Abstract
This study explores the production of prebiotic xylooligosaccharide (XOS) from cassava pulp waste and its effectiveness for the growth of Lactobacillus acidophilus (L. acidophilus). We successfully produced and characterized XOS from cassava pulp xylan using a Bacillus sp. endo-β-1,4-D-xylanase. The XOS was added to modify the MRS medium (MRSm) in various concentrations (0, 1, 3 and 5%) in which the L. acidophilus was inoculated. The growth of L. acidophilus was observed every 12 h for 2 days, and the fermentation products were analyzed for pH, sugar content, and short-chain fatty acids (SCFA) in terms of types and amount. The study showed that L. acidophilus grew well in MRSm. The optimum XOS concentration in MRSm was 5%, indicated by the highest growth of L. acidophilus (8.61 log CFU mL−1). The profile of SCFA products is 14.42 mM acetic acid, 0.25 mM propionic acid, 0.13 mM isobutyric acid, 0.41 mM n-butyric acid, 0.02 mM n-valeric acid, 0.25 mM isovaleric acid, and 25.08 mM lactic acid.
1. Introduction
The adult human digestive tract contains more than 500 different bacterial species, which play an important role in colon function [1]. These beneficial bacteria can support and benefit the host’s health by helping to digest complex carbohydrates and maintaining the balance between various types of intestinal bacteria [2,3,4], especially pathogens. They also produce vitamins, short chains of fatty acids (SCFA), and other nutrients for their host, providing up to 15% of total daily caloric intake. It has been reported that a balanced microflora is essential for bowel function, including resistance to infection by pathogens [5]. Prebiotics are defined as “undigested food ingredients that affect the host by stimulating selective growth and activity of one or a limited number of beneficial bacteria in the large intestine, thereby improving host healthiness” [6,7]. Many studies have confirmed that merging prebiotics with food is a good approach to altering colon microflora [7,8]. Accordingly, popular interest in human gut microbes has increased, followed by an interest in improving host health through dietary intervention influencing the gut microbiome, particularly using “non-digestible oligosaccharides” (NDOs) as prebiotics.
NDOs, by definition, belong to the broad category of dietary fiber, which is not digested or absorbed in the small intestine of humans, but might be completely or partially fermented in the large intestine. Besides their prebiotic effects, NDOs are believed to relieve symptoms of diseases such as diabetes, arteriosclerosis, and colon cancer [9,10]. Many positive health effects are related to microbial fermentation of NDOs in the large intestine. The fermentation rate of NDOs depends on the nature of the substrate.
Bifidobacteria and Lactobacilli, essential and beneficial microbes that inhabit the human gut, are common target species for food intervention studies [11]. Bacterial enzymes will ferment NDO to produce short-chain fatty acids (SCFA), including acetate, propionate, and butyrate. These molecules represent the major flow of carbon from the diet through the microbiome to the host and contribute to the acidification of the intestine [10]. Acidification influences bacterial species’ balance, metabolic activity, and product formation. For example, acetate is mainly metabolized in human muscles, the kidneys, the heart, and the brain, while propionate is a possible gluconeogenic precursor suppressing cholesterol synthesis [12]. In addition, butyrate is known to have anti-proliferation and anti-angiogenic effects in colonocytes [13].
NDO can be formed not only by acidic hydrolysis of polysaccharides resulting in non-specific oligosaccharides, but also by using enzymes to produce specific ones. For example, xylan can be hydrolyzed enzymatically by endo-β-1,4-D-xylanase to produce prebiotic xylooligosaccharide (XOS), including xylotriose (X3), xylotetraose (X4), xylopentaose (X5) and small amounts of xylose (X1) [14,15]. XOS has a degree of polymerization (DP) between 2 and 10, and is applicable to stimulate the growth of probiotic bacteria in the intestine, such as Bifidobacteria and Lactobacilli [16,17,18]. Besides prebiotic effects, XOS stimulates antioxidant activity [19]. This is important because XOS supports the prevention of digestive diseases such as colorectal cancer, Crohn’s disease, and ulcerative colitis [20,21,22]. Several studies have discovered the production of XOS from various biomass sources, such as corn hull, garlic bagasse, and cassava peel [16,23,24]. The potential market for XOS application mainly corresponds to ingredients for functional and healthy foods, such as combinations with milk, soft drinks, etc. [25]
The consumption of oligosaccharides in foods will maintain the symbiotic relationship between Lactobacillus sp. and the host [20,26,27] As such, the production of XOS by utilizing beneficial bacteria is currently under scrutiny in the academic community. In these trials, lactic acid bacteria, such as Bifidobacterium sp., and Lactobacillus sp., are commonly used. For example, Rycroft et al. [28] conducted research using fructooligosaccharide (FOS), Inulin, XOS, lactulose, isomalto-oligosaccharides (IMO), galactooligosaccharides (GOS), and soybean oligosaccharides (SOS) using several probiotics, one of which is Lactobacillus sp. Like other analogous probiotics, Lactobacillus can inhibit the growth of pathogenic bacteria, increase body immunity, and yield anticarcinogenic effects in the human intestine. For example, Ooi and Liong [29] reduced cholesterol levels by 22.6% using L. acidophilus. In addition, Probiotic L. acidophilus can inhibit the growth of C. Albicans in the mouth because it can produce short-chain fatty acids and bacteriocins [30]. On the other hand, the probiotic effect of xylooligosaccharide has been studied less compared to fructooligosaccharide (FOS) and galactooligosaccharides (GOS) [31].
Probiotic intervention shows great promise in supporting human health. Therefore, various methods have been employed to cultivate the production of specific probiotics. Here, we focus on providing the appropriate raw materials for the cultivation of these probiotics with a particular emphasis on exploring alternative ways to utilize agricultural wastes from the tapioca production industry in Indonesia. Like many other food production processes, this production results in tremendous waste: The production of 250–300 tons of tapioca results in approximately 280 tons of pulp and 1.6 tons of peel as by-products [32]. As such, this study explores how cassava pulp and peel can be utilized to stimulate probiotic growth.
Cassava pulp contains (w/w%) 36.6% cellulose, 21.3% hemicellulose, 17.3% lignin, 2.4% protein, and 7.0% ash [33]. Hemicellulose of cassava pulp is a good source of xylan, which is estimated to contain 6.23% of xylan from cassava pulp, using 10% NaOH extraction [15]. Cassava pulp xylan can be hydrolyzed enzymatically to produce prebiotic XOS. There are no detailed reports concerning the production of XOS from cassava pulp waste.
