Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Bacteria, Yeast Consortium in Different Milks
2.2. Preliminary Kefir Fermentation for Yeast Consortium Setup and Evaluation of Yeasts’ Coexistence with LbS
2.3. Yeasts and LbS Detection and Growth Kinetics
2.4. Improved Yeast Consortium-LbS in Milks Fermentation
2.5. Main Analytical Characters and By-Products of Fermentation of Kefir
2.6. Sensorial Analyses of Kefìr
2.7. Statistical Analyses
3. Results
3.1. Preliminary Kefir Fermentation to Test Yeast Consortium–LbS Coexistence
3.2. Assessment of the Improved Yeast Consortium in Different Milks
3.3. Analytical Characters and Fermentation By-Products of Different Kefir Fermented by the Improved Yeast Consortium–LbS
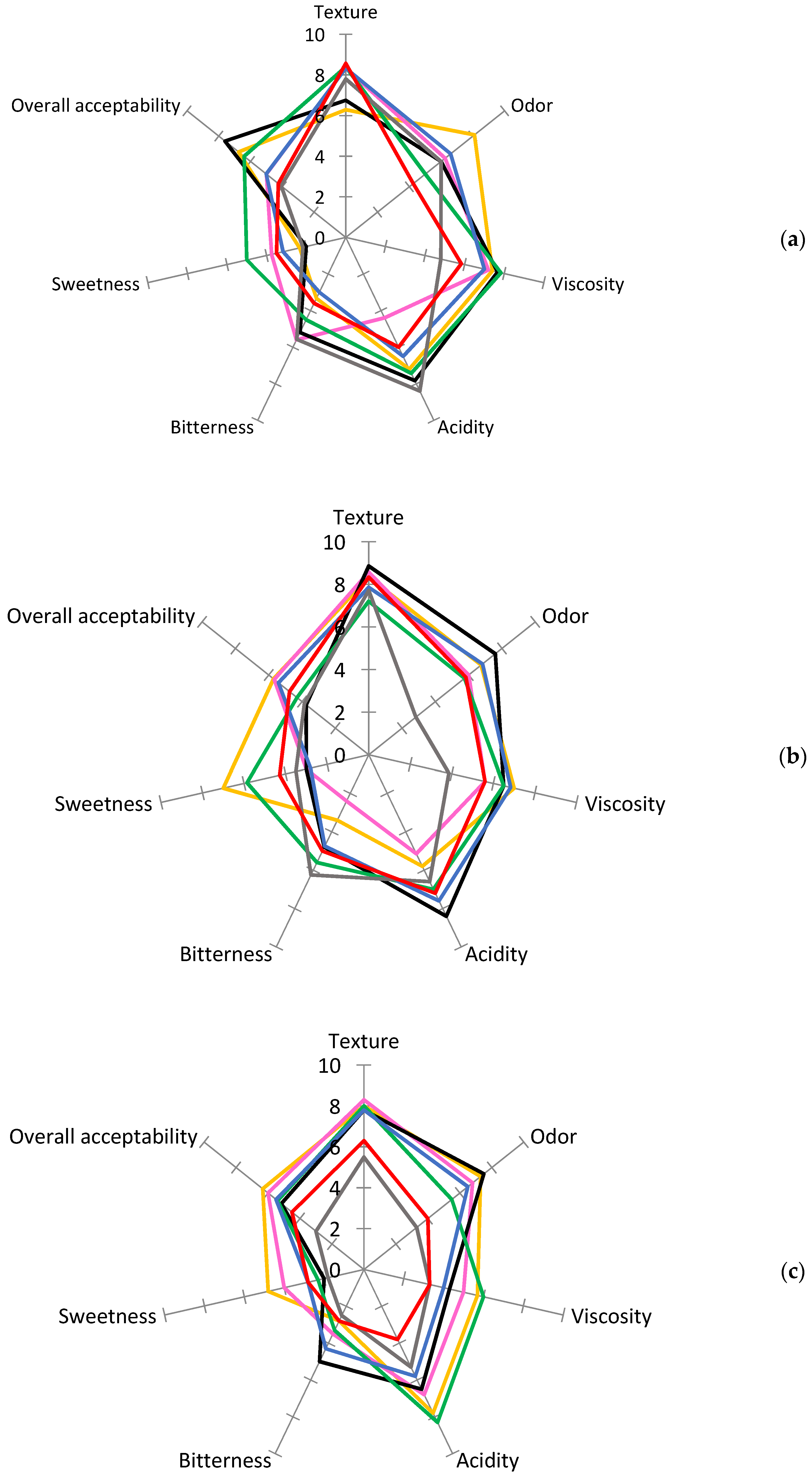
3.4. Sensorial Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [Green Version]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, V. Phytochemistry and functional food: The needs of healthy life. J. Phytochem. Biochem. 2017, 1, 103. [Google Scholar]
- Homayouni, A.; Alizadeh, M.; Alikhah, H.; Zijah, V. Functional dairy probiotic food development: Trends, concepts and products. Intech 2012, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.; Mortadza, S.; Alitheen, N. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Blasche, S.; Kim, Y.; Mars, R.A.T.; Machado, D.; Maansson, M.; Kafkia, E.; Milanese, A.; Zeller, G.; Teusink, B.; Nielsen, J.; et al. Metabolic cooperation and spatiotemporal niche partitioning in a kefir microbial community. Nat. Microbiol. 2021, 6, 196–208. [Google Scholar] [CrossRef]
- Bourrie, B.C.; Ju, T.; Fouhse, J.M.; Forgie, A.J.; Sergi, C.; Cotter, P.D.; Willing, B.P. Kefir microbial composition is a deciding factor in the physiological impact of kefir in a mouse model of obesity. Br. J. Nutr. 2021, 125, 129–138. [Google Scholar] [CrossRef]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.S.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Maria do Carmo, G.P. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a functional beverage gaining momentum towards its health promoting attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Codex Alimentarius. Milk and Milk Products, CODEX STAN 243-2003, 2nd ed.; World Health Organization Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.; El-Seedi, H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [Green Version]
- Guzel-Seydim, Z.B.; Gokirmaklı, C.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Leite, A.M.D.O.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P.A. Big world in small grain: A review of natural milk kefir starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Terpou, A. Ethnic Selected Fermented Foods of Greece. In Fermented Food Products, 1st ed.; Sankaranarayanan, N.A., Dhanasekaran, D., Eds.; CRC Press: London, UK; Taylor & Francis Group: New York, NY, USA, 2020; p. 10. [Google Scholar]
- Meydani, S.N.; Ha, W.K. Immunologic effects of yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef]
