Bioconversion of Glycerol into Lactic Acid by a New Bacterial Strain from the Brazilian Cerrado Soil
Abstract
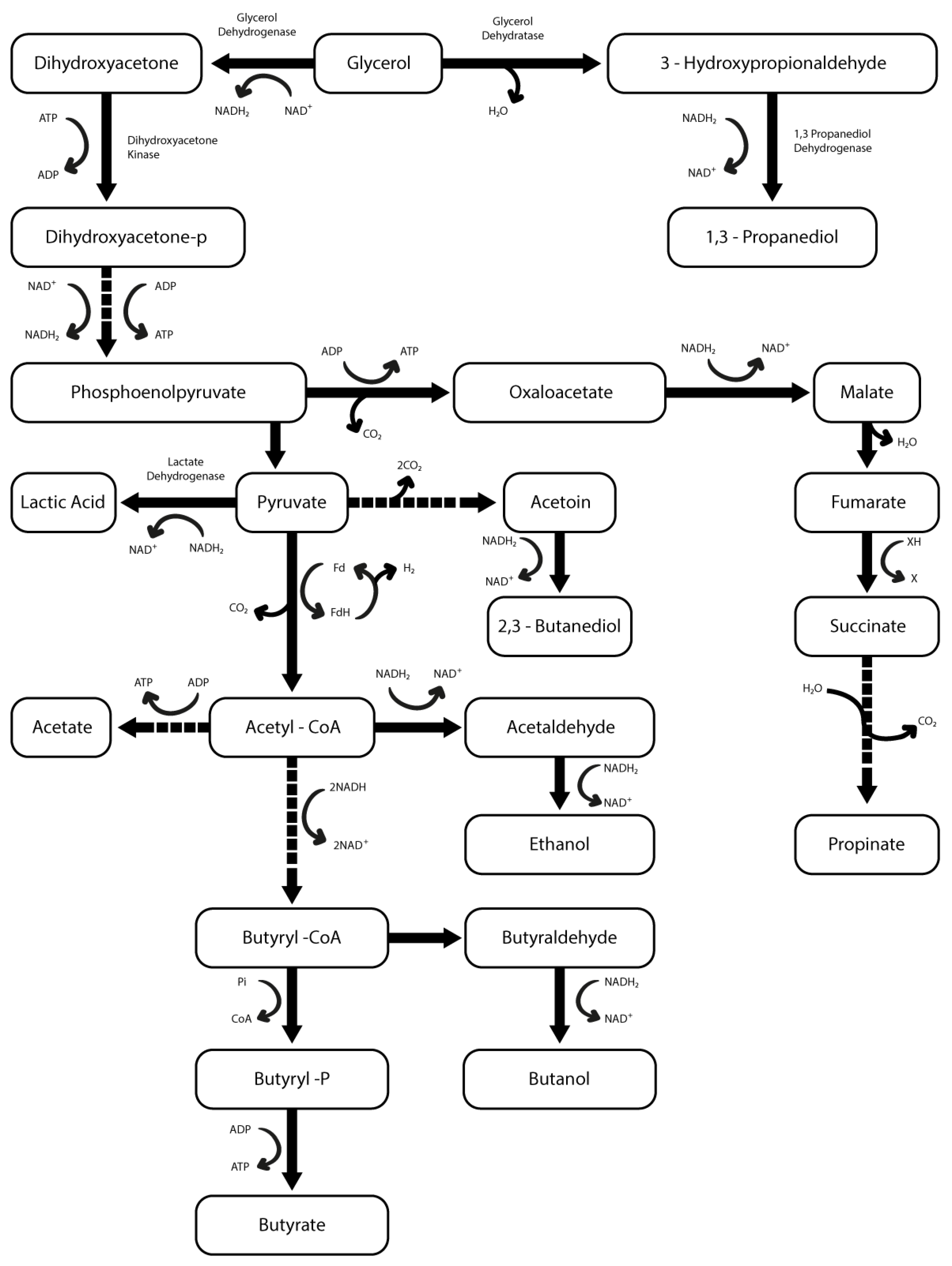
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Microbial Isolation
2.2. Culture Conditions and Selection of Bacterial Strains Producing Lactic Acid under Microaerobiosis
2.3. 16S rRNA Gene Analysis
2.4. Evaluation of Inoculum Concentration for the Lactic Acid Synthesis
2.5. Experimental Design
2.5.1. Factorial Design
2.5.2. Rotatable Central Composite Design
2.6. Analysis of Cell Growth and Determination of Glycerol and Metabolic Byproducts
2.7. Fermentation Yield
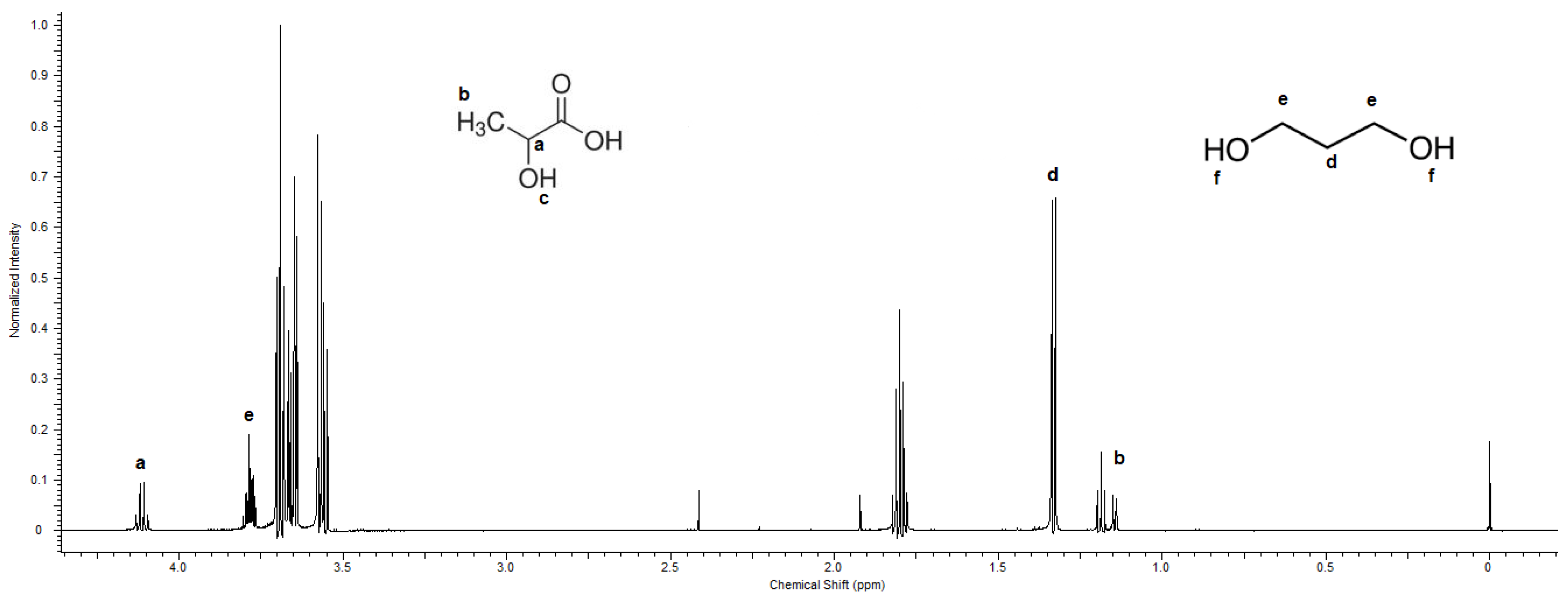
2.8. Proton Nuclear Magnetic Resonance
3. Results and Discussion
3.1. Selection of Bacteria in Microaerobiosis
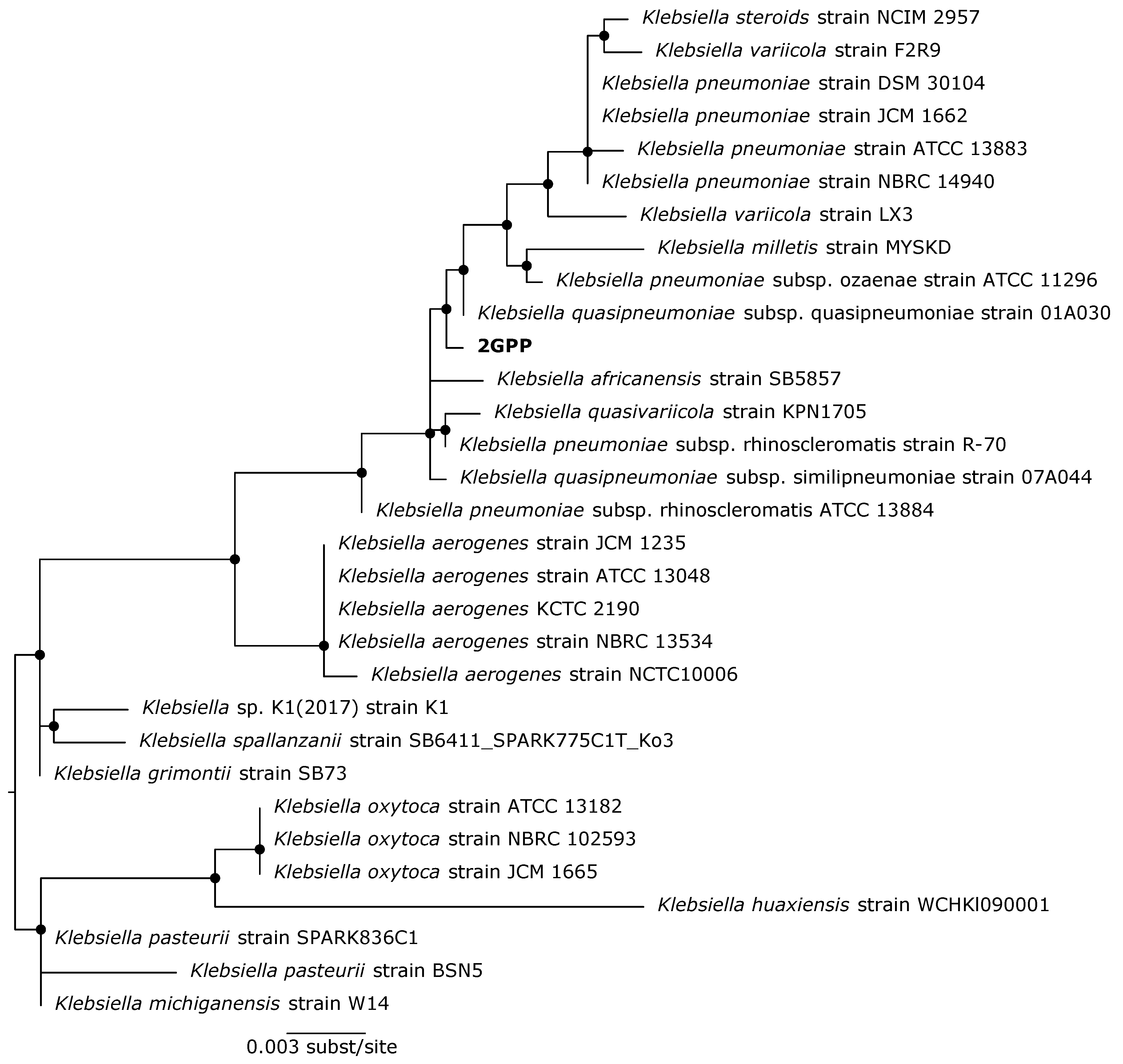
3.2. Phylogenetic Analysis of the 2GPP Selected Strain
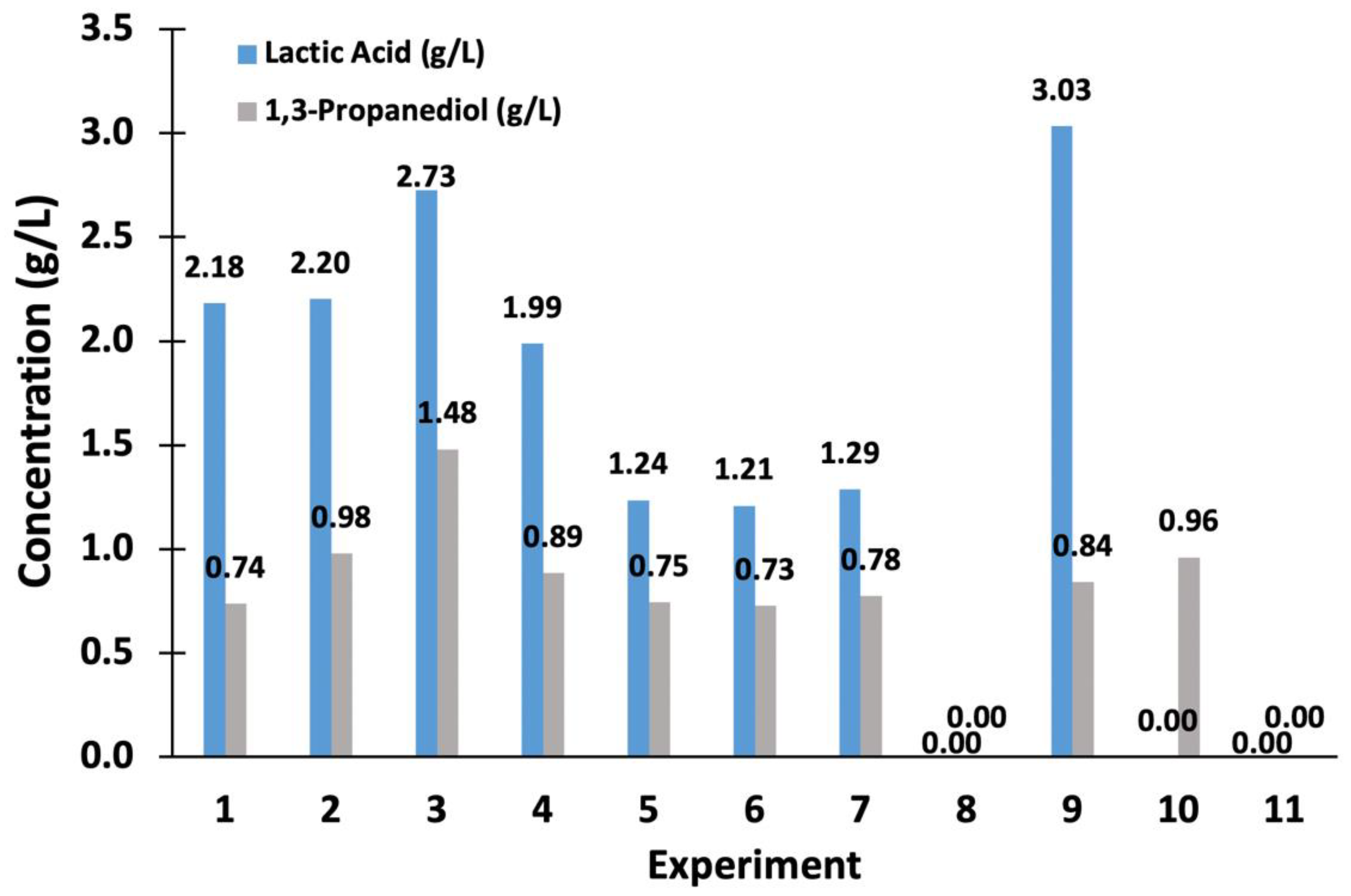
3.3. Evaluation of the Inoculum Concentration and Agitation in Lactic Acid Production by Klebsiella sp. 2GPP
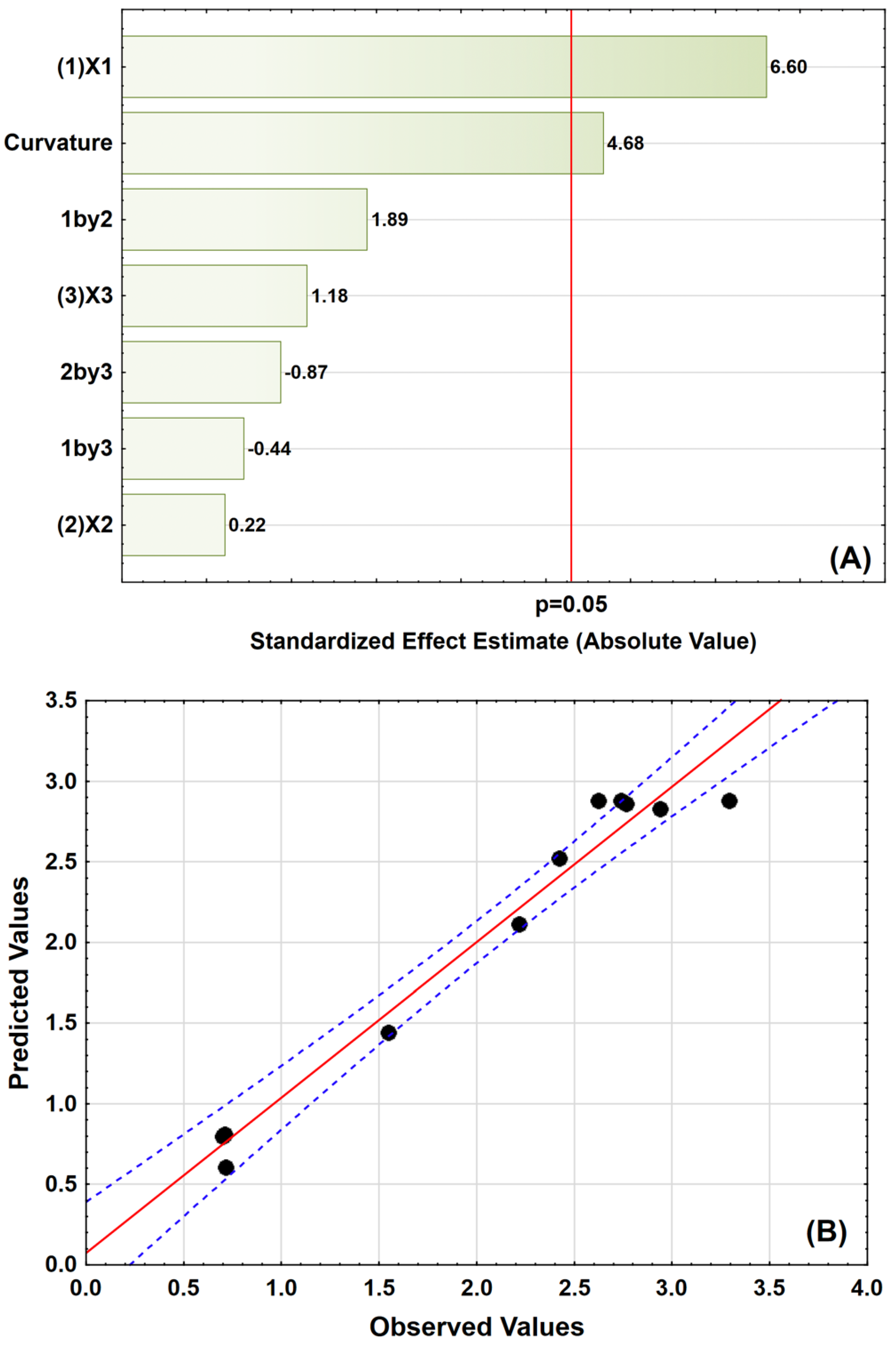
3.4. 23 Factorial Design
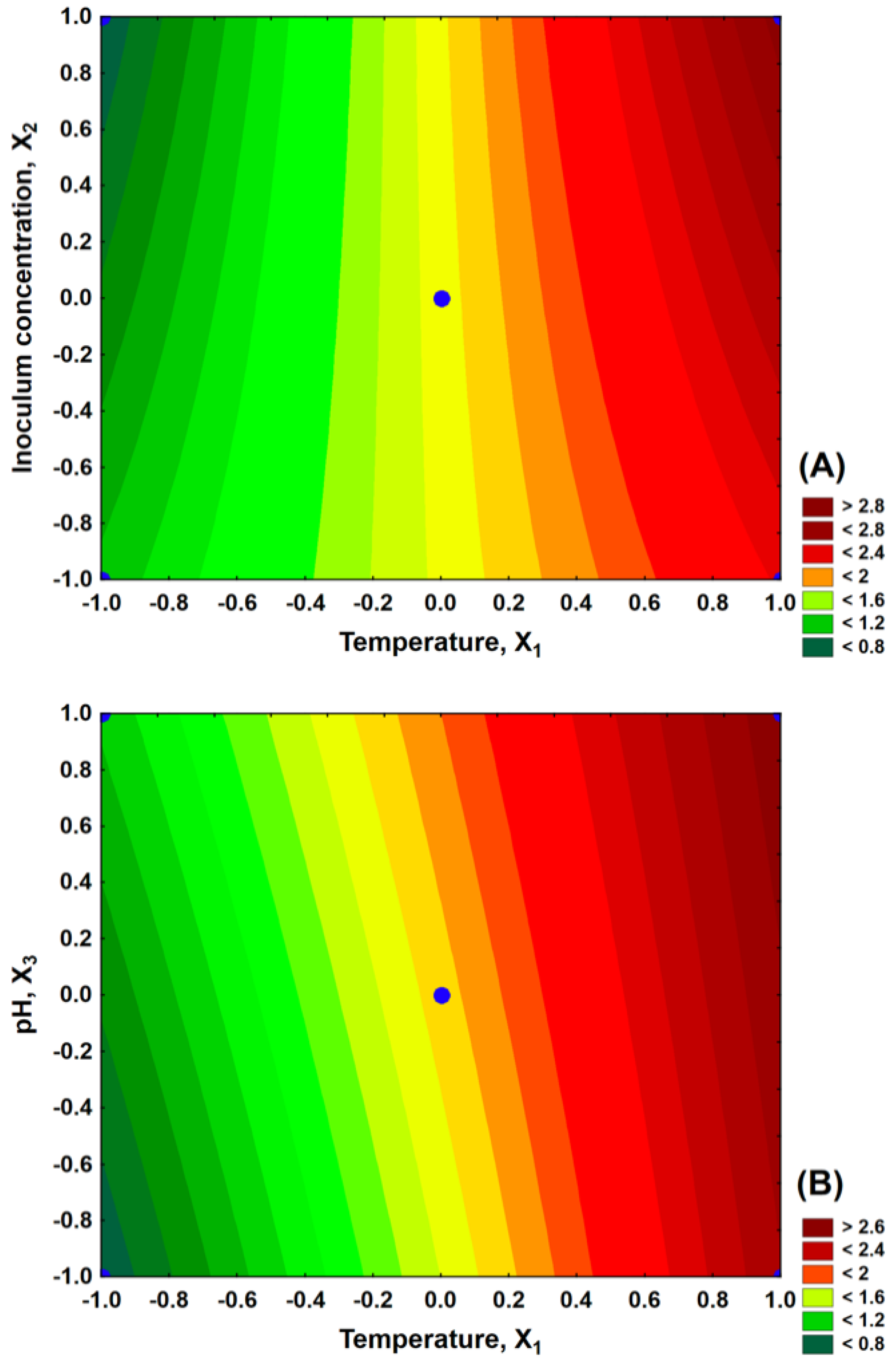
3.5. Rotatable Central Composite Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, A.C.; Zhang, Q.; Lyne, P.W. Ethanol-diesel fuel blends—A review. Bioresour. Technol. 2005, 96, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Moka, S.; Pande, M.; Rani, M.; Gakhar, R.; Sharma, M.; Rani, J.; Bhaskarwar, A.N. Alternative fuels: An overview of current trends and scope for future. Renew. Sustain. Energy Rev. 2014, 32, 697–712. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Liu, H.L.; Huang, S.Y.; Fu, Y.S.; Fang, B.S. Metabolic profiles analysis of 1, 3-propanediol production process by Clostridium butyricum through repeated batch fermentation coupled with activated carbon adsorption. Biotechnol. Bioeng. 2018, 115, 684–693. [Google Scholar] [CrossRef]