Previously, we isolated endo-β-1,4-D-xylanase from a Bacillus sp. isolated from the soil termite abdomen, potentially a hydrolytic enzyme for xylan [15,34] In this research, we wanted to analyze the potential enzyme for cassava pulp, and XOS compounds obtained from cassava pulp were produced by enzymatic hydrolysis using endo-β-1,4-D-xylanase. Specifically, we tried to examine the feasibility of XOS cassava pulp as a prebiotic for the cultivation of the probiotic L. acidophilus. The growth of L. acidophilus was examined in various XOS concentrations added to the MRS modification medium. Observations also included pH, sugar content, and the profile of SCFA being produced.
2. Materials and Methods
2.1. Materials
Cassava pulp was obtained from a home industry of a cassava mill in the Jember area. The pulp was dried in an oven at 65 °C, ground, and sieved (200 mesh). The powder was extracted for its xylan content. Then, the xylan was stored in containers at room temperature. Endo-β-1,4-D-xylanase was produced by Bacillus sp. isolated from soil termites’ abdomen and purified by Ni-NTA chromatography [34]. L. acidophilus was purchased commercially from Institut Pertanian Bogor (IPB), Indonesia. The protein content in crude enzyme was determined by the Bradford method [35] using BSA (bovine serum albumin) as a standard. At the same time, the Miller method measured the activity by determining reduction in sugars by DNS (dinitrosalicylic acid). The probiotics and media used were L. acidophilus (pure culture) and MRSB (Merck). All reagents and chemicals used in this study were of analytical grade (Merck, Sigma). The standard XOS, xylose (X1), xylobiose (X2), xylotriose (X3), xylotetraose (X4), and xylopentaose (X5), were obtained from Megazyme, Ireland. Silica Gel 60 F254 TLC plate was obtained from Merck, Germany. Dialysis tubes were obtained from Elkay.
2.2. Production of XOS from Cassava Pulp
Cassava pulp xylan solution 1.1% (w/v) was used as the substrate. Endo-β-1,4-D-xylanase enzyme was added at a ratio of 1:1. Endo-β-1,4-D-xylanase has a specific activity of 31.30 U/mg and total protein content of 0.454 mg. The mixture was then incubated at 40 °C for 16 h and then centrifuged at 7826× g for 10 min at 4 °C. Negative control was prepared by the same method as described before by using an inactivated endo-β-1,4-D-xylanase (heated at 100 °C) [15].
2.3. Analysis of XOS by Thin Layer Chromatography (TLC) and High-Performance Liquid Chromatography (HPLC)
TLC was conducted to analyze the obtained XOS composition qualitatively. Up to 4 µL of XOS products were placed on the activated chromatoplate. The chromatoplate was developed in the mixture of n-butanol, acetic acid, and distilled water with a ratio of 2:1:1 (v:v:v) as the mobile phase. After running, spots of XOS were detected by naphthol and H2SO4 in ethanol, followed by drying at 100 °C. Standard and negative control was also running in the same TLC plate.
On the other hand, HPLC analysis was carried out to observe XOS composition quantitatively. The HPLC column was the Waters Sugarpak carbohydrate column (6.5 × 300 mm). The water flow rate in the mobile phase was 0.5 L/min. The standard XOS were xylose (X1), xylobiose (X2), xylotriosa (X3), xylopentaose (X4), and xylohexose (X5).
2.4. MRS-Modified Media (MRSm) Production
MRSm was produced by mixing 1 g of tryptophan, 0.8 g of meat extract, 0.4 g of yeast extract, 0.2 g of K2HPO4, 0.1 g of Tween 80, 0.2 g of sodium acetate, 0.02 g of magnesium sulfate, 0.004 g of manganese sulfate, and 0.2 g of (NH4)2CO3. Then, the obtained XOS was added to the media with different concentrations (0, 1, 3 and 5%).
2.5. Fermentation of L. acidophilus
Fermentation of L. acidophilus was carried out in MRS-modified media (MRSm) with various concentrations of cassava pulps XOS (0%, 1%, 3% and 5%, v/v). The culture was incubated at 37 °C, 150 rpm shaking for 2 days with sampling at 0, 12, 24, 36, and 48 h. Samples were analyzed for the population of L. acidophilus, pH, and sugar content. For the sugar content, as the total reducing sugar, measured by the Miller method, 1000 μL of the fermented sample was heated for 1 min. Then, it was centrifuged for 10 min (4 °C/7826× g). Into 250 μL of the supernatant, 750 μL of Miller reagents were added. The mixture was heated for 15 min (100 °C), cooled for 20 min, and measured for the absorbance at 550 nm.
2.6. Calculation of Lactic Acid Bacterial Populations
A sample from fermentation (1 mL) was added to 9 mL of saline solution, followed by serial dilutions (10−1–10−8). Finally, 1 mL solution from the last two series of dilutions (10−7 and 10−8) was poured into MRSB and MRSm agar media and incubated at 37 °C for 48 h. Lactic acid bacterial colonies were calculated according to the Bacteriological Analytical Manual (BAM) 2001 standard based on FAO/ WHO (Food and Agriculture Organization/World Health Organization).
2.7. SCFA and Lactic Acid Analysis
SCFA profile and lactic acid were determined after 48 h of incubation. A total of 0.003 g of sulfur 5-salicylate dihydrate was added to the 1 mL sample. The mixture was centrifuged at 11,269 × g (7 °C for 5 min). The supernatant was analyzed for the SCFA profile by gas chromatography. Lactic acid levels were measured by titration using 0.1 N NaOH.
3. Results
3.1. Production and Characterization of XOS from Cassava Pulp
XOS was produced through hydrolysis of cassava pulp xylan by endo-1.4-D-xylanase (31.3 U/mg) in an incubation condition of 40 °C, pH 5, and 24 h. Yield XOS was 0.237% ± 0.002 (w/w) from 1 g cassava pulp.
