Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Operation Mode
2.2. Substrates and Inoculum
2.3. Analytical Methods
2.4. Calculations
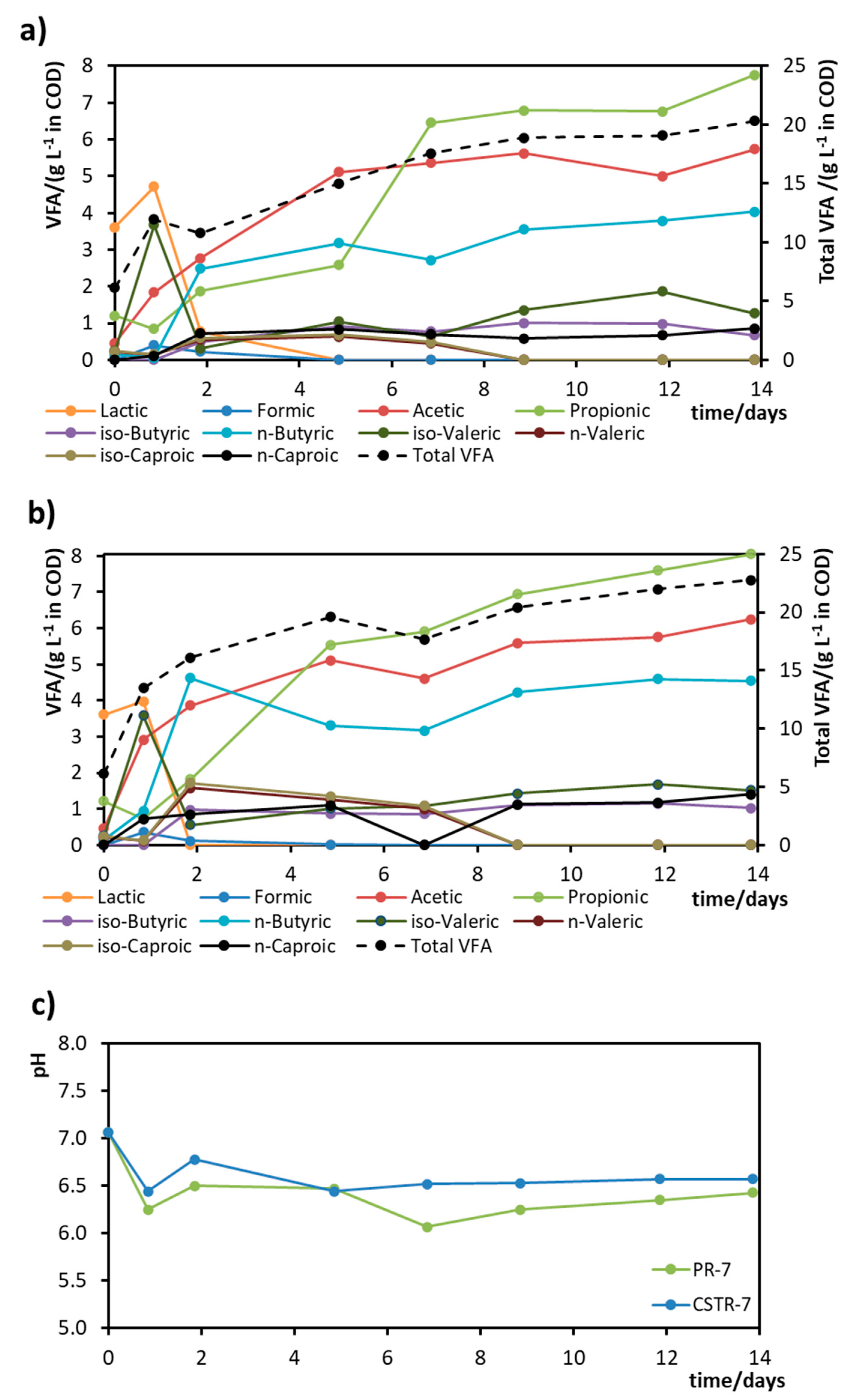
3. Results
- Experiment I
- Experiment II
4. Discussion
| Type of Substrate | Type of Reactor | pH | Salinity | COD Fed | ηa | VFA | % VFA | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g L−1) | (g L−1) | % | (g L−1) | Acetate | Propionate | Butyrate | Valerate | Caproate | Lactate | ||||
| FW | Batch (Lab-scale anaerobic reactors, Vw = 4 L) | 6 | 0 | 200 | n.d. | 41.06 ± 0.92 | 47. 6± 1.5 | 8.9 ± 0.4 | 26.4 ± 0.7 | - | - | - | [29] |
| 3 | n.d. | 36.18 ± 0.62 | 42.7 ± 1.9 | 10.3 ± 0.3 | 27.1 ± 1.2 | - | - | - | |||||
| 6 | n.d. | 33.37 ± 0.61 | 40.6 ± 0.4 | 44.0 ± 0.1 | 35.3 ± 0.5 | - | - | - | |||||
| 9 | n.d. | 22.65 ± 0.19 | 25.5 ± 0.2 | 8.5 ± 0.1 | 51.2 ± 0.1 | - | - | - | |||||
| 12 | n.d. | 4.14 ± 0.44 | 25.2 ± 0.1 | 2.7 ± 0.1 | 18.9 ± 3.0 | - | - | - | |||||
| FW | Batch (Vials, Vw = 0.5 L) | 6 | 0 | 27 | n.d. | 26.61 | 65 | 6 | 29 | - | - | - | [36] |
| 10 | n.d. | 30.36 | 53 | 19 | 28 | - | - | - | |||||
| 30 | n.d. | 26.93 | 51 | 27 | 22 | - | - | - | |||||
| 50 | n.d. | 26.19 | 57 | 33 | 10 | - | - | - | |||||
| 70 | n.d. | 24.65 | 46 | 51 | 3 | - | - | - | |||||
| FW | Fed-batch (Bench scale reactors, Vw = 0.5 L) | 6 | 0 | 15 | 38.27 | 4.603 | 38.9 | 34.7 | 31.5 | 0 | - | - | [37] |
| 1 | 44.76 | 5.384 | 49 | 15 | 32 | 4 | - | - | |||||
| 5 | 54.65 | 6.25 | 42 | 19 | 34 | 5 | - | - | |||||
| 10 | 53.36 | 6.12 | 43 | 18 | 32 | 7 | - | - | |||||
| 20 | 60.71 | 6.488 | 33 | 16 | 40 | 11 | - | - | |||||
| 30 | 63.35 | 6.52 | 27 | 15 | 44 | 14 | - | - | |||||
| 40 | 65.38 | 6.578 | 25 | 11 | 47 | 17 | - | - | |||||
| 50 | 59.05 | 5.988 | 26 | 14.9 | 44 | 12.3 | - | - | |||||
| FW and WAS | Batch (Stirred reactors, Vw = 0.8 L) | 7 | 0 | 40 | n.d. | 7.4 ± 1.4 (in COD) | - | - | Dominant | Dominant | - | - | [35] |
| 10 | n.d. | 12.0 ± 1.5 (in COD) | - | - | Dominant | Dominant | - | Dominant | |||||
| 20 | n.d. | 12.4 ± 1.1 (in COD) | - | - | Dominant | Dominant | - | Dominant | |||||
| 30 | n.d. | 7.2 to 7.5 (in COD) | Dominant | - | - | - | - | Dominant | |||||
| 40 | n.d. | 7.2 to 7.5 (in COD) | Dominant | - | - | - | - | Dominant | |||||
| 50 | n.d. | 7.2 to 7.5 (in COD) | Dominant | - | - | - | - | - | |||||
| FW, Biodiesel WW and Brine | Batch (GSBC, Vw = 2L) | 5 | 12.3 ± 1.5 | 50 | 24 | 11.9 (in COD) | 7.6 | 5.6 | 7.6 | 12 | 0 | 67.2 | This study |
| Batch (BSR, Vw = 2.5 L) | 5 | 12.2 ± 2.2 | 22 | 11.0 (in COD) | 16.2 | 9.3 | 0 | 17.3 | 0 | 57.2 | |||
| Batch (BSR, Vw = 2.5 L) | 7 | 18.4 ± 1.5 | 46 | 22.8 (in COD) | 27.4 | 35.3 | 24.4 | 6.7 | 6.2 | 0 | |||