- Li, K.-T.; Li, H.-H. Glycerol Conversion to Lactic Acid with Unsupported Copper Salts and Bulk Cupric Oxide in Aqueous Alkali Media. Appl. Biochem. Biotechnol. 2020, 191, 125–134. [Google Scholar] [CrossRef]
- Petrov, K.; Petrova, P. Enhanced production of 2, 3-butanediol from glycerol by forced pH fluctuations. Appl. Microbiol. Biotechnol. 2010, 87, 943–949. [Google Scholar] [CrossRef]
- De Santana, J.S.; da Silva, J.L.; Dutra, E.D.; Menezes, R.S.C.; de Souza, R.B.; Pinheiro, I.O. Production of 1,3-propanediol by Lactobacillus diolivorans from agro-industrial residues and cactus cladode acid hydrolyzate. Appl. Biochem. Biotechnol. 2021, 193, 1585–1601. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, A.; Dong, H.; Li, Q.; Niu, C. A novel process for recovery and refining of L-lactic acid from fermentation broth. Bioresour. Technol. 2012, 112, 280–284. [Google Scholar] [CrossRef]
- Joglekar, H.; Rahman, I.; Babu, S.; Kulkarni, B.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Sousdaleff, M.; Lima, M.V.S.; LIMA, H.O. Lactic acid production by fermentation from sugarcane molasses with Lactobacillus casei. Braz. J. Food Technol. VII BMCFB 2009, 2, 34–40. [Google Scholar]
- Sakata, M.M.; Alberto-Rincon, M.d.C.; Duek, E.A. Estudo da interação polímero/cartilagem/osso utilizando poli (Ácido Lático-co-Ácido Glicólico) e poli (p-Dioxanona) em condilo femural de coelhos. Polímeros 2004, 14, 176–180. [Google Scholar] [CrossRef]
- Ji, X.-J.; Huang, H.; Ouyang, P.-K. Microbial 2, 3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.H.; Kim, C.H.; Heo, S.-Y.; Oh, B.-R.; Hong, W.-K.; Kim, S.; Kim, D.-H.; Seo, J.-W. Production of 3-hydroxypropionic acid through propionaldehyde dehydrogenase PduP mediated biosynthetic pathway in Klebsiella pneumoniae. Bioresour. Technol. 2012, 103, 1–6. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, X.; Tan, W.-S.; Zhu, M.-L. Characteristics of fermentative hydrogen production with Klebsiella pneumoniae ECU-15 isolated from anaerobic sewage sludge. Int. J. Hydrog. Energy 2010, 35, 71–80. [Google Scholar] [CrossRef]