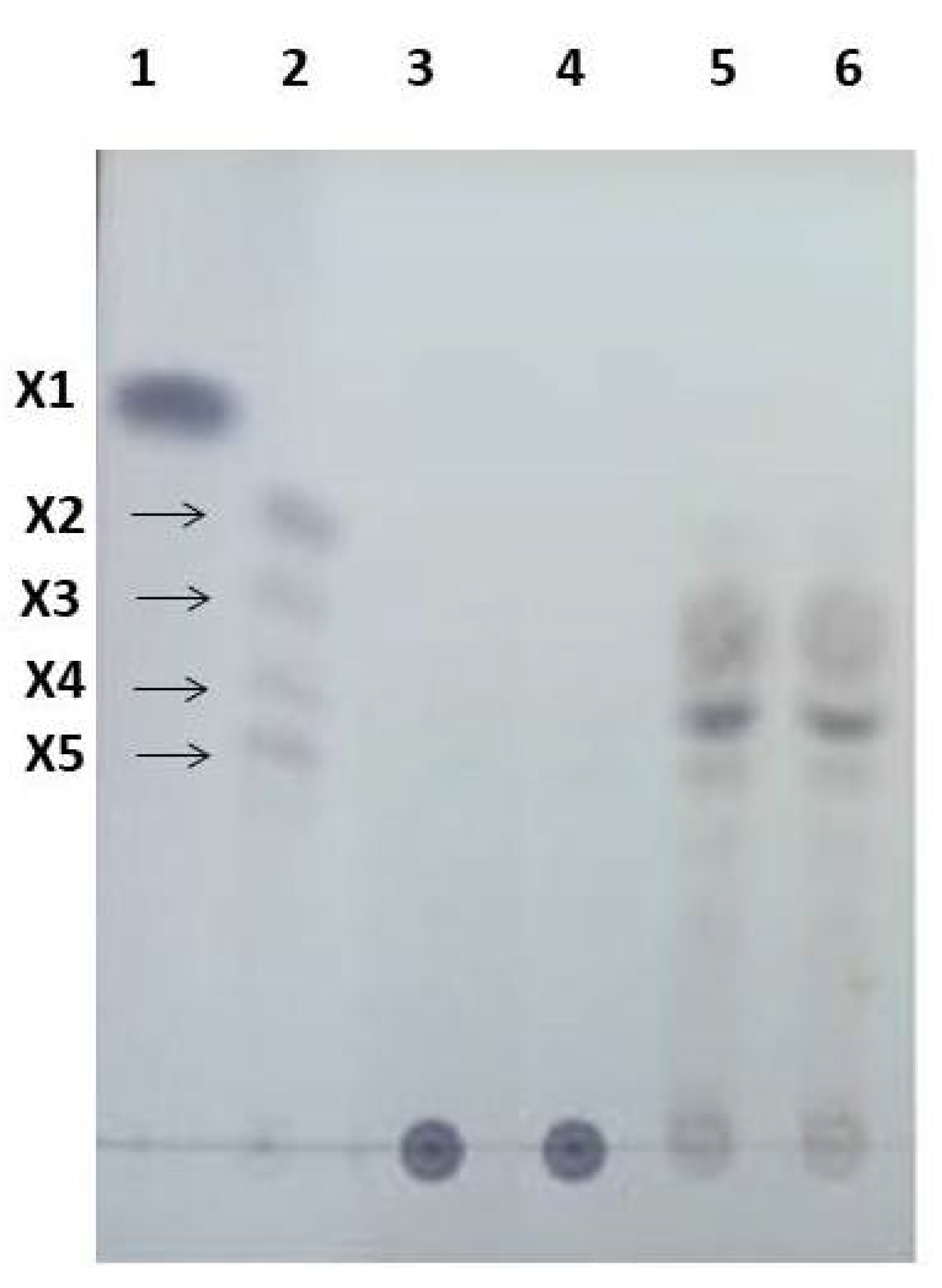
The product composition was analyzed by TLC (Figure 1). The resulting spots were identified based on the similarity of the position (Rf) with standard XOS, confirming xylotriose, xylotetraose, and xylopentaose as enzymatic products and a lack of xylose and xylobiose.
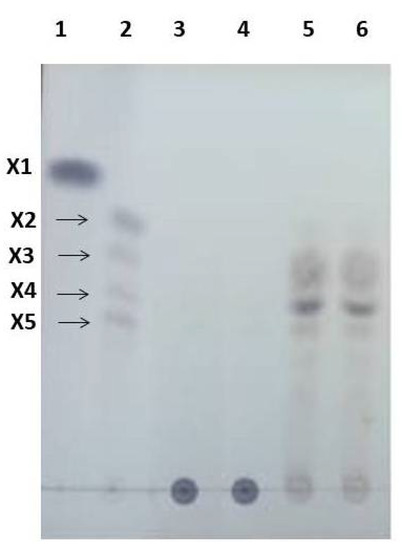
Figure 1.
TLC chromatogram of XOS after enzymatic hydrolysis of cassava pulp xylan by endo-1.4-D-xylanase (in lanes 5 and 6), xylotetraose being the major product. Standard XOS: X1 (lane 1); and mixtures X2–X5 (lane 2). The control from the inactive enzyme (lanes 3 and 4).
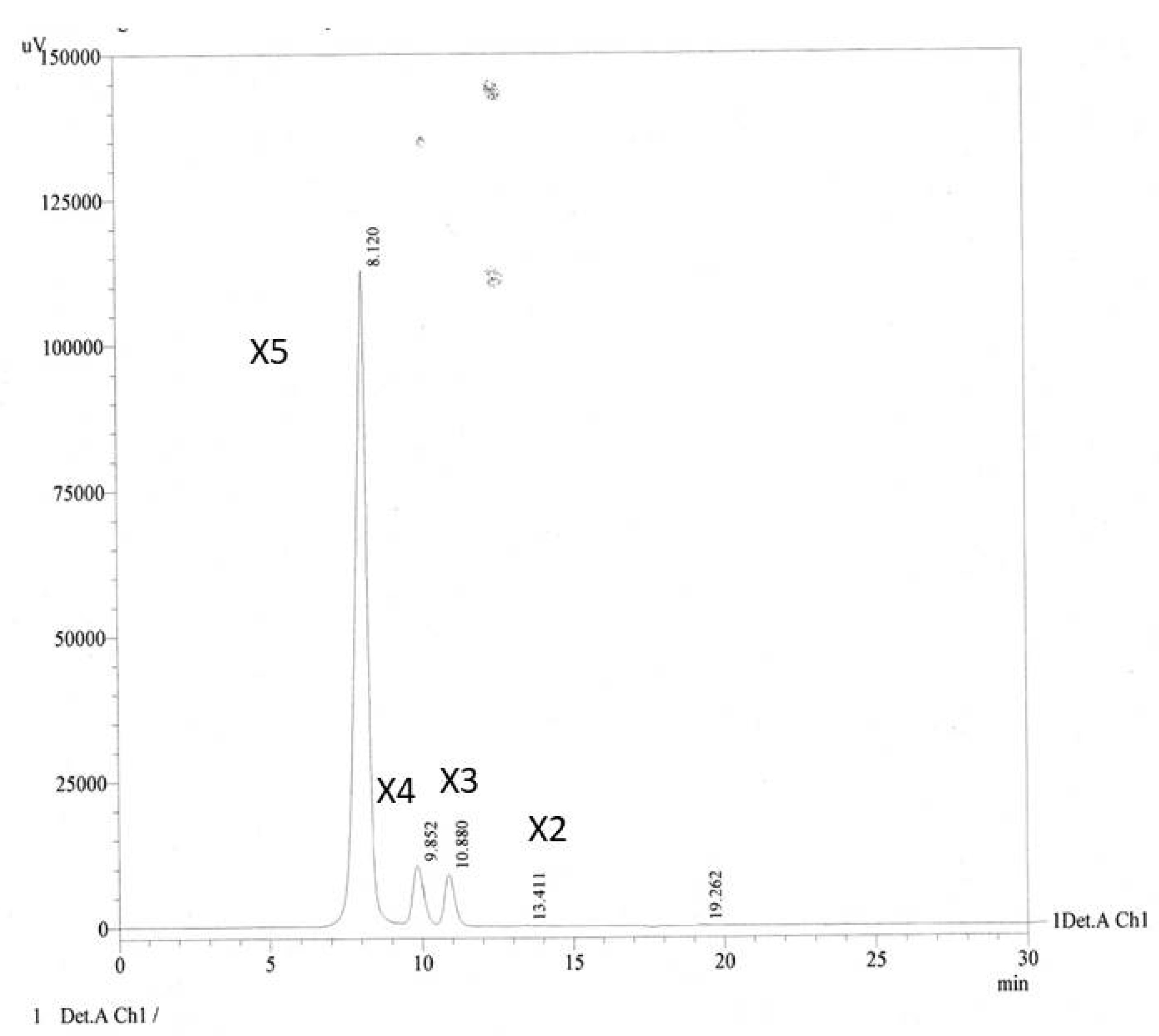
Quantitative analysis by HPLC (Figure 2) revealed the concentrations of XOS in hydrolysate to be 7.43, 89.80, 35.17 and 5591.15 ppm for xylobiose (X2), xylotriose (X3), xylotetraose (X4) and xylopentaose (X5), respectively.