| Batch (PBSR, Vw = 1.2 L) | 7 | 17.8 ± 1.5 | 41 | 20.3 (in COD) | 28.2 | 38.1 | 23.2 | 6.2 | 4.2 | 0 | |||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perimenis, A.; Nicolay, T.; Leclercq, M.; Gerin, P.A. Comparison of the acidogenic and methanogenic potential of agroindustrial residues. Waste Manag. 2018, 72, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion processes in anaerobic digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Bermúdez-Penabad, N.; Kennes, C.; Veiga, M.C. Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag. 2017, 68, 96–102. [Google Scholar] [CrossRef]
- De Vrieze, J.; Coma, M.; Debeuckelaere, M.; Van der Meeren, P.; Rabaey, K. High salinity in molasses wastewaters shifts anaerobic digestion to carboxylate production. Water Res. 2016, 98, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Luo, J.; Guo, G.; Mackey, H.R.; Hao, T.; Chen, G. Seawater-based wastewater accelerates development of aerobic granular sludge: A laboratory proof-of-concept. Water Res. 2017, 115, 210–219. [Google Scholar] [CrossRef]
- Pólvora, S.; Aníbal, J.; Martins, A. INCREaSE 2019; Monteiro, J., João Silva, A., Mortal, A., Aníbal, J., Moreira da Silva, M., Oliveira, M., Sousa, N., Eds.; Springer International Publishing: Faro, Portugal, 2020; ISBN 978-3-030-30937-4. [Google Scholar]
- Xiao, Y.; Roberts, D.J. A review of anaerobic treatment of saline wastewater. Environ. Technol. 2010, 31, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater—A review. Bioresour. Technol. 2010, 101, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; van Lier, J.B.; de Kreuk, M.K. Growth media in anaerobic fermentative processes: The underestimated potential of thermophilic fermentation and anaerobic digestion. Biotechnol. Adv. 2018, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Wang, S.; Yuan, Z.; Peng, Y. Enhanced volatile fatty acids production of waste activated sludge under salinity conditions: Performance and mechanisms. J. Biosci. Bioeng. 2016, 121, 293–298. [Google Scholar] [CrossRef] [PubMed]
- van Lier, J.B.; Mahmoud, N.; Zeeman, G. Anaerobic wastewater treatment. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M., van Loosdrecht, M., Ekama, G., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 415–456. [Google Scholar]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Morgan-Sagastume, F.; Pratt, S.; Karlsson, A.; Cirne, D.; Lant, P.; Werker, A. Production of volatile fatty acids by fermentation of waste activated sludge pre-treated in full-scale thermal hydrolysis plants. Bioresour. Technol. 2011, 102, 3089–3097. [Google Scholar] [CrossRef]
