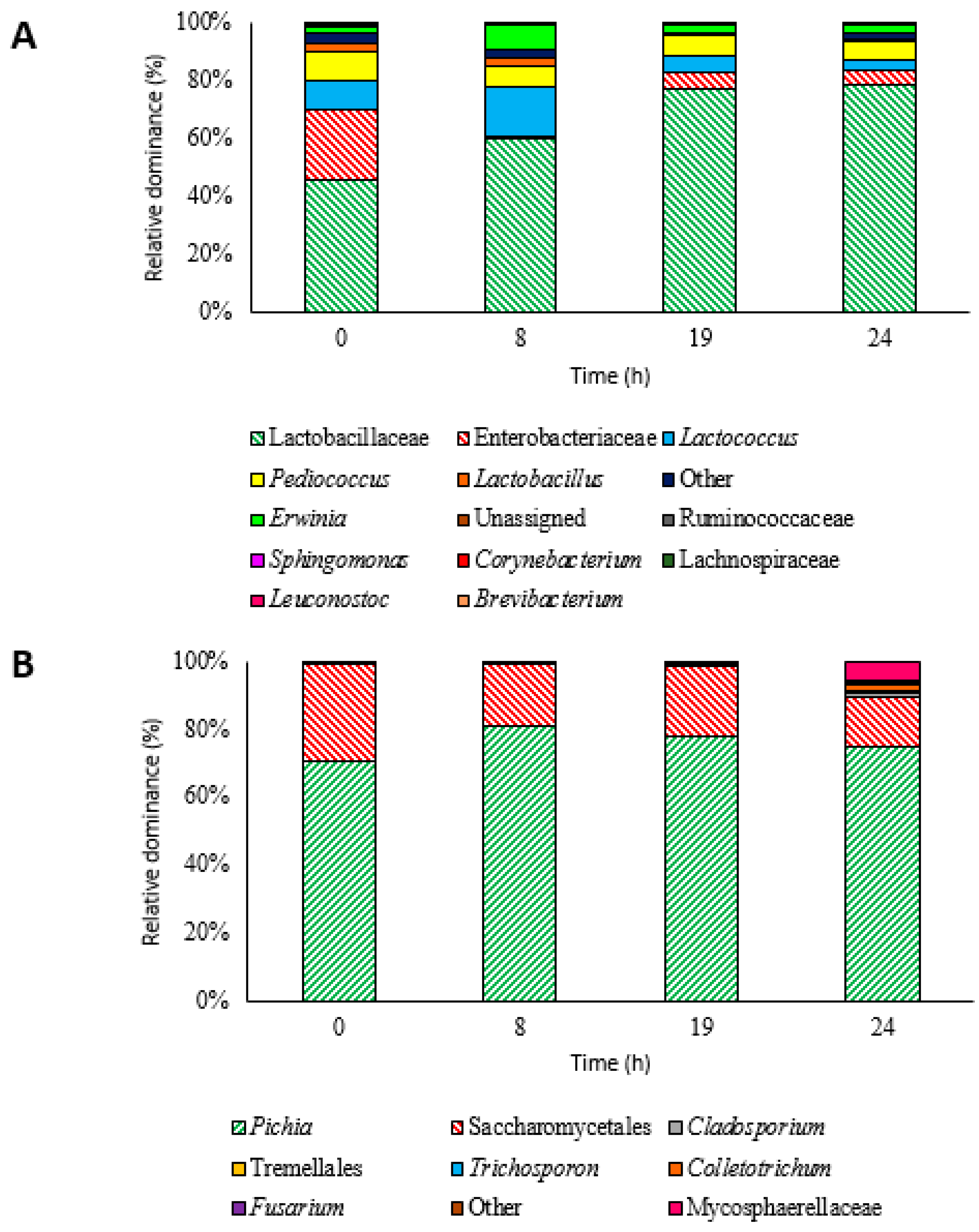
3.2. Microbiota Dynamics during Inoculated Coffee Fermentation
3.3. Chemical Analysis of the Fermentation Liquid Fraction and Coffee Beans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ICO. Coffee year production by country. Data 2021, 2021, 7–9. [Google Scholar]
- de Melo Pereira, G.V.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of co ff ee beans–A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Tanobe, V.O.A.; Soccol, V.T.; da Silva, B.J.G.; Rodrigues, C.; Soccol, C.R. Yeast diversity and physicochemical characteristics associated with coffee bean fermentation from the Brazilian Cerrado Mineiro region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuysta, L. Exploring the Impacts of Postharvest Processing on the Microbiota and. Appl. Environ. Microbiol. 2017, 83, 1–16. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Coral Medina, J.D.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; De Vuyst, L. Following coffee production from cherries to cup: Microbiological and metabolomic analysis of wet processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef] [Green Version]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Sorto, R.D.; Goés-Neto, A.; Kato, R.; Soccol, C.R. Facility-specific ‘house’ microbiome ensures the maintenance of functional microbial communities into coffee beans fermentation: Implications for source tracking. Environ. Microbiol. Rep. 2021, 13, 470–481. [Google Scholar] [CrossRef]
- Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; da Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- de Carvalho Neto, D.P.; Vinícius De Melo Pereira, G.; Finco, A.M.O.; Rodrigues, C.; De Carvalho, J.C.; Soccol, C.R. Microbiological, physicochemical and sensory studies of coffee beans fermentation conducted in a yeast bioreactor model. Food Biotechnol. 2020, 34, 172–192. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.P.; Andrade Lara, J.M.R.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and biochemical performances of six yeast species as potential starter cultures for wet fermentation of coffee beans. LWT 2021, 137, 110430. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of lactic acid bacteria to improve the fermentation and quality of coffee during on-farm processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; da Cruz Pedrozo Miguel, M.G.; Martinez, S.J.; Bressani, A.P.P.; Evangelista, S.R.; e Batista, C.F.S.; Schwan, R.F. The use of mesophilic and lactic acid bacteria strains as starter cultures for improvement of coffee beans wet fermentation. World J. Microbiol. Biotechnol. 2020, 36, 1–15. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Silva, C.F.; da Cruz Miguel, M.G.P.; de Sauza Cordeiro, C.