- Agarbati, A.; Marini, E.; Galli, E.; Canonico, L.; Ciani, M.; Comitini, F. Characterization of wild yeasts isolated from artisan dairies in the Marche region, Italy, for selection of promising functional starters. LWT Food Sci. Technol. 2020, 139, 110531. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Dertli, E.; Çon, A.H. Microbial diversity of traditional kefir grains and their role on kefir aroma. LWT Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Yıldız-Akgül, F.; Yetişemiyen, A.; Şenel, E.; Yıldırım, Z. Microbiological, physicochemical, and sensory characteristics of kefir produced by secondary fermentation. Mljekarstvo 2018, 68, 3. [Google Scholar] [CrossRef]
- Diosma, G.; Romanin, D.E.; Rey-Burusco, M.F.; Londero, A.; Garrote, G.L. Yeasts from kefir grains: Isolation, identification, and probiotic characterization. World J. Microbiol. Biotechnol. 2014, 30, 43–53. [Google Scholar] [CrossRef]
- Fleet, G.H.; Balia, R. The Public Health and Probiotic Significance of Yeasts in Foods and Beverages. In Yeasts in Food and Beverages: The Yeast Handbook; Querol, A., Fleet, G.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Czerucka, D.; Piche, T.; Rampal, P. Yeast as probiotics–Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; de Rezende, L.C.D.; Pereira, T.D.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C. Identification of new bioactive peptides from Kefir milk through proteopeptidomics: Bioprospection of antihypertensive molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef]
- Petersen, K.M.; Westall, S.; Jespersen, L. Microbial succession of Debaryomyces hansenii strains during the production of Danish surfaced-ripened cheeses. J. Dairy Sci. 2002, 85, 478–486. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Athanasiadis, I.; Bekatorou, A.; Iconomopoulou, M.; Blekas, G. Kefir yeast technology: Scale-up in SCP production using milk whey. Biotechnol. Bioeng. 2005, 89, 788–796. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Athanasiadis, I.; Bekatorou, A.; Psarianos, C.; Kanellaki, M.; Agouridis, N.; Blekas, G. Kefir-yeast technology: Industrial scale-up of alcoholic fermentation of whey, promoted by raisin extracts, using kefir-yeast granular biomass. Enzyme Microb. Technol. 2007, 41, 576–582. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.; Kok-Tas, T.; Ertekin-Filiz, B.; Seydim, A.C. Effect of different growth conditions on biomass increase in kefir grains. J. Dairy Sci. 2011, 94, 1239–1242. [Google Scholar] [CrossRef]
- Lopitz-Ostoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A sym- biotic yeast-bacteria community with alleged healthy capabilities. Rev. Iberoam Micol. 2006, 23, 67–74. [Google Scholar]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004, 22, 197–200. [Google Scholar] [CrossRef]
- Kök-Taş, T.; Seydim, A.C.; Özer, B.; Guzel-Seydim, Z.B. Effects of different fermentation parameters on quality characteristics of kefir. J. Dairy Sci. 2013, 96, 780–789. [Google Scholar] [CrossRef] [Green Version]