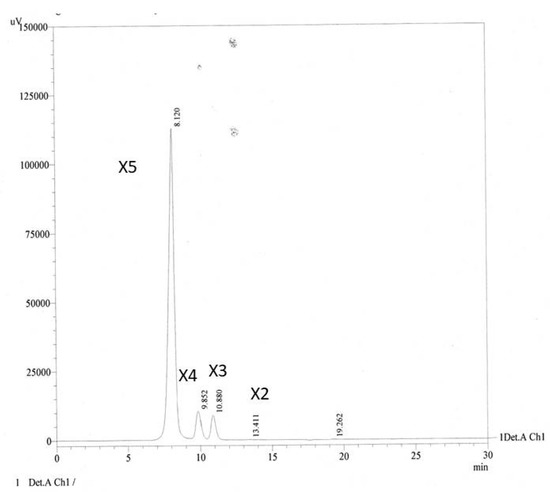
Figure 2.
HPLC chromatogram of XOS from enzymatic hydrolysis of cassava pulp xylan by endo-1.4-D-xylanase, Xn indicates the xylose subunits in the oligomers.
Lanes 5 and 6 of TLC show a thicker spot between X4 and X5 than standard line 2. Further analysis by HPLC referring to this thicker spot is X5 as the most abundant product of hydrolyses. The hydrolysis products of corn cob xylan in alkaline conditions were xylobiose and xylotriose as the main components [35]. Yield of XOS was 30% (w/v).
3.2. Growth of L. acidophilus
Prebiotic properties of XOS are already present in its crude solution, which stimulates the growth of the intestinal microflora. Furthermore, in the human digestion system, XOS encourages the growth of beneficial bacteria, such as Bifidobacterium sp. and Lactobacillus sp. [20,23,36,37]. Thus, the benefits of XOS are protecting against infection via the inhibition of pathogenic bacterial growth, decreasing intestinal pH, producing nutrients, increasing mineral absorption, and other biological benefits, including antioxidants, anti-inflammatory, and anti-allergic effects [20,24]
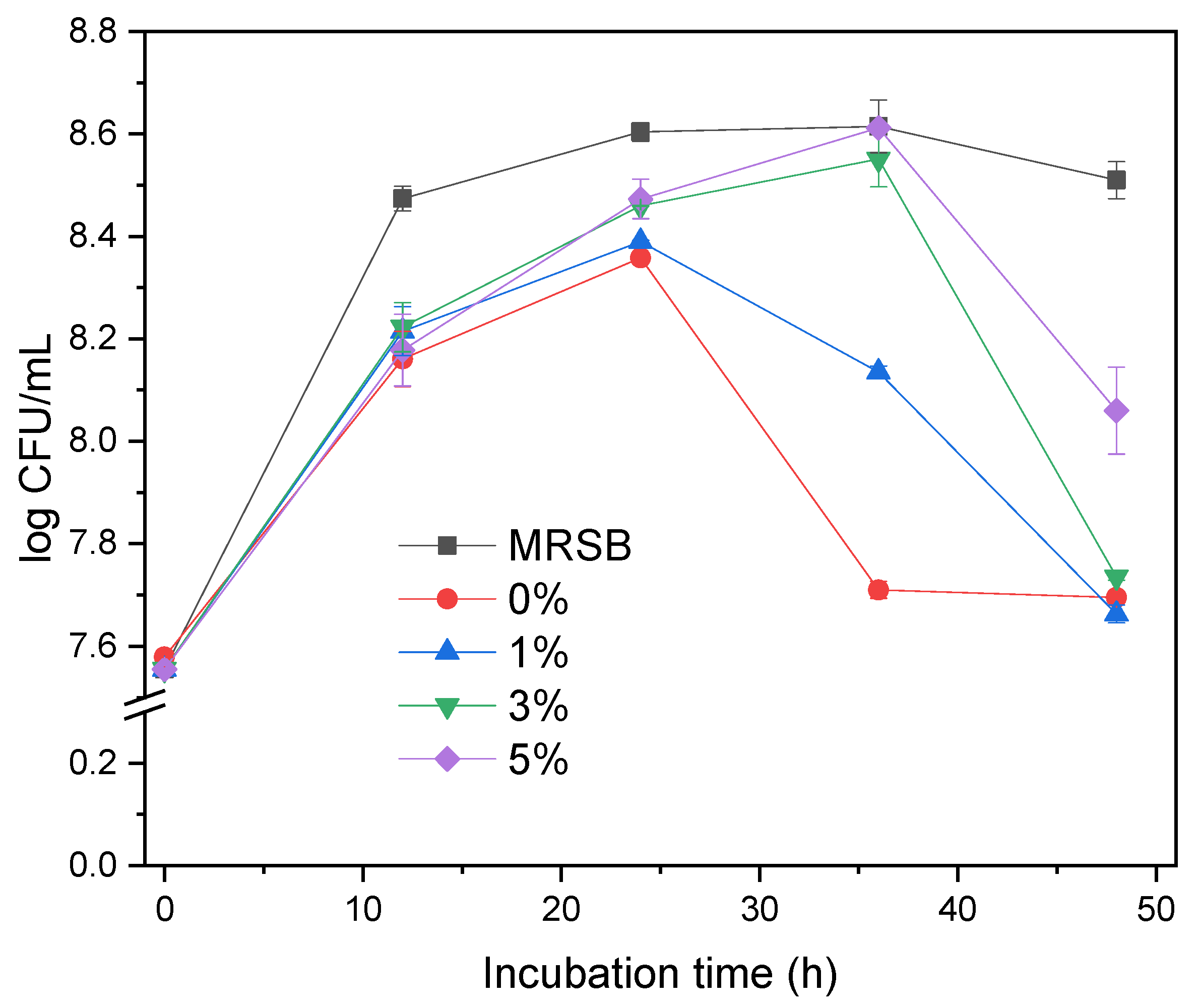
The bacterial growth profile (Figure 3) shows the population of L. acidophilus in various concentrations of cassava pulp XOS (MRSm 0, 1, 3, 5%) during 48 h observation. In general, the growth of L. acidophilus in MRSm with the addition of cassava pulp XOS is better.