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Vale, A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with pichia fermentans and pediococcus acidilactici on metabolite produced during fermentation and volatile composition of coffee beans. Fermentation 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Muynarsk, E.S.M.; de Melo Pereira, G.V.; Mesa, D.; Thomaz-soccol, V.; Pagnoncelli, M.G.B.; Soccol, C.R. Draft Genome Sequence of Pediococcus acidilactici Strain. Microbiol. Resour. Announc. 2019, 8, e00332-19. [Google Scholar] [CrossRef] [Green Version]
- Feltrin, V.P.; Sant’Anna, E.S.; Porto, A.C.S.; Torres, R.C.O. Produçao de lactobacillus plantarum em melaço de cana-de-açûcar. Braz. Arch. Biol. Technol. 2000, 43, 119–124. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; de Carvalho, J.C.; Soccol, V.T.; Soccol, C.R. High-throughput rRNA gene sequencing reveals high and complex bacterial diversity associated with brazilian coffee bean fermentation. Food Technol. Biotechnol. 2018, 56, 90–95. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Rantsiou, K.; Iliopoulos, V.; Cocolin, L.; Nychas, G.J.E. Bacterial species associated with sound and Botrytis-infected grapes from a Greek vineyard. Int. J. Food Microbiol. 2011, 145, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Pinho, D.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Unravelling the diversity of grapevine microbiome. PLoS ONE 2014, 9, e85622. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Ingram, L.O. Synergistic hydrolysis of carboxymethyl cellulose and acid-swollen cellulose by two endoglucanases (CelZ and CelY) from Erwinia chrysanthemi. J. Bacteriol. 2000, 182, 5676–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueule, D.; Fourny, G.; Ageron, E.; Le Flèche-Matéos, A.; Vandenbogaert, M.; Grimont, P.A.D.; Cilas, C. Pantoea coffeiphila sp. nov., cause of the ‘potato taste’ of Arabica coffee from the African great lakes region. Int. J. Syst. Evol. Microbiol. 2015, 65, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-Term Coffee Monoculture Alters Soil Chemical Properties and Microbial Communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef]
- Cabrera-Rodríguez, A.; Trejo-Calzada, R.; García-De la Peña, C.; Arreola-Ávila, J.G.; Nava-Reyna, E.; Vaca-Paniagua, F.; Díaz-Velásquez, C.; Meza-Herrera, C.A. A metagenomic approach in the evaluation of the soil microbiome in coffee plantations under organic and conventional production in tropical agroecosystems. Emir. J. Food Agric. 2020, 32, 263–270. [Google Scholar] [CrossRef]