- Verachtert, H.; Kumara, H.M.C.; Dawoud, E. Yeast in Mixed Cultures with Emphasis on Lambic Beer Brewing. In Yeast—Biotechnology and Biocatalysis; Verachtert, H., De Mot, R., Eds.; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Beshkova, D.M.; Simova, E.D.; Simov, Z.I.; Frengova, G.I.; Spasov, Z.N. Pure cultures for making kefir. Food Microbiol. 2002, 19, 537–544. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, S.Y.; Chen, K.N.; Liu, J.R.; Chen, M.J. Microbiological and chemical properties of kefir manufactured by entrapped microorganisms isolated from kefir grains. J. Dairy Sci. 2009, 92, 3002–3013. [Google Scholar] [CrossRef] [Green Version]
- Smid, E.J.; Lacroix, C. Microbe–microbe interactions in mixed culture food fermentations. Curr. Opin. Biotechnol. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Microbial Interactions in Kefir: A Natural Probiotic Drink. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Blackwell Publishing: Iowa, IA, USA, 2010. [Google Scholar]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation-A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Duitschaever, C.L.; Toop, D.H.; Buteau, C. Consumer acceptance of sweetened and flavoured kefir. Milchwissenschaft 1991, 46, 227–229. [Google Scholar]
- Sulmiyati, S.; Said, N.S.; Fahrodi, D.U.; Malaka, R.; Maruddin, F. The physicochemical, microbiology, and sensory characteristics of kefir goat milk with different levels of kefir grain. Trop. Anim. Sci. J. 2019, 42, 152–158. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef]
- Biagiotti, C.; Ciani, M.; Canonico, L.; Comitini, F. Occurrence and involvement of yeast biota in ripening of Italian Fossa cheese. Eur. Food Res. Technol. 2018, 244, 1921–1931. [Google Scholar] [CrossRef]
- Yalçin, S.K.; Ozbas, Z.Y. Yeasts from Traditional Cheeses for Potential Applications. In Global Food Security and Wellness; Springer: New York, NY, USA, 2017; pp. 277–293. [Google Scholar]
 ), LbS with D. hansenii 36 (
), LbS with D. hansenii 36 (  ), LbS with D. hansenii 78 (
), LbS with D. hansenii 78 (  ), LbS with C. zeylanoides 13 (
), LbS with C. zeylanoides 13 (  ), LbS with K. Lactis 80 (
), LbS with K. Lactis 80 (  ), LbS with Y. lipolytica 92 (
), LbS with Y. lipolytica 92 (  ), and LbS with yeast consortium (
), and LbS with yeast consortium (  ). At the bottom of the graph (a), the trend of each yeast was represented: C. zeylanoides 13 (
). At the bottom of the graph (a), the trend of each yeast was represented: C. zeylanoides 13 (  ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. Lactis 80 (
), K. Lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. Graph (b) represents the growth kinetics of the single species forming the yeast consortium: D. hansenii 38 + 78 (
), without distinction among the species. Graph (b) represents the growth kinetics of the single species forming the yeast consortium: D. hansenii 38 + 78 (  ), C. zeylanoides 13 (
), C. zeylanoides 13 (  ), K. lactis 80 (
), K. lactis 80 (  ), and Y. lipolytica 92 (
), and Y. lipolytica 92 (  ). Data means ± standard deviations are represented as error bars.