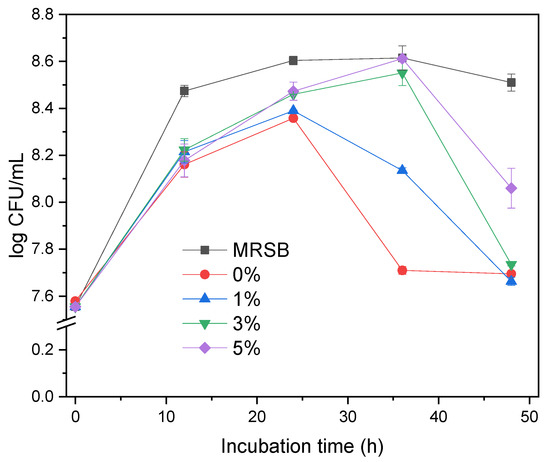
Figure 3.
Growth Curve of Lactobacillus acidophilus in MRSB and MRSm media with different concentration of XOS as a function of incubation time.
Figure 3 shows that L. acidophilus increased from 7 log CFU mL−1. The total population of bacterial colonies reported in the small intestine was 5–6 log CFU mL−1 [38]. An increase of 8.61 log 10 CFU mL−1 indicates that the added XOS cassava pulp was successfully used as a carbon source for L. acidophilus.
The growth profiles are affected well by the concentration of XOS in MRSm. L. acidophilus grows up in all media at the first 24 h and then decreases for the MRSm 0 and 1%. The bacteria in MRSm 3 and 5% still grow up until 36 h, and then decrease in different levels, in which the population in MRSm 5% is higher than that in 3%. The growth of L. acidophilus in MRSB increases in the first 12 h and is stagnant till the end of the 48 h observation. This is because MRSB media has sufficient glucose as a carbon source to support the growth of L. acidophilus within 48 h of observation time. Its fast growth in the first 12 h is due to the simpler glucose sugar than XOS. Meanwhile, the amount of sugar in MRSm in the form of XOS is limited to 24 h for 1% and 36 h for 3 and 5%. The amount of sugar in all mediums at every 12 h of observation is presented in Figure 3.
3.3. Total Reducing Sugar
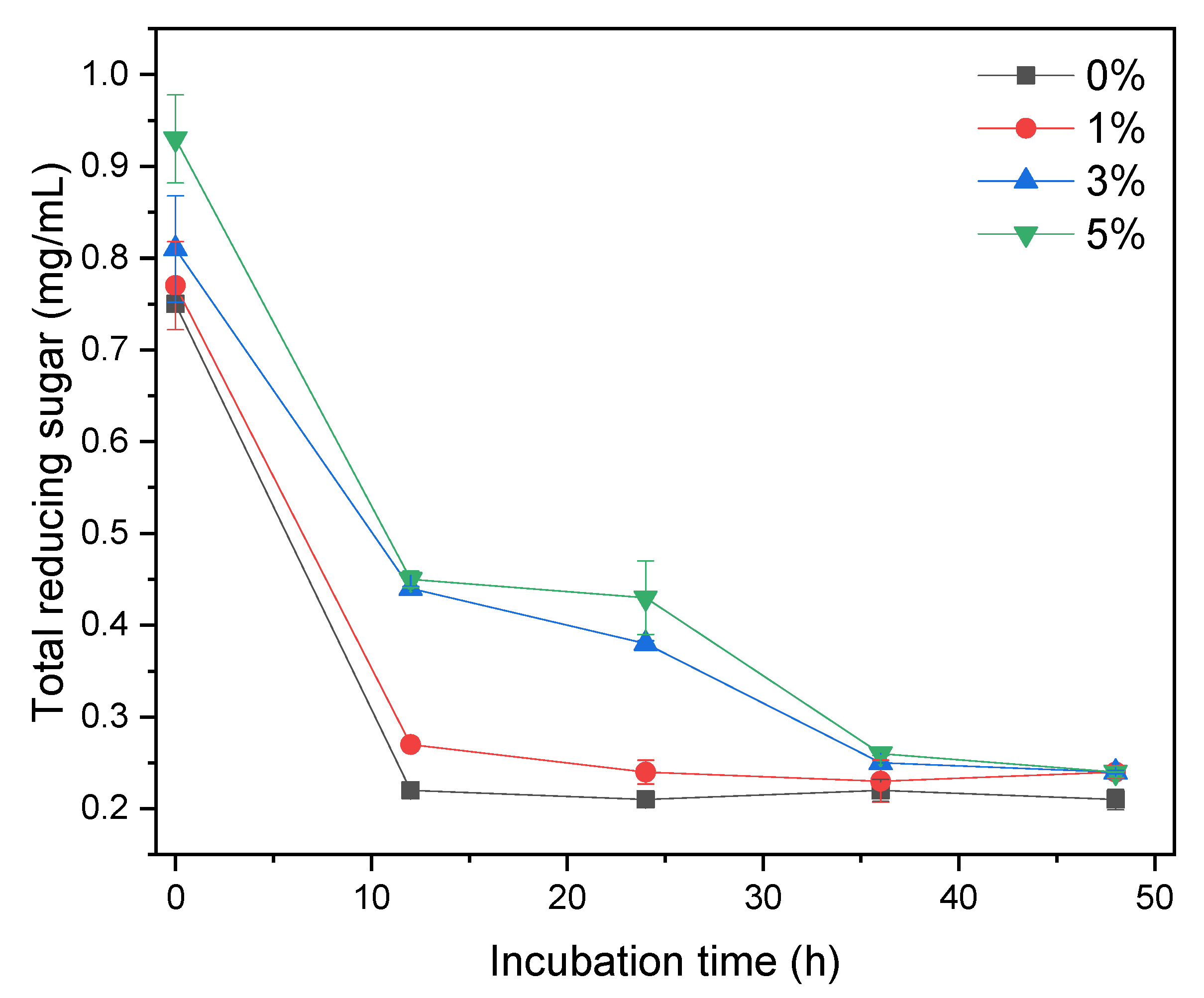
This study examined the potential of XOS as a prebiotic in L. acidophilus growth in various MRSm. XOS is metabolically oxidized as a carbon source to generate energy for bacterial growth. XOS content can be determined by total reducing sugar measurement. Figure 4 shows the total reducing sugar content in MRSm media with different concentrations of XOS as a function of incubation time. At an incubation time of 0 h, MRSm with a higher concentration of XOS has a higher total reducing sugar content. The total reducing sugar content in MRSm 0 and 1% significantly decreased at the first 12 h and remained constant for the rest of the incubation. Meanwhile, for MRSm 3 and 5%, the total reducing sugar content also significantly decreases in the first 12 h, gradually decreases from 12 to 36 h, and remains constant after 36 h of incubation.