- Park, J.M.; Song, H.; Lee, H.J.; Seung, D. Genome-scale reconstruction and in silico analysis of Klebsiella oxytoca for 2, 3-butanediol production. Microb. Cell Factories 2013, 12, 20. [Google Scholar] [CrossRef]
- Rossi, D.M.; da Costa, J.B.; de Souza, E.A.; Peralba, M.d.C.R.; Ayub, M.A.Z. Bioconversion of residual glycerol from biodiesel synthesis into 1, 3-propanediol and ethanol by isolated bacteria from environmental consortia. Renew. Energy 2012, 39, 223–227. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, J.; Fu, H.; Mu, Y.; Sun, Y.; Xu, Y.; Xiu, Z. A Klebsiella pneumoniae bacteriophage and its effect on 1, 3-propanediol fermentation. Process. Biochem. 2016, 51, 1323–1330. [Google Scholar] [CrossRef]
- Wei, D.; Xu, J.; Sun, J.; Shi, J.; Hao, J. 2-Ketogluconic acid production by Klebsiella pneumoniae CGMCC 1.6366. J. Ind. Microbiol. Biotechnol. 2013, 40, 561–570. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Du, C.; Li, Y.; Cao, Z.A. Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab. Eng. 2009, 11, 101–106. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, S.; Huang, J.; Guo, H.; Bi, X.; Hou, M.; Chen, X.; Hou, S.; Lin, H.; Lu, Y.; et al. Optimization of Initial Cation Concentrations for L-Lactic Acid Production from Fructose by Lactobacillus pentosus Cells. Appl. Biochem. Biotechnol. 2021, 193, 1496–1512. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Ma, K.; Cui, Y.; Zhao, K.; Yang, Y.; Wang, Y.; Hu, G.; He, M. d-Lactic acid production from agricultural residues by membrane integrated continuous fermentation coupled with B vitamin supplementation. Biotechnol. Biofuels Bioprod. 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and characterization of PLA-based green composites: A review. J. Thermoplast. Compos. Mater. 