). Data means ± standard deviations are represented as error bars.
 ), LbS with D. hansenii 36 (
), LbS with D. hansenii 36 (  ), LbS with D. hansenii 78 (
), LbS with D. hansenii 78 (  ), LbS with C. zeylanoides 13 (
), LbS with C. zeylanoides 13 (  ), LbS with K. Lactis 80 (
), LbS with K. Lactis 80 (  ), LbS with Y. lipolytica 92 (
), LbS with Y. lipolytica 92 (  ), and LbS with yeast consortium (
), and LbS with yeast consortium (  ). At the bottom of the graph (a), the trend of each yeast was represented: C. zeylanoides 13 (
). At the bottom of the graph (a), the trend of each yeast was represented: C. zeylanoides 13 (  ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. Lactis 80 (
), K. Lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. Graph (b) represents the growth kinetics of the single species forming the yeast consortium: D. hansenii 38 + 78 (
), without distinction among the species. Graph (b) represents the growth kinetics of the single species forming the yeast consortium: D. hansenii 38 + 78 (  ), C. zeylanoides 13 (
), C. zeylanoides 13 (  ), K. lactis 80 (
), K. lactis 80 (  ), and Y. lipolytica 92 (
), and Y. lipolytica 92 (  ). Data means ± standard deviations are represented as error bars.
). Data means ± standard deviations are represented as error bars.
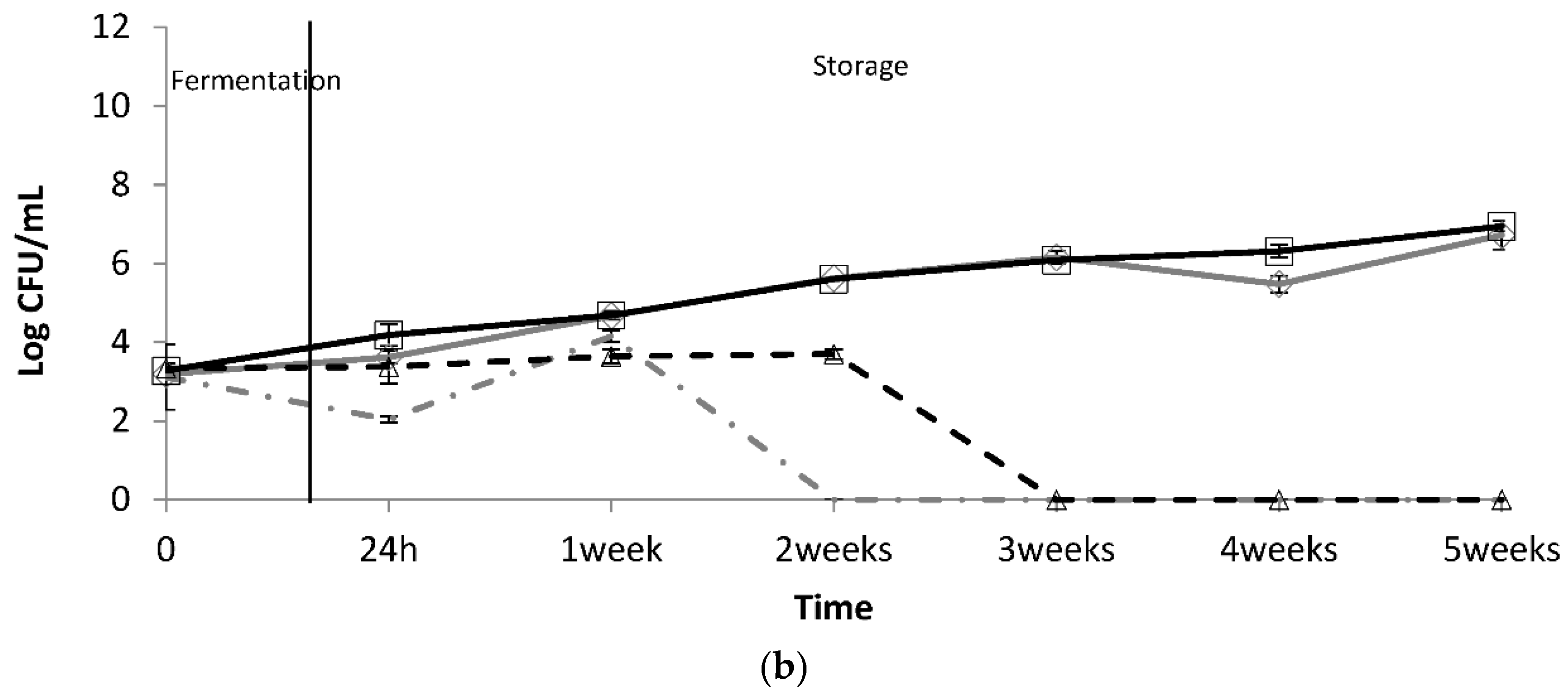
 ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. lactis 80 (
), K. lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. Data means ± standard deviations are represented as error bars.
