Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Bacterial Viability
2.4. Acid Tolerance Test
2.5. Bile Tolerance Test
2.6. Enumeration of S. thermophilus and L. bulgaricus
2.7. Protease Activity
2.8. Statistical Analysis
3. Results and Discussion
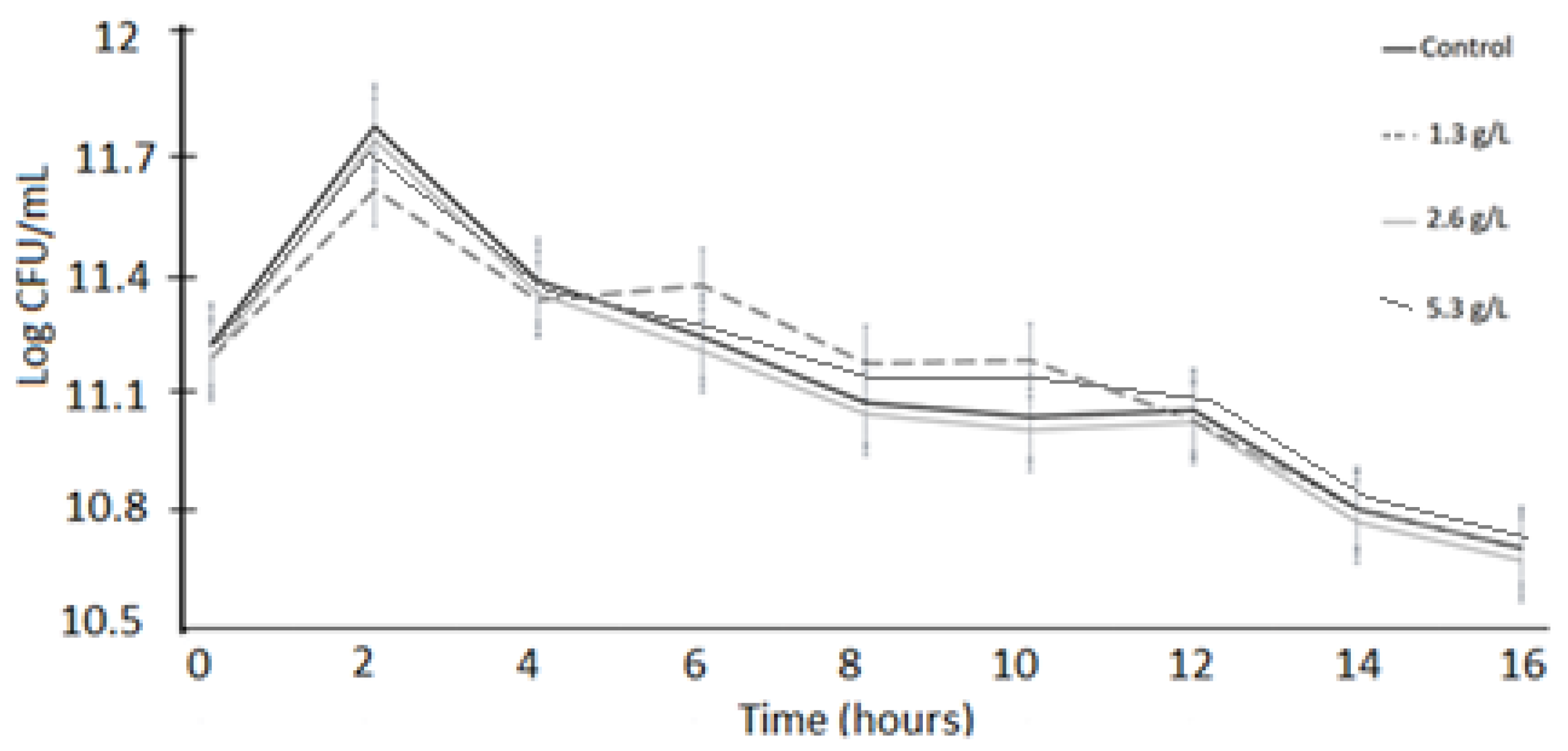
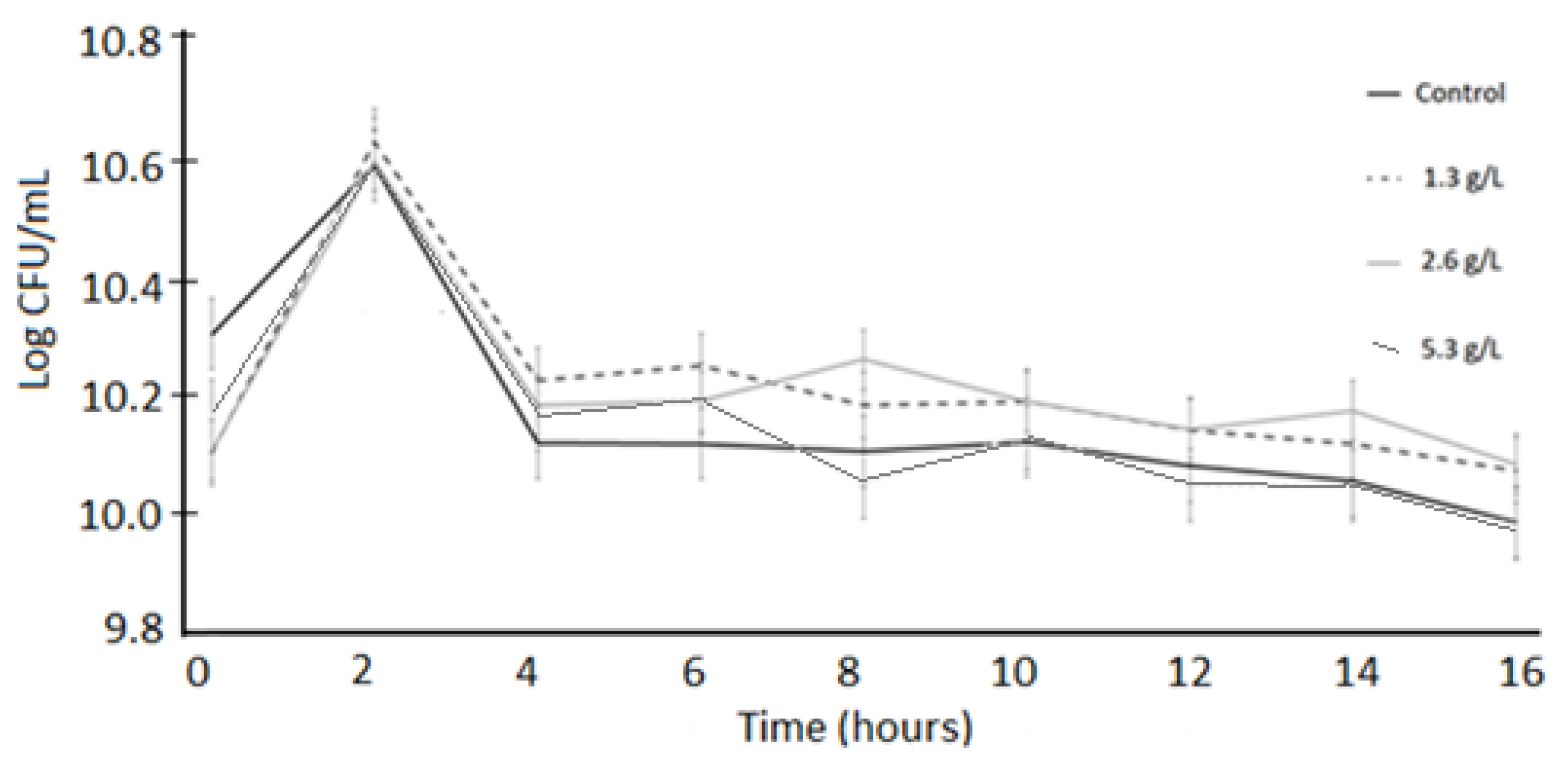
3.1. Bacterial Viability
3.2. Acid Tolerance
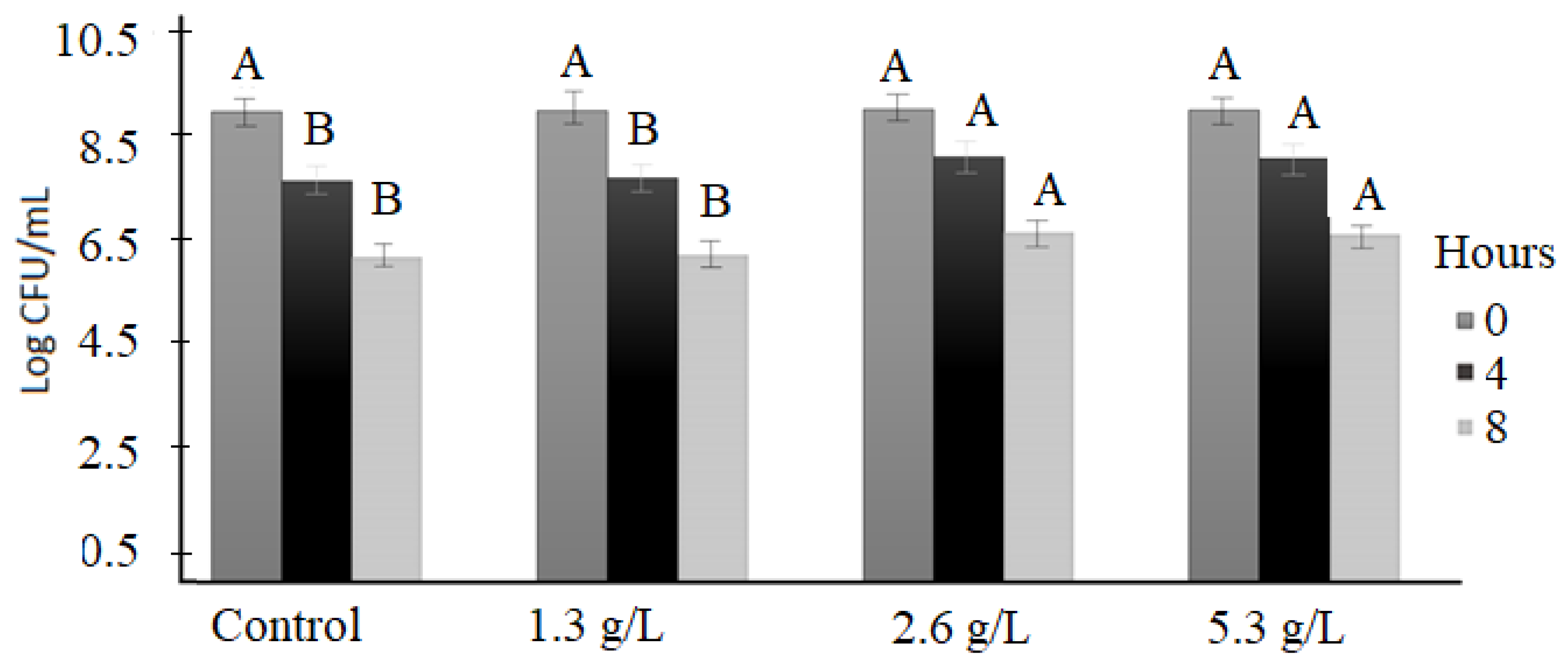
3.3. Bile Tolerance
3.4. Protease Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plaza, J.; Ruiz, F.; Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products:possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Fiocco, D.; Longo, A.; Arena, M.; Russo, P.; Spano, G.; Capozzi, V. How probiotics face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr. 2019, 60, 1552–1580. [Google Scholar] [CrossRef]
- Rishi, P.; Mavi, S.; Bharrhan, S.; Shukla, G.; Tewari, R. Protective efficacy of probiotic alone or in conjunction with a prebiotic in Salmonella-induced liver damage. FEMS Microbiol. Ecol. 2009, 69, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W. Microecology of the gastrointestinal tract in relation to lactic acid bacteria. Int. Dairy J. 1995, 5, 1059–1070. [Google Scholar] [CrossRef]
- Campeotto, F.; Waligora-Dupriet, A.J.; Doucet-Populaire, F.; Kalach, N.; Dupont, C.; Butel, M.J. Mise en place de la flore intestinale du nouveau-né flore intestinale mise au point. Gastroenterol. Clin. Biol. 2007, 31, 533–542. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1. [Google Scholar] [CrossRef]
- Lafourcade, A.; Achod, L.; Keita, H.; Carvalho, J.; de Souza, T.; Amado, J. Development, pharmacological and toxicological evaluation of a new tablet formulation based on Cassia grandis fruit extract. Sustain. Chem. Pharm. 2020, 16, 00244. [Google Scholar] [CrossRef]