- Veloso, T.G.R.; da Silva, M.d.C.S.; Cardoso, W.S.; Guarçoni, R.C.; Kasuya, M.C.M.; Pereira, L.L. Effects of environmental factors on microbiota of fruits and soil of Coffea arabica in Brazil. Sci. Rep. 2020, 10, 14692. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Vale, A.S.; Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic acid bacteria: What coffee industry should know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Zhao, K.; Wang, X.L.; Wang, X.; Zhao, X.H.; Yu, B.; Wang, H.X. Co-inoculation of yeast and lactic acid bacteria to improve cherry wines sensory quality. Int. J. Food Sci. Technol. 2013, 48, 1783–1790. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Batista, N.N.; Simão, J.B.P.; Dias, D.R.; Schwan, R.F. Co-inoculation of yeasts starters: A strategy to improve quality of low altitude Arabica coffee. Food Chem. 2021, 361, 130133. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Endo, A. Taxonomic status of lactic acid bacteria in wine and key characteristics to differentiate species. S. Afr. J. Enol. Vitic. 2009, 30, 72–90. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.L.; Pereira, P.V.; Bertoli, L.D.; Silveira, D.L.; Batista, N.N.; Pinheiro, P.F.; de Souza Carneiro, J.; Schwan, R.F.; de Assis Silva, S.; Coelho, J.M.; et al. Fermentation of Coffea canephora inoculated with yeasts: Microbiological, chemical, and sensory characteristics. Food Microbiol. 2021, 98. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on coffee aroma: II. Roasted coffee. J. Food Compos. Anal. 2007, 20, 297–307. [Google Scholar] [CrossRef]

| Compound | 0 h | 8 h | 19 h | 24 h |
|---|---|---|---|---|
| GC-MS (area) | ||||
| Higher alcohols (2) | ||||
| 1-Butanol, 3-methyl | 0.27 ± 0.02 a | 0.39 ± 0.02 b | 0.63 ± 0.00 c | 0.60 ± 0.00 c |
| 2-Heptanol | 0.17 ± 0.01 a | 0.13 ± 0.00 a | ND | ND |
| Ester (4) | ||||
| Ethyl acetate | 4.10 ± 0.13 a | 2.24 ± 0.78 b | 3.05 ± 0.10 c | 4.80 ± 0.02 a |
| Methyl acetate | ND | ND | 3.80 ± 0.41 a | 0.49 ± 0.04 b |
| Ethyl isobutyrate | ND | ND | 0.63 ± 0.00 | ND |
| Isoamyl acetate | ND | 0.25 ± 0.00 a | 0.37 ± 0.08 a | 0.27 ± 0.00 a |
| Aldehyde (3) | ||||
| Butanal, 3 methyl | 0.86 ± 0.06 a | 0.78 ± 0.05 ab | 0.21 ± 0.00 b | 0.74 ± 0.28 ab |
| Butanal, 2 methyl | 0.63 ± 0.26 a | 0.34 ± 0.00 a | 0.13 ± 0.01 a | 0.32 ± 0.00a |
| Benzeneacetaldehyde | 0.23 ± 0.02 a | 0.33 ± 0.10 a | ND | ND |
| HPLC (g/L) | ||||
| Glucose | 1.08 ± 0.11 a | 0.75 ± 0.09 ab | 0.52 ± 0.14 b | 0.62 ± 0.21 b |
| Fructose | 2.52 ± 0.22 a | 1.78 ± 0.49 b | 1.26 ± 0.13 b | 1.47 ± 0.06 b |
| Lactic acid | ND | 0.43 ± 0.04 a | 1.24 ± 0.21 b | 1.41 ± 0.12 b |
| Ethanol | ND | 0.37 ± 0.12 a | 0.50 ± 0.09 a | 0.81 ± 0.08 b |
| pH | 5.16 | 4.50 | 4.17 | 4.13 |
| Compound (Area) | 0 h | 8 h | 19 h | 24 h |
|---|---|---|---|---|
| Organic acid (3) | ||||
| Acetic acid | 5.35 ± 0.04 a | 6.03 ± 0.86 a | 9.99 ± 0.33 b | 9.73 ± 0.84 b |
| Butanoic acid, 3-methyl | 0.33 ± 0.03 a | 0.44 ± 0.24 ab | 0.58 ± 0.08 ab | 0.85 ± 0.18 b |
| Isovaleric acid | 0.36 ± 0.06 a | 1.39 ± 0.18 b | 0.54 ± 0.12 ac | 0.77 ± 0.04 c |