- Abreu, A.A.; Alves, J.I.; Pereira, M.A.; Sousa, D.Z.; Alves, M.M. Strategies to suppress hydrogen-consuming microorganisms affect macro and micro scale structure and microbiology of granular sludge. Biotechnol. Bioeng. 2011, 108, 1766–1775. [Google Scholar] [CrossRef] [Green Version]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999; ISBN 0875532357. [Google Scholar]
- Saritpongteeraka, K.; Boonsawang, P.; Sung, S.; Chaiprapat, S. Co-fermentation of oil palm lignocellulosic residue with pig manure in anaerobic leach bed reactor for fatty acid production. Energy Convers. Manag. 2014, 84, 354–362. [Google Scholar] [CrossRef]
- Wu, Q.L.; Guo, W.Q.; Zheng, H.S.; Luo, H.C.; Feng, X.C.; Yin, R.L.; Ren, N.Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: The mechanism and microbial community analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Duber, A.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Conversion of organic waste into volatile fatty acids—The influence of process operating parameters. Chem. Eng. J. 2018, 345, 395–403. [Google Scholar] [CrossRef]
- Higgins, M.J.; Novak, J.T. The effect of cations on the settling and dewatering of activated sludges: Laboratory results. Water Environ. Res. 1997, 69, 215–224. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Xing, L.; Li, B.; Peng, Y. The effect of salinity on waste activated sludge alkaline fermentation and kinetic analysis. J. Environ. Sci. (China) 2016, 43, 80–90. [Google Scholar] [CrossRef]
- Fra-Vázquez, A.; Pedrouso, A.; Val del Rio, A.; Mosquera-corral, A.; Val, A.; Mosquera-corral, A. Volatile fatty acid production from saline cooked mussel processing wastewater at low pH. Sci. Total Environ. 2020, 732, 139337. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Murto, M.; Read, J.S.; Mattiasson, B. Profile of hydrolases and biogas production during two-stage mesophilic anaerobic digestion of solid potato waste. Process Biochem. 2005, 40, 2945–2952. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Q.; Jiang, J.; Zhang, H. Effects of salt and oil concentrations on volatile fatty acid generation in food waste fermentation. Renew. Energy 2017, 113, 1523–1528. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Wang, D.; Li, X.; An, H.; Xie, T.; Chen, F.; Xu, Q.; Sun, Y.; Zeng, G.; et al. Revealing the underlying mechanisms of how sodium chloride affects short-chain fatty acid production from the cofermentation of waste activated sludge and food waste. ACS Sustain. Chem. Eng. 2016, 4, 4675–4684. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential impact of salinity on methane production from food waste anaerobic digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzyme Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Design of experiments to assess pre-treatment and co-digestion strategies that optimize biogas production from macroalgae Gracilaria vermiculophylla. Bioresour. Technol. 