), without distinction among the species. Data means ± standard deviations are represented as error bars.
 ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. lactis 80 (
), K. lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. Data means ± standard deviations are represented as error bars.
), without distinction among the species. Data means ± standard deviations are represented as error bars.
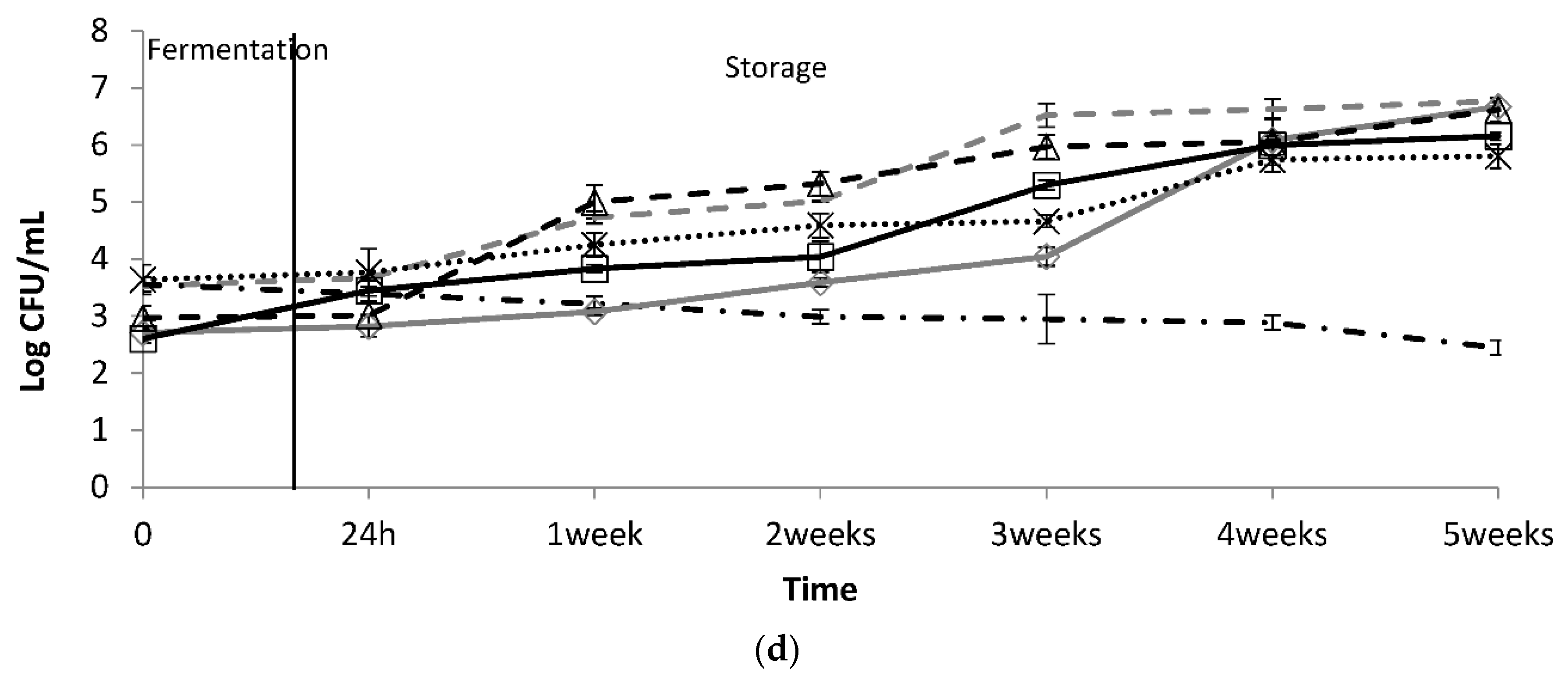


 ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. Lactis 80 (
), K. Lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. The control trial was indicated as LbS (
), without distinction among the species. The control trial was indicated as LbS (  ).