- Fuentes, J.; Aleman, R.; Linares, I.; Sánchez, J. The Carao (Cassia grandis L.): Its potential usage in pharmacological, nutritional, and medicinal applications. Innovations in biotechnology. In Innovations in Biotechnology for a Sustainable Future; Maddela, N.R., García, L.C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Fuentes, J.; Fernández, I.; Fernández, H.; Sánchez, J.; Alemán, R.; Alarcon, M. Quantification of bioactive molecules, minerals and bromatological analysis in carao (Cassia grandis). J. Agric. Sci. 2020, 12, 88. [Google Scholar] [CrossRef]
- Fuentes, J.; Salas, L.; Linares, I.; Alarcón, M.; Carretero, A.; Lozano, J. Development of an innovative pressurized liquid extraction procedure by response surface methodology to recover bioactive compounds from carao tree seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Montero, F.I.; Fuentes, J.; Cascos, G.; Saravia, M.S.; Lozano, J.; Martín, D. Masking effect of Cassia grandis sensory defect with flavoured stuffed olives. Foods 2022, 11, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Olson, D.; Aryana, K. Whey protein isolate improves acid and bile tolerances of Streptococcus thermophilus ST-M5 and Lactobacillus delbrueckii ssp. bulgaricus LB-12. J. Dairy Sci. 2015, 98, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Loghavi, L.; Sastry, S.; Yousef, A. Effect of moderate electric field on the metabolic activity and growth kinetics of Lactobacillus acidophilus. Biotechnol. Bioeng. 2007, 98, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Gibson, G. Cholesterol assimilation by lactic acid bacteria and Bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.I.; Shah, N.P. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus and Btfidobacterium spp. J. Dairy Sci. 1996, 79, 1529–1536. [Google Scholar] [CrossRef]