2014, 27, 52–81. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.; Zhang, Y.; Chen, Y.; Hang, H.; Chu, J.; Zhuang, Y. Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour. Technol. 2021, 323, 124549. [Google Scholar] [CrossRef] [PubMed]
- Thitiprasert, S.; Piluk, J.; Tolieng, V.; Tanaka, N.; Shiwa, Y.; Fujita, N.; Tanasupawat, S.; Thongchul, N. Draft genome sequencing of Sporolactobacillus terrae SBT-1, an efficient bacterium to ferment concentrated sugar to d-lactic acid. Arch. Microbiol. 2021, 203, 3577–3590. [Google Scholar] [CrossRef]
- Tamires Moreira Melo, N.; Pontes, G.C.; Procópio, D.P.; de Gois e Cunha, G.C.; Eliodório, K.P.; Costa Paes, H.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Zeng, J.; Jian, J.-H.; Zhu, J.-F.; Zhang, G.-X.; Liu, D.-H. Model-based temperature control for improving lactic acid production from glycerol. RSC Adv. 2019, 9, 11614–11620. [Google Scholar] [CrossRef]
- Jovan, Ć.; Nataša, J.; Slavica, I.; Sandra, K.; Dragiša, S.; Vlada, V. Production of lactic acid by Enterococcus faecalis on waste glycerol from biodiesel production. Chem. Ind. Chem. Eng. Q. 2020, 26, 151–156. [Google Scholar] [CrossRef]
- Singh, K.; Ainala, S.K.; Park, S. Metabolic engineering of Lactobacillus reuteri DSM 20,016 for improved 1,3-propanediol production from glycerol. Bioresour. Technol. 2021, 338, 125590. [Google Scholar] [CrossRef]
- Doi, Y. Lactic acid fermentation is the main aerobic metabolic pathway in Enterococcus faecalis metabolizing a high concentration of glycerol. Appl. Microbiol. Biotechnol. 2018, 102, 10183–10192. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Sousa, S.; Gomes, A.M.; Lorenzo, J.M.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Utilization of glycerol during consecutive cycles of Lactobacillus reuteri fermentation under pressure: The impact on cell growth and fermentation profile. Process. Biochem. 2018, 75, 39–48. [Google Scholar] [CrossRef]