).
 ), D. hansenii 36 (
), D. hansenii 36 (  ), D. hansenii 78 (
), D. hansenii 78 (  ), K. Lactis 80 (
), K. Lactis 80 (  ), Y. lipolytica 92 (
), Y. lipolytica 92 (  ), and the yeast consortium (
), and the yeast consortium (  ), without distinction among the species. The control trial was indicated as LbS (
), without distinction among the species. The control trial was indicated as LbS (  ).
).

| Milks | Nutritional Values for 1 L of Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Energy (Kcal) | Total Fat (g) | Saturated Fat (g) | Total Carbohydrates (g) | Sugars (g) | Fibers (g) | Protein (g) | Salt (g) | Calcium (g) | |
| Whole cow’s milk | 640 | 36 | 26 | 49 | 49 | 0 | 31 | 1 | 1.2 |
| Lactose-free Cow’s milk | 340 | 35 | 24 | 51 | 51 | 0 | 32 | 1 | 1.2 |
| Goat’s milk | 450 | 16 | 12 | 44 | 44 | 0 | 32 | 2 | 1.2 |
| Sheep’s milk | 1030 | 69 | 48 | 51 | 51 | 0 | 53 | 3 | 1.8 |
| LbS (Control) | Dh 36 | Dh 78 | Cz 13 | Kl 80 | Yl 92 | Y. Consortium | ||
|---|---|---|---|---|---|---|---|---|
| K_C | pH | 3.95 ± 0.02 ab | 3.94 ± 0.02 a | 3.92 ± 0.04 a | 3.96 ± 0.01 a | 3.92 ± 0.01 a | 3.96 ± 0.03 a | 3.92 ± 0.03 ab |
| Lactose % w/w | 3.02 ± 0.01 c | 3.95 ± 0.14 a | 3.74 ± 0.07 a | 1.01 ± 0.05 e | 1.98 ± 0.04 d | 3.88 ± 0.08 a | 3.27 ± 0.01 b | |
| Lactic acid g/L | 9.32 ± 0.01 b | 8.59 ± 0.03 d | 9.52 ± 0.02 a | 9.13 ± 0.07 c | 8.62 ± 0.03 d | 9.39 ± 0.01 b | 8.36 ± 0.02 e | |
| Acetic acid g/L | 0.71 ± 0.01 a | 0.54 ± 0.01 d | 0.62 ± 0.00 c | 0.53 ± 0.01 d | 0.43 ± 0.01 f | 0.49 ± 0.01 e | 0.66 ± 0.00 b | |
| EtOH % v/v | nd | nd | nd | nd | nd | nd | nd | |
| K_HD | pH | 3.97 ± 0.03 a | 4.02 ± 0.01 a | 4.03 ± 0.00 a | 4.05 ± 0.01 a | 3.97 ± 0.01 a | 3.99 ± 0.02 a | 4.03 ± 0.01 a |
| Lactose % w/w | nd | nd | nd | nd | nd | nd | nd | |
| Lactic acid g/L | 7.95 ± 0.01 cd | 8.33 ±0.07 a | 8.36 ± 0.04 a | 7.95 ± 0.05 d | 8.07 ± 0.04 bc | 8.07 ± 0.11 b | 8.43 ± 0.06 a | |
| Acetic acid g/L | 0.47 ± 0.01 a | 0.28 ± 0.01 d | 0.40 ± 0.01 b | 0.40 ± 0.01 b | 0.38 ± 0.01 b | 0.32 ± 0.01 c | 0.32 ± 0.00 c | |
| EtOH % v/v | nd | nd | nd | nd | nd | nd | nd | |
| K_G | pH | 3.97 ± 0.00 bc | 3.99 ± 0.01 bc | 3.91 ± 0.00 c | 3.96 ± 0.01 bc | 3.95 ± 0.01 bc | 4.21 ± 0.01 a | 4.08 ± 0.02 b |
| Lactose % w/w | 4.02 ± 0.01 c | 4.49 ± 0.04 a | 3.45 ± 0.00 e | 4.09 ± 0.01 b | 4.09 ± 0.03 b | 2.69 ± 0.01 f | 3.95 ± 0.04 d | |
| Lactic acid g/L | 7.24 ± 0.01 c | 8.04 ± 0.07 a | 8.04 ± 0.06 a | 7.56 ± 0.01 b | 7.11 ± 0.01 d | 6.22 ± 0.01 e | 7.08 ± 0.02 d | |