2014, 162, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindeboom, R.E.F.; Fermoso, F.G.; Weijma, J.; Zagt, K.; Van Lier, J.B. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sadiq, S.; Zhang, W.; Chen, Y.; Xu, X.; Abbas, A.; Chen, S.; Zhang, R.; Xue, G.; Sobotka, D.; et al. Salinity enhances high optically active L-lactate production from co-fermentation of food waste and waste activated sludge: Unveiling the response of microbial community shift and functional profiling. Bioresour. Technol. 2021, 319, 124124. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, J.; Liu, J.; Chen, T.; Shen, D. Characteristics of acidogenic fermentation for volatile fatty acid production from food waste at high concentrations of NaCl. Bioresour. Technol. 2019, 271, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Kiran Katari, J.; Chatterjee, S.; Venkata Mohan, S. Salinity induced acidogenic fermentation of food waste regulates biohydrogen production and volatile fatty acids profile. Fuel 2020, 276, 117794. [Google Scholar] [CrossRef]
- Yasser Farouk, R.; Li, L.; Wang, Y.; Li, Y.; Melak, S. Influence of pretreatment and pH on the enhancement of hydrogen and volatile fatty acids production from food waste in the semi-continuously running reactor. Int. J. Hydrogen Energy 2020, 45, 3729–3738. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Shin, S.G.; Hwang, S.; Lee, C. Continuous fermentation of food waste leachate for the production of volatile fatty acids and potential as a denitrification carbon source. Bioresour. Technol. 2016, 207, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Keshav, A.; Chand, S.; Wasewar, K.L. Recovery of propionic acid from aqueous phase by reactive extraction using quarternary amine (Aliquat 336) in various diluents. Chem. Eng. J. 2009, 152, 95–102. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, X.; Wang, H. Overall process of using a valerate-dominant sludge hydrolysate to produce high-quality polyhydroxyalkanoates (PHA) in a mixed culture. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mohan, S.V. Effect of substrate load and nutrients concentration on the polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour. Technol. 2012, 114, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef]