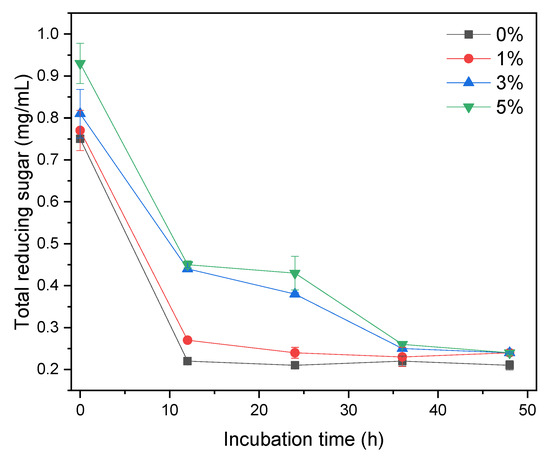
Figure 4.
Total reducing sugar content in MRSm media with different concentration of XOS as a function of incubation time.
3.4. Short-Chain Fatty Acids (SCFA)
Various SCFA were produced during XOS fermentation by L. acidophilus [37,39]. Table 1 shows the SCFA amount produced after 48 h fermentation time of XOS by L.acidophilus in MRSm 0 and 5%. The observed SCFA were lactic acid, acetic acid, propionic acid, isobutyric acid, n-butyric acid, isovaleric acid, and n-valeric acid, as revealed by gas chromatography. The total amount of SCFA produced in MRSm 5% was higher than of MRSm 0%. However, the amount of each SCFA for those media varied. A shorter chain of SCFA (e.g., acetic acid and propionic acid) was produced more in MRSm 0%. In contrast, a longer chain of SCFA (e.g., n-butyric acid, isovaleric acid, and lactic acid) was produced more in MRSm 5%.

Table 1.
Type and content of SCFA after fermentation of L. acidophilus with addition of XOS (5%) as carbon source compared to medium containing glucose (0%).
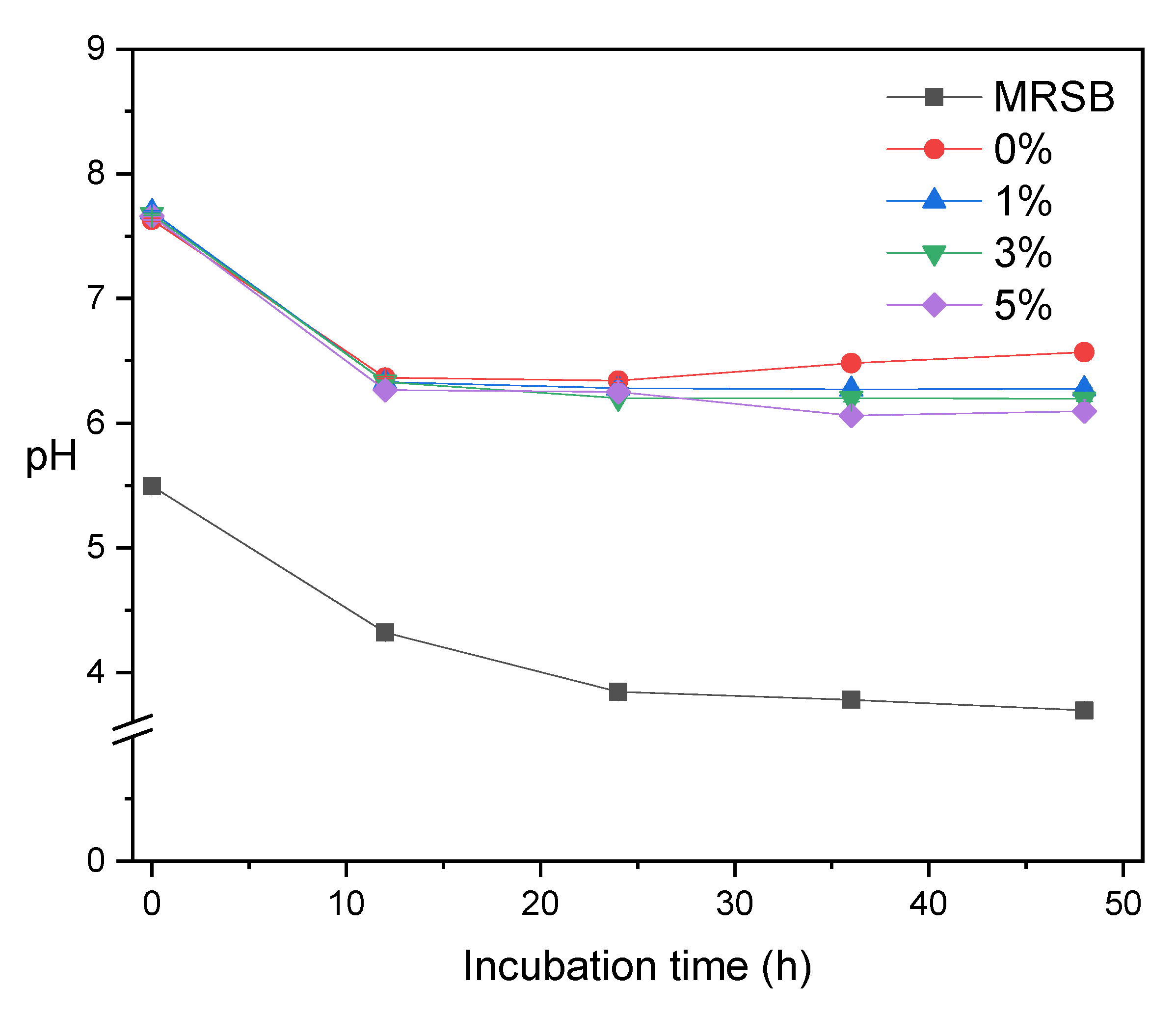
Figure 5 shows the pH profile during the fermentation process. In general, the pH value of all media decreases as the fermentation occurs. The reduction of pH is due to the SCFA production from bacterial fermentation as a function of incubation time [37]. The pH value also decreases as the concentration of XOS in media increases, which is in good agreement with the total amount of SCFA (Table 1). We observed an anomaly at MRSm 0%, where the pH increased after 24 h of incubation. It is due to the production of ammonia from meat extract. The main products of Lactobacilli carbohydrate fermentation are acetic acid and lactic acid with pKa 4.8 and 3.8, respectively [36]. The pH profiles obtained in this fermentation were slightly higher than the study of corncob XOS fermentation by L. acidophilus. Chapla et al. [40] reported that the pH after 24 h of XOS fermentation of corncobs by L. acidophilus reached 5.9 ± 0.09.