| Acetic acid g/L | 0.94 ± 0.00 a | 0.70 ± 0.02 c | 0.66 ± 0.01 d | 0.78 ± 0.00 b | 0.62 ± 0.00 e | 0.56 ± 0.00 f | 0.56 ± 0.01 f | |
| EtOH % v/v | nd | nd | nd | nd | nd | nd | nd | |
| K_S | pH | 3.82 ± 0.02 ab | 3.87 ± 0.02 a | 3.90 ± 0.01 a | 3.78 ± 0.03 ab | 3.91 ± 0.02 a | 3.78 ± 0.00 ab | 3.77 ± 0.01 b |
| Lactose % w/w | 2.09 ± 0.01 e | 3.00 ± 0.01 a | 2.01 ± 0.01 f | 2.20 ± 0.02 d | 2.36 ± 0.02 c | 2.70 ± 0.01 b | 1.86 ± 0.01 g | |
| Lactic acid g/L | 11.76 ± 0.03 c | 13.14 ± 0.03 b | 10.06 ± 0.00 f | 10.45 ± 0.01 e | 10.54 ± 0.02 d | 14.26 ± 0.00 a | 11.73 ± 0.00 c | |
| Acetic acid g/L | 0.53 ± 0.01 c | 0.41 ± 0.00 f | 0.70 ± 0.01 a | 0.49 ± 0.01 d | 0.55 ± 0.01 b | 0.47 ± 0.00 e | 0.40 ± 0.01 f | |
| EtOH % v/v | nd | nd | nd | nd | nd | nd | nd |
| Fermentation By-Products (mg/L) | LbS (Control) | Dh 36 | Dh 78 | Cz 13 | Kl 80 | Yl 92 | Y. Consortium | |
|---|---|---|---|---|---|---|---|---|
| K_C | Esters | |||||||
| Ethyl acetate | nd | nd | nd | nd | nd | nd | nd | |
| Alcohols | ||||||||
| n-propanol | 12.00 ± 0.04 a | 9.91 ± 0.03 f | 10.26 ± 0.03 e | 11.70 ± 0.02 b | 10.69 ± 0.03 d | 10.26 ± 0.02 e | 11.40 ± 0.02 c | |
| Isobutanol | nd | nd | nd | nd | nd | nd | nd | |
| Amyl alcohol | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.22 ± 0.01 a | 0.00 ± 0.00 b | |
| Isoamyl alcohol | 2.83 ± 0.02 a | 2.68 ± 0.04 c | 0.00 ± 0.00 d | 2.68 ± 0.01 c | 0.00 ± 0.00 d | 2.75 ± 0.01 b | 2.70 ± 0.02 c | |
| Carbonyl Compounds | ||||||||
| Acetaldehyde | 0.49 ± 0.01 c | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.06 ± 0.01 d | 0.00 ± 0.00 e | 13.44 ± 0.03 a | 2.96 ± 0.03 b | |
| Acetoin | 0.39 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | |
| K_HD | Esters | |||||||
| Ethyl acetate | 0.11 ± 0.01 f | 2.52 ± 0.04 c | 3.45 ± 0.03 b | 0.00 ± 0.00 g | 1.05 ± 0.03 e | 1.55 ± 0.02 d | 15.97 ± 0.01 a | |
| Alcohols | ||||||||
| n-propanol | 14.88 ± 0.07 b | 12.11 ± 0.01 f | 12.68 ± 0.04 d | 12.42 ± 0.04 e | 12.86 ± 0.03 c | 18.11 ± 0.01 a | 10.44 ± 0.07 g | |
| Isobutanol | nd | nd | nd | nd | nd | nd | nd | |
| Amyl alcohol | 0.04 ± 0.00 e | 2.10 ± 0.02 f | 0.00 ± 0.00 f | 7.59 ± 0.02 a | 2.43 ± 0.02 c | 0.00 ± 0.00 f | 4.91 ± 0.01 b | |
| Isoamyl alcohol | 2.70 ± 0.02 ab | 2.73 ± 0.04 ab | 0.00 ± 0.00 c | 2.69 ± 0.25 b | 0.00 ± 0.00 c | 2.82 ± 0.02 a | 2.99 ± 0.08 a | |
| Carbonyl Compounds | ||||||||
| Acetaldehyde | 3.99 ± 0.02 d | 0.36 ± 0.02 f | 1.09 ± 0.02 e | 0.00 ± 0.00 g | 5.43 ± 0.02 b | 4.32 ± 0.03 c | 11.11 ± 0.02 a | |
| Acetoin | nd | nd | nd | nd | nd | nd | nd | |
| K_G | Esters | |||||||