- Bai, Z.; Gao, Z.; Sun, J.; Wu, B.; He, B. d-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresour. Technol. 2016, 207, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Ardagh, M.A.; Shetty, M.; Chatzidimitriou, A.; Kumar, G.; Vlaisavljevich, B.; Dauenhauer, P.J. On the Spatial Design of Co-Fed Amines for Selective Dehydration of Methyl Lactate to Acrylates. ACS Catal. 2021, 11, 5718–5735. [Google Scholar] [CrossRef]
- Makshina, E.V.; Canadell, J.; van Krieken, J.; Sels, B.F. Potassium-Modified ZSM-5 Catalysts for Methyl Acrylate Formation from Methyl Lactate: The Impact of the Intrinsic Properties on Their Stability and Selectivity. ACS Sustain. Chem. Eng. 2022, 10, 6196–6204. [Google Scholar] [CrossRef]
- Pang, Y.; Lee, C.S.; Vlaisavljevich, B.; Nicholas, C.; Dauenhauer, P. Multifunctional Amine Modifiers for Selective Dehydration of Methyl Lactate to Acrylates. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics, Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1991; pp. 115–176. [Google Scholar]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Shimodaira, H. An Approximately Unbiased Test of Phylogenetic Tree Selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef]
- Almeida, I.C.; Pacheco, T.F.; Machado, F.; Gonçalves, S.B. Evaluation of different strains of Saccharomyces cerevisiae for ethanol production from high-amylopectin BRS AG rice (Oryza sativa L.). Sci. Rep. 2022, 12, 2122. [Google Scholar] [CrossRef] [PubMed]
- Vivek, N.; Pandey, A.; Binod, P. Biological valorization of pure and crude glycerol into 1, 3-propanediol using a novel isolate Lactobacillus brevis N1E9. 3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Camacho, H.; Aguilar-Vera, A.; Beltran-Rojel, M.; Aguilar-Vera, E.; Duran-Bedolla, J.; Rodriguez-Medina, N.; Lozano-Aguirre, L.; Perez-Carrascal, O.M.; Rojas, J.; Garza-Ramos, U. Molecular epidemiology of Klebsiella variicola obtained from different sources. Sci. Rep. 2019, 9, 10610. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Oh, B.-R.; Lee, S.-M.; Heo, S.-Y.; Seo, J.-W.; Kim, C.H. Efficient production of 1, 3-propanediol from crude glycerol by repeated fed-batch fermentation strategy of a lactate and 2, 3-butanediol deficient mutant of Klebsiella pneumoniae. Microb. Cell Factories 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Zhang, A.H.; Huang, S.Y.; Zhuang, X.Y.; Wang, K.; Yao, C.Y.; Fang, B.S. A novel kinetic model to describe 1, 3-propanediol production fermentation by Clostridium butyricum. AIChE J. 2019, 65, e16587. [Google Scholar] [CrossRef]
- Hong, A.A.; Cheng, K.K.; Peng, F.; Zhou, S.; Sun, Y.; Liu, C.M.; Liu, D.H. Strain isolation and optimization of process parameters for bioconversion of glycerol to lactic acid. J. Chem. Technol. Biotechnol. 2009, 84, 1576–1581. [Google Scholar] [CrossRef]
- Sangproo, M.; Polyiam, P.; Jantama, S.S.; Kanchanatawee, S.; Jantama, K. Metabolic engineering of Klebsiella oxytoca M5a1 to produce optically pure d-lactate in mineral salts medium. Bioresour. Technol. 2012, 119, 191–198. [Google Scholar] [CrossRef]
- Ochoa, D.H.; Braga, J.W.B.; Machado, F. Optimization of the Functional Characteristics of Cleaning Products Through Experimental Design. J. Surfactants Deterg. 2017, 20, 467–481. [Google Scholar] [CrossRef]
- Mota, M.F.; Rodrigues, M.G.F.; Machado, F. Oil–water separation process with organoclays: A comparative analysis. Appl. Clay Sci. 2014, 99, 237–245. [Google Scholar] [CrossRef]
- Suemar, P.; Fonseca, E.F.; Coutinho, R.C.; Machado, F.; Fontes, R.; Ferreira, L.C.; Lima, E.L.; Melo, P.A.; Pinto, J.C.; Nele, M. Quantitative Evaluation of the Efficiency of Water-in-Crude-Oil Emulsion Dehydration by Electrocoalescence in Pilot-Plant and Full-Scale Units. Ind. Eng. Chem. Res. 2012, 51, 13423–13437. [Google Scholar] [CrossRef]