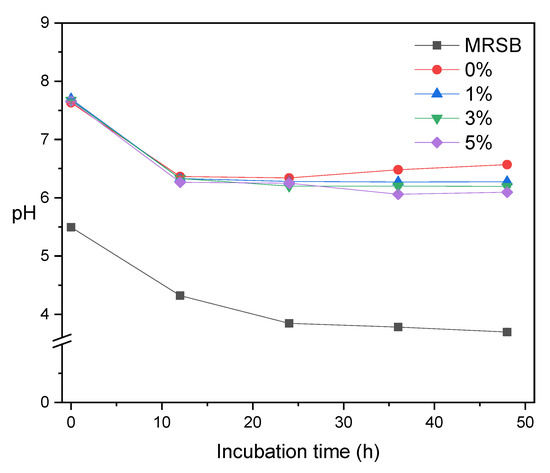
Figure 5.
pH profile of MRSB and several MRSm as a function of incubation time.
Aachary and Prapulla [25] found that fermentation by L. acidophilus increased butyric acid and total volatile fatty acids. In this study, the number of n-butyric acid in 5% was higher than 0%. This was also observed in the total amount of SCFA. Propionic acid and butyric acid produced from fermentation can prevent colon cancer [28]. In addition, SCFA can protect the digestive system against diseases, such as colorectal cancer, Chron’s disease, and ulcerative colitis [22]. Lactic acid produced during fermentation is an intermediate product and then converted to acetic acid, propionate, and butyrate [24].
4. Discussion
The differences in the growth profiles (Figure 3) are likely due to the content and composition of XOS. MRSB has simpler sugar (glucose), while cassava pulp XOS has a high X5 content. A high glucose concentration in MRSB boosted the growth during the first 12 h, while the group with the lower growth rate had bacteria digesting XOS to produce X1. The insignificant bacteria growth in MRSm 0 and 1% after 24 h can be explained by a lack of xylose to support the further development of bacteria. However, MSRm 3 and 5% cases are still increasing with similar populations of MRSB. The decline of growth after 24 and 36 h indicates that the XOS as bacterial food is nearly finished, while in MRSB, there is still enough substance to support till 48 h. The bacteria in MRSB reached maximum growth and become stagnant after 12 h because of other conditions beyond the carbon sources. The bacterial growth profile showed that cassava pulp XOS had an increased prolonging effect for L. acidophilus growth. As L. acidophilus is a human probiotic bacterium, we can provisionally conclude that XOS from enzymatically hydrolyzed cassava pulp xylan has a prebiotic effect. The higher concentration of XOS added, the better the impact on the growth.
These results show that commercial XOS can be used as a bacterial growth medium. Lactobacillus sp. growth was measured to be lower when compared to the growth of probiotic strains of Bifidobacteria, Clostridia, Bacteroides, and Streptococci. Lactobacillus growth in commercial XOS media reached 8.93 log CF/gram feces [28]. Bengal gram husk XOS and Wheat bran XOS were utilized effectively by all the microorganisms, except L. plantarum NDRI strain 184 [41]. The growth of L. acidophilus with the addition of XOS cassava pulp (5%) reached 8.61 log CFU/mL.
We believe that the significant decrease in the total reducing sugar content (Figure 4) is likely due to the simple XOS (X2–X4), which was easily digested and consumed by bacteria during the first 12 h. After 12 h, this simple XOS depleted, and bacteria began to consume the higher XOS, i.e., X5. It is also confirmed by HPLC results (Figure 2) that the obtained XOS mainly consists of X5. This profile also aligns with the growth curve of bacteria in Figure 3, where the bacteria significantly grow in the first 12 h and then remain constant. It indicates that the main metabolism pathway in both media was different.
The variation of the obtained SCFA amount in different media (Table 1) is due to the other XOS type consumed by L. acidophilus leading to different produced SCFA. For example, in MRSm 5%, the high amount of X5 consumed by L. acidophilus produces a longer chain of SCFA and vice versa. This SCFA is known to have a supporting effect on the digestive system. For example, n-butyric acid could increase the metabolic efficiency process in the epithelium of the large intestine and prevent colon cancer [42]. This is because SCFA is absorbed by intestinal epithelial cells and used as an energy source. Rycroft et al. [28] reported that commercial XOS fermentation for 24 h produced SCFA in the form of lactic acid (17.91 mM), acetic acid (26.70 mM), propionic acid (7.21 mM), and butyric acid (1.75 mM). This indicated that SCFA produced from fermented XOS cassava pulp (Table 1) has slightly lower yields but has the same composition.
5. Conclusions
From the experiment results, we conclude that XOS obtained from enzymatic hydrolyses of Cassava pulp xylan by endo-β-1,4-D-xylanase has a prebiotic effect due to its effect on probiotic L. acidophilus growth. The addition of 5% XOS to the medium increased the growth of L. acidophilus to 8.61 log CFU/mL after 36 h incubation. This value is similar to the change in MRSB media. Total reduced sugar levels support the growth profile explanation. Furthermore, the addition of XOS also affected the profile of SCFA produced by bacteria, in which the lactic acid was dominant in a medium with XOS of 5%. In contrast, acetic acid was predominant without XOS.