- Oberg, C.; Wang, A.; Moyes, L.; Brown, R.; Richardson, G. Effects of proteolytic activity of thermolactic cultures on physical properties of mozzarella cheese. J. Dairy Sci. 1991, 74, 389–397. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Joung, J.; Lee, J.; Ha, Y.; Shin, Y.; Kim, Y.; Kim, S.; Oh, N. Enhanced microbial, functional and sensory properties of herbal yogurt fermented with korean traditional plant extracts. Korean J. Food Sci. Anim. Resour. 2016, 36, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Pelicano, E.R.L.; De Souza, P.A.; De Souza, H.B.A.; Leonel, F.R.; Zeola, N.M.B.L.; Boiago, M.M. Productive traits of broiler chickens fed diets containing different growth promoters. Braz. J. Poult. Sci. 2004, 6, 177–182. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Dawson, K.A.; Newman, K.E. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the caeca of Salmonella-challenged broiler chicks. Poult. Sci. 2000, 79, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Sandine, W.E.; Elliker, P.R. Microbially induced flavours and fermented foods flavour in fermented dairy products. J. Agric. Food Chem. 1970, 18, 557–562. [Google Scholar] [CrossRef]
- Radke, L.; Sandine, W. Influence of temperature on associative growth of Streptococcus thermophilus and Lactobacillus bulgaricus. J. Dairy Sci. 1986, 69, 2558–2568. [Google Scholar] [CrossRef]
- Wu, W.; Li, H. Metabolites of Lactic Acid Bacteria. In Lactic Acid Bacteria in Foodborne Hazards Reduction; Springer: Singapore, 2018; pp. 87–113. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, Y.; Zhang, Y.; Leng, C.; Sun, J.; Chen, H.; Zhang, Y.; Li, A.; Feng, Z. Profiles of Streptococcus thermophilus MN-ZLW-002 nutrient requirements in controlled pH batch fermentations. Microbiologyopen 2018, 8, 00633. [Google Scholar] [CrossRef]
- Santos, E.; Fernández, C.; Jaime, I.; Rovira, J. Comparative study of lactic acid bacteria house flora isolated in different varieties of ‘chorizo’. Int. J. Food Microbiol. 1998, 39, 123–128. [Google Scholar] [CrossRef]