- Ji, X.-J.; Huang, H.; Zhu, J.-G.; Hu, N.; Li, S. Efficient 1, 3-propanediol production by fed-batch culture of Klebsiella pneumoniae: The role of pH fluctuation. Appl. Biochem. Biotechnol. 2009, 159, 605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, P.; Li, Y.; Xu, L.; Tian, P. Engineering CRISPR interference system in Klebsiella pneumoniae for attenuating lactic acid synthesis. Microb. Cell Factories 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Microorganism | Substrate | Lactic Acid Concentration (g·L−1) | Yield (g·g−1) | Productivity (g·L−1·h−1) | Reference |
|---|---|---|---|---|---|
| Komagataella phiffi Strains | glycerol | 70.0 | 0.665 | 1.39 | [27] |
| Escherichia coli AC-521 | glycerol | 90.4 | 0.88 | 1.13 | [28] |
| Enterococcus faecalis | waste glycerol from biodiesel production | 15.8 | 0.37 | 0.61 | [29] |
| Lactobacillus reuteri DSM 20,016 Δadh2 | glycerol and glucose | 50.44 | 0.78 | 1.50 * | [30] |
| Enterococcus faecalis strain W11 | glycerol | 135.0 | 0.9 | 0.87 | [31] |
| Lactobacillus reuteri | glycerol and glucose | 6.0 | 0.536 | 0.28 | [32] |
| Variables | −1 | 0 | +1 |
|---|---|---|---|
| X1: Temperature (°C) | 27 | 32 | 37 |
| X2: Inoculum concentration (g·L−1) | 5 | 12.5 | 20 |
| X3: pH | 6.4 | 7.4 | 8.5 |
| Experiment | X1 | X2 | X3 |
| 1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 |
| 3 | −1 | +1 | −1 |
| 4 | +1 | +1 | −1 |
| 5 | −1 | −1 | +1 |
| 6 | +1 | −1 | +1 |
| 7 | −1 | +1 | +1 |
| 8 | +1 | +1 | +1 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 |
| Variables | −1.41 | −1 | 0 | +1 | +1.41 |
|---|---|---|---|---|---|
| X1: Inoculum concentration (g·L−1) | 6.1 | 7.5 | 10 | 12.5 | 14.9 |
| X2: pH | 7.6 | 9 | 9.5 | 10 | 11.4 |
| Experiment | X1 | X2 | |||
| 1 | −1 | −1 | |||
| 2 | +1 | −1 | |||
| 3 | −1 | +1 | |||
| 4 | +1 | +1 | |||