Author Contributions
Conceptualization, A.A.I.R. and A.B.S.; Methodology, M.T.R. and N.N.; Validation, A.L. and K.S.; Format Analysis, A.A.I.R. and M.R.; Investigation, A.A.I.R.; Writing—original draft, A.A.I.R., A.L. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Direktorat Riset dan Penmgabdian Masyarakat Kementerian Riset dan Teknologi/Badan Riset dan Inovasi Nasional The Republic of Indonesia through Program Fundamental Research 2021 grant number 023/E5/PG.02.00.PT/2022.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We thank Direktorat Riset dan Pengabdian Masyarakat Kementerian Riset dan Teknologi/Badan Riset dan Inovasi Nasional The Republic of Indonesia through Program Fundamental Research 2021 and DAAD program “Sustainable International Teaching and Research in Bioengineering and Biotechnology” in Flensburg University of Applied Science for facility research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuhl, J.P.; Corbett, M. Keeping the Gut Microflora at Bay. Science 2004, 303, 1624–1625. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996, 4, 430–435. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo JVan Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Klessen, B.; Sykura, B.; Zunft, H.-J.; Blaut, M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated. Am. J. Clin. Nutr. 1997, 65, 1397–1402. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Liao, J.-W.; Chung, Y.-C.; Hsieh, C.-P.; Chan, Y.-C. Xylooligosaccharides and Fructooligosaccharides Affect the Intestinal Microbiota and Precancerous Colonic Lesion Development in Rats. J. Nutr. 2004, 134, 1523–1528. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R. Dietary Modulation of the Human Gut Microflora Using the Prebiotics Oligofructose and Inulin. J. Nutr. 1999, 129, 1438S–1441S. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Morris, J.G. Colonic Bacterial Flora: Changing Understandings in the Molecular Age. J. Nutr. 2004, 134, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ratnadewi, A.A.I.; Masruroh, H.; Suwardiyanto; Santoso, A.B. Application of coffee peel waste as raw material for Xylooligosaccharide Production. Coffe Sci. 2019, 14, 446–454. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.I.; Santoso, A.B.; Sulistyaningsih, E.; Handayani, W. Application of Cassava Peel and Waste as Raw Materials for Xylooligosaccharide Production Using Endoxylanase from Bacillus subtilis of Soil Termite Abdomen. Procedia Chem. 2016, 18, 31–38. [Google Scholar] [CrossRef]
- Hafidah, A.H.; Sulistyaningsih, E.; Handayani, W.; Ratnadewi, A.A.I. Prebiotic Potential of Xylooligosaccharides Derived from Cassava Dregs in Balb/c Mice Colon. Pertanika J. Trop. Agric. Sci. 2018, 41, 1021–1031. [Google Scholar]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Finegold, S.M. In Vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp. and Lactobacillus spp. Int. J. Food Sci. Nutr. 2015, 66, 919–922. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.I.; Zain, M.H.A.; Kusuma, A.A.N.N.; Handayani, W.; Nugraha, A.S.; Siswoyo, T.A. Lactobacillus casei fermentation towards xylooligosaccharide (XOS) obtained from coffee peel enzymatic hydrolysate. Biocatal. Agric. Biotechnol. 2020, 23, 101446. [Google Scholar] [CrossRef]
- Lasrado, L.D.; Gudipati, M. Antioxidant property of synbiotic combination of Lactobacillus sp. and wheat bran xylo-oligosaccharides. J. Food Sci. Technol. 2014, 52, 4551–4557. [Google Scholar] [CrossRef]
- Buruiană, C.T.; Vizireanu, C. Prebiotic xylooligosaccharides from lignocellulosic materials: Production, purification and applications-An overview. Ann. Univ. Dunarea De Jos Galati 2014, 38, 18–31. [Google Scholar]
- Fiorini, D.; Boarelli, M.C.; Gabbianelli, R.; Ballini, R.; Pacetti, D. A quantitative headspace–solid-phase microextraction–gas chromatography–flame ionization detector method to analyze short chain free fatty acids in rat feces. Anal. Biochem. 2016, 508, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.M.; Barnes, M.B.; Gras, S.L.; McSweeney, C.; Lockett, T.; Augustin, M.A.; Gooley, P.R. Esterification of high amylose starch with short chain fatty acids modulates degradation by Bifidobacterium spp. J. Funct. Foods 2014, 6, 137–146. [Google Scholar] [CrossRef]
- Moura, P.; Barata, R.; Carvalheiro, F.; Gírio, F.; Loureiro-Dias, M.C.; Esteves, M.P. In Vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT Food Sci. Technol. 2007, 40, 963–972. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Sims, I.M.; Ryan, J.L.J.; Kim, S.H. In Vitro fermentation of prebiotic oligosaccharides by Bifidobacterium lactis HN019 and Lactobacillus spp. Anaerobe 2014, 25, 11–17. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Wang, C. In Vitro fermentation of xylooligosaccharides from wheat bran insoluble dietary fiber by Bifidobacteria. Carbohydr. Polym. 2010, 82, 419–423. [Google Scholar] [CrossRef]
- Rycroft, C.E.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in Vivo and in Vitro Findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef]
- Pertami, S.D.; Pancasiyanuar, M.; Irasari, S.A.; Rahardjo, M.B.; Wasilah, W. Lactobacillus acidophilus Probiotic Inhibits the Growth of Candida albicans. J. Dent. Indones. 2013, 20, 64–67. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Kok, N. Effects of fructans-type prebiotics on lipid metabolism. Am. J. Clin. Nutr. 2001, 73, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.S.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Amenaghawon, N.A.; Ogbeide, S.E.; Okieimen, C.O. Application of statistical experimental design for the optimisation of dilute sulphuric acid hydrolysis of Cassava Bagasse. Acta Polytech. Hung. 2014, 11, 239–250. [Google Scholar]
- Safitri, E.; Hanifah; Previta; Sudarko; Puspaningsih, N.N.T.; Ratnadewi, A.A.I. Cloning, purification, and characterization of recombinant endo- β-1,4-D-xylanase of Bacillus sp. From soil termite abdomen. Biocatal. Agric. Biotechnol. 2020, 31, 101877. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; de Oliva Neto, P.; da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- McLaughlin, H.P.; Motherway, M.O.C.; Lakshminarayanan, B.; Stanton, C.; Paul Ross, R.; Brulc, J.; Menon, R.; O’Toole, P.W.; van Sinderen, D. Carbohydrate catabolic diversity of bifidobacteria and lactobacilli of human origin. Int. J. Food Microbiol. 2015, 203, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhou, H.; Wang, Z. Effects of xylooligosaccharide on growth performance, activities of digestive enzymes, and intestinal microflora of juvenile Pelodiscus sinensis. Front. Agric. China 2011, 5, 612–617. [Google Scholar] [CrossRef]
- Kedia, G.; Vázquez, J.A.; Pandiella, S.S. Enzymatic digestion and in vitro fermentation of oat fractions by human lactobacillus strains. Enzyme Microb. Technol. 2008, 43, 355–361. [Google Scholar] [CrossRef]
- Reddy, S.S.; Krishnan, C. Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT Food Sci. Technol. 2016, 65, 237–245. [Google Scholar] [CrossRef]
- Chapla, D.; Pandit, P.; Shah, A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour. Technol. 2012, 115, 215–221. [Google Scholar] [CrossRef]
- Madhukumar, M.S.; Muralikrishna, G. Fermentation of xylo-oligosaccharides obtained from wheat bran and Bengal gram husk by lactic acid bacteria and bifidobacteria. J. Food Sci. Technol. 2012, 49, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The Effects of Short-Chain Fatty Acids on Human Colon Cancer Cell Phenotype Are Associated with Histone Hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).