| Ethyl acetate | 13.98 ± 0.07 a | 6.66 ± 0.21 d | 0.00 ± 0.00 g | 0.24 ± 0.01 f | 4.04 ± 0.03 e | 4.04 ± 0.03 e | 8.87 ± 0.15 c | |
| Alcohols | ||||||||
| n-propanol | 15.95 ± 0.14 a | 11.92 ± 0.35 c | 9.83 ± 0.28 f | 10.98 ± 0.14 d | 10.18 ± 0.01 e | 10.18 ± 0.01 e | 12.06 ± 0.12 b | |
| Isobutanol | nd | nd | nd | nd | nd | nd | nd | |
| Amyl alcohol | 0.01 ± 0.00 c | 3.62 ± 0.28 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 1.62 ± 0.03 b | 1.62 ± 0.03 b | 0.00 ± 0.00 c | |
| Isoamyl alcohol | 0.00 ± 0.00 c | 2.73 ± 0.14 b | 0.00 ± 0.00 c | 2.72 ± 0.03 b | 2.79 ± 0.02 b | 2.79 ± 0.02 b | 0.00 ± 0.00 c | |
| Carbonyl Compounds | ||||||||
| Acetaldehyde | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.17 ± 0.01 c | 0.17 ± 0.01 c | 5.97 ± 0.42 a | |
| Acetoin | 28.39 ± 0.07 a | 16.79 ± 0.21 b | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 1.54 ± 0.01 c | |
| K_S | Esters | |||||||
| Ethyl acetate | 0.00 ± 0.00 b | 12.31 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | |
| Alcohols | ||||||||
| n-propanol | 10.12 ± 0.03 f | 13.73 ± 0.02 b | 11.19 ± 0.02 e | 12.84 ± 0.36 c | 15.99 ± 0.16 a | 9.29 ± 0.03 g | 11.95 ± 0.26 d | |
| Isobutanol | 2.66 ± 0.04 c | 0.00 ± 0.00 d | 2.69 ± 0.03 bc | 2.76 ± 0.03 b | 2.95 ± 0.03 a | 2.64 ± 0.04 bc | 2.65 ± 0.03 c | |
| Amyl alcohol | 0.15 ± 0.01 e | 0.54 ± 0.03 b | 0.04 ± 0.00 f | 0.30 ± 0.03 d | 0.39 ± 0.01 c | 0.78 ± 0.00 a | 0.06 ± 0.00 f | |
| Isoamyl alcohol | 2.73 ± 0.03 c | 2.70 ± 0.02 c | 3.04 ± 0.03 b | 2.73 ± 0.04 c | 3.04 ± 0.06 b | 3.23 ± 0.01 a | 2.74 ± 0.02 c | |
| Carbonyl Compounds | ||||||||
| Acetaldehyde | 0.00 ± 0.00 b | 0.74 ± 0.02 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | |
| Acetoin | 0.00 ± 0.00 b | 41.67 ± 0.22 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarbati, A.; Ciani, M.; Canonico, L.; Galli, E.; Comitini, F. Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation 2022, 8, 9. https://doi.org/10.3390/fermentation8010009
Agarbati A, Ciani M, Canonico L, Galli E, Comitini F. Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation. 2022; 8(1):9. https://doi.org/10.3390/fermentation8010009
Chicago/Turabian StyleAgarbati, Alice, Maurizio Ciani, Laura Canonico, Edoardo Galli, and Francesca Comitini. 2022. "Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium" Fermentation 8, no. 1: 9. https://doi.org/10.3390/fermentation8010009
APA StyleAgarbati, A., Ciani, M., Canonico, L., Galli, E., & Comitini, F. (2022). Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation, 8(1), 9. https://doi.org/10.3390/fermentation8010009