| 5 | 0 | 0 | |||
| 6 | 0 | 0 | |||
| 7 | 0 | 0 | |||
| 8 | −1.41 | 0 | |||
| 9 | +1.41 | 0 | |||
| 10 | 0 | −1.41 | |||
| 11 | 0 | +1.41 | |||
| 24 h | 48 h | |||||
|---|---|---|---|---|---|---|
| Inoculum Concentration (g·L−1) | Lactic Acid (g·L−1) | 1,3-Propanediol (g·L−1) | Lactic Acid (g·L−1) | 1,3-Propanediol (g·L−1) | YLA/Gly | Y1,3-PDO/Gly |
| 5.0 (120 rpm) | 1.00 ± 0.09 | 0.61 ± 0.09 | 1.94 ± 0.41 | 1.28 ± 0.20 | 0.54 | 0.36 |
| 4.2 (120 rpm) | 1.10 ± 0.13 | 0.87 ± 0.14 | 1.43 ± 0.21 | 1.09 ± 0.21 | 0.41 | 0.31 |
| 2.0 (120 rpm) | 0.79 ± 0.12 | 0.53 ± 0.11 | 1.34 ± 0,13 | 0.97 ± 0.27 | 0.38 | 0.28 |
| 1.0 (120 rpm) | 0.81 ± 0.19 | 0.68 ± 0.25 | 1.29 ± 0.13 | 0.94 ± 0.59 | 0.37 | 0.26 |
| 4.2 (unstirred) | 2.29 ± 0.02 | 1.48 ± 0.01 | 2.88 ± 0.39 | 1.91 ± 0.27 | 0.81 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, R.G.M.R.; Lima, J.R.C.; Fávaro, L.C.L.; Machado, F.; Gonçalves, S.B. Bioconversion of Glycerol into Lactic Acid by a New Bacterial Strain from the Brazilian Cerrado Soil. Fermentation 2022, 8, 477. https://doi.org/10.3390/fermentation8100477
Barroso RGMR, Lima JRC, Fávaro LCL, Machado F, Gonçalves SB. Bioconversion of Glycerol into Lactic Acid by a New Bacterial Strain from the Brazilian Cerrado Soil. Fermentation. 2022; 8(10):477. https://doi.org/10.3390/fermentation8100477
Chicago/Turabian StyleBarroso, Raissa G. M. R., Jamille R. C. Lima, Léia C. L. Fávaro, Fabricio Machado, and Sílvia B. Gonçalves. 2022. "Bioconversion of Glycerol into Lactic Acid by a New Bacterial Strain from the Brazilian Cerrado Soil" Fermentation 8, no. 10: 477. https://doi.org/10.3390/fermentation8100477
APA StyleBarroso, R. G. M. R., Lima, J. R. C., Fávaro, L. C. L., Machado, F., & Gonçalves, S. B. (2022). Bioconversion of Glycerol into Lactic Acid by a New Bacterial Strain from the Brazilian Cerrado Soil. Fermentation, 8(10), 477. https://doi.org/10.3390/fermentation8100477