| Higher alcohols (7) | ||||
| Propanol, 2-methyl | 0.16 ± 0.00 a | 0.13 ± 0.00 ab | 0.11 ± 0.00 b | 0.11 ± 0.02 b |
| 1-Octen-3-ol | 0.41 ± 0.03 a | 0.29 ± 0.05 b | 0.22 ± 0.00 b | 0.20 ± 0.03 b |
| 2-Hexanol, 5 methyl | 0.59 ± 0.10 a | 0.59 ± 0.06 a | 0.46 ± 0.05 ab | 0.33 ± 0.01 b |
| 1-Butanol, 3-methyl | - | 0.60 ± 0.24 a | 0.28 ± 0.00 a | 0.59 ± 0.12 a |
| 2-Heptanol, 3-methyl | - | - | - | 0.13 ± 0.03 |
| 1-Butanol, 2-methyl | - | - | - | 0.28 ± 0.00 |
| Phenylethyl Alcohol | - | - | - | 0.26 ± 0.03 |
| Aldehyde (8) | ||||
| Butanal, 3 methyl | 3.33 ± 0.46 ab | 4.39 ± 0.33 b | 2.48 ± 0.18 c | 1.50 ± 0.79 c |
| Butanal, 2 methyl | 0.70 ± 0.07 ab | 0.76 ± 0.07 b | 0.26 ± 0.00 ac | 0.39 ± 0.00 c |
| Hexanal | 2.01 ± 0.32 a | 3.49 ± 0.56 bc | 3.97 ± 0.52 c | 2.44 ± 0.27 ab |
| Heptanal | 0.20 ± 0.02 a | 0.07 ± 0.01 b | - | - |
| Benzeacetaldehyde | 1.06 ± 0.06 a | 1.07 ±0.03 a | 0.94 ± 0.05 ab | 0.81 ± 0.02 b |
| Benzaldehyde | 1.56 ± 0.06 a | 1.23 ± 0.12 b | 0.90 ± 0.03 c | 0.89 ± 0.06 c |
| Pentanal | 0.61 ± 0.06 a | 0.58 ± 0.21 a | 0.58 ± 0.04 a | 0.49 ± 0.04 a |
| Methional | 0.70 ± 0.00 a | 0.79 ± 0.22 a | 0.67 ± 0.12 a | 0.30 ± 0.04 b |
| Ester (1) | ||||
| Ethyl acetate | - | - | 0.13 ± 0.01 a | 0.25 ± 0.00 b |
| Terpenes (2) | ||||
| Linalol | 0.65 ± 0.00 a | 0.71 ± 0.04 ab | 0.95 ± 0.00 b | 0.61 ± 0.16 a |
| Limonene | - | - | 0.17 ± 0.01 | - |
| Pyrazine (1) | ||||
| 2-Isobuttyl-3-methoxypyrazine | 0.23 ± 0.11 | - | - | - |
| Ketones (1) | ||||
| Acetoin | 0.47 ± 0.05 a | 3.49 ± 0.51 bc | 4.15 ± 0.08 c | 3.30 ± 0.09 b |
| Hydrocarbons (2) | ||||
| Toluene | 0.23 ± 0.10 a | 0.22 ± 0.01 a | - | - |
| Nonane, 3-methyl-5-propyl | 0.27 ± 0.00 | - | - | - |
| Furanone (1) | ||||
| Furan, 2-pentyl | - | 0.33 ± 0.07 a | 0.27 ± 0.06 a | 0.15 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pregolini, V.B.; de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Soccol, C.R. Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation. Fermentation 2021, 7, 278. https://doi.org/10.3390/fermentation7040278
Pregolini VB, de Melo Pereira GV, da Silva Vale A, de Carvalho Neto DP, Soccol CR. Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation. Fermentation. 2021; 7(4):278. https://doi.org/10.3390/fermentation7040278
Chicago/Turabian StylePregolini, Vanessa Bassi, Gilberto Vinícius de Melo Pereira, Alexander da Silva Vale, Dão Pedro de Carvalho Neto, and Carlos Ricardo Soccol. 2021. "Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation" Fermentation 7, no. 4: 278. https://doi.org/10.3390/fermentation7040278
APA StylePregolini, V. B., de Melo Pereira, G. V., da Silva Vale, A., de Carvalho Neto, D. P., & Soccol, C. R. (2021). Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation. Fermentation, 7(4), 278. https://doi.org/10.3390/fermentation7040278