- Bengtsson, S.; Karlsson, A.; Alexandersson, T.; Quadri, L.; Hjort, M.; Johansson, P.; Morgan-Sagastume, F.; Anterrieu, S.; Arcos-Hernandez, M.; Karabegovic, L.; et al. A process for polyhydroxyalkanoate (PHA) production from municipal wastewater treatment with biological carbon and nitrogen removal demonstrated at pilot-scale. New Biotechnol. 2017, 35, 42–53. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: Effect of redox potential and inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Selvam, A.; Wong, J.W.C. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.; Jia, M.; Hu, J.; Zheng, Y.; Xiong, W.P.; Cao, J. Enhanced mesophilic anaerobic digestion of waste sludge with the iron nanoparticles addition and kinetic analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]


| FW | Biodiesel WW | |
|---|---|---|
| tCOD (g kg−1) | 169.7 ± 19.8 | 61.4 ± 1.9 |
| TS (g kg−1) | 163.2 ± 5.3 | 39.3 ± 0.2 |
| VS (g kg−1) | 146.1 ± 6.7 | 15.2 ± 0.1 |
| GSBC -5 | BSR-5 | PBSR-7 | BSR-7 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | VFA | sCOD | VFA/sCOD | ηa | VFA | sCOD | VFA/sCOD | ηa | VFA | sCOD | VFA/sCOD | ηa | VFA | sCOD | VFA/sCOD | ηa |
| (d) | (g/L in COD) | (g/L) | % | % | (g/L in COD) | g/L | % | % | (g/L in COD) | (g/L) | % | % | (g/L in COD) | (g/L) | % | % |
| 0 | 0.9 | 38.4 | 3 | 2 | 1.1 | 35.9 | 3 | 2 | 6.1 | 28.6 | 21 | 12 | 6.1 | 28.6 | 21 | 12 |
| 1 | 9.1 | 40.4 | 23 | 18 | 8.2 | 37.8 | 22 | 16 | 11.9 | 30.7 | 39 | 24 | 13.5 | 33.8 | 40 | 27 |
| 2 | 9.9 | 41.2 | 24 | 20 | 14.1 | 38.5 | 37 | 28 | 10.8 | 32.2 | 34 | 22 | 16.1 | 33.3 | 48 | 32 |
| 5 | 11.9 | 39.4 | 30 | 24 | 11.0 | 36.0 | 30 | 22 | 15.0 | 36.1 | 42 | 30 | 19.6 | 36.1 | 54 | 39 |
| 7 | - | - | - | - | - | - | - | - | 17.6 | 36.3 | 48 | 35 | 17.7 | 36.9 | 48 | 35 |
| 9 | - | - | - | - | - | - | - | - | 18.9 | 35.9 | 53 | 38 | 20.4 | 36.5 | 56 | 41 |
| 12 | - | - | - | - | - | - | - | - | 19.1 | 36.5 | 52 | 38 | 22.0 | 36.4 | 60 | 44 |
| 14 | - | - | - | - | - | - | - | - | 20.3 | 37.2 | 55 | 41 | 22.8 | 34.7 | 66 | 46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, M.S.; Oliveira, J.V.; Pereira, C.; Carvalho, M.; Mesquita, D.P.; Alves, M.M. Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration. Fermentation 2021, 7, 303. https://doi.org/10.3390/fermentation7040303
Duarte MS, Oliveira JV, Pereira C, Carvalho M, Mesquita DP, Alves MM. Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration. Fermentation. 2021; 7(4):303. https://doi.org/10.3390/fermentation7040303
Chicago/Turabian StyleDuarte, Maria Salomé, João V. Oliveira, Carla Pereira, Miguel Carvalho, Daniela P. Mesquita, and Maria Madalena Alves. 2021. "Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration" Fermentation 7, no. 4: 303. https://doi.org/10.3390/fermentation7040303
APA StyleDuarte, M. S., Oliveira, J. V., Pereira, C., Carvalho, M., Mesquita, D. P., & Alves, M. M. (2021). Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration. Fermentation, 7(4), 303. https://doi.org/10.3390/fermentation7040303