- Zhai, Z.; Douillard, F.; An, H.; Wang, G.; Guo, X.; Luo, Y.; Hao, Y. Proteomic characterization of the acid tolerance response in Lactobacillus delbrueckii subsp. bulgaricus CAUH1 and functional identification of a novel acid stress-related transcriptional regulator Ldb0677. Environ. Microbiol. 2014, 16, 1524–1537. [Google Scholar] [CrossRef]
- Fernandez, A.; Ogawa, J.; Penaud, S.; Boudebbouze, S.; Ehrlich, D.; van de Guchte, M.; Maguin, E. Rerouting of pyruvate metabolism during acid adaptation in Lactobacillus bulgaricus. Proteomics 2008, 8, 3154–3163. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Gunn, J. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000, 2, 907–913. [Google Scholar] [CrossRef]
- Letort, C.; Juillard, V. Development of a minimal chemically-defined medium for the exponential growth of Streptococcus thermophilus. J. Appl. Microbiol. 2001, 91, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Rul, F.; Monnet, V. Presence of additional peptidases in Streptococcus thermophilus CNRZ 302 compared to Lactococcus lactis. J. Appl. Microbiol. 1997, 82, 695–704. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Zhang, Y.; Yang, Z. Growth and exopolysaccharide production by Streptococcus thermophilus ST1 in skim milk. Braz. J. Microbiol. 2011, 42, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Nurdini, L.; Nuraida, L.; Suwanto, A.; Suliantari. Microbial growth dynamics during tempe fermentation in two different home industries. Int. Food Res. J. 2015, 22, 1668–1674. [Google Scholar]
- Shah, N.; Jelen, P. Survival of lactic acid bacteria and their lactases under acidic conditions. J. Food Sci. 1990, 55, 506–509. [Google Scholar] [CrossRef]
- Silva, J.; Carvalho, A.; Pereira, H.; Teixeira, P.; Gibbs, P. Induction of stress tolerance in Lactobacillus delbrueckii ssp. bulgaricus by the addition of sucrose to the growth medium. J. Dairy Res. 2004, 71, 121–125. [Google Scholar] [CrossRef] [Green Version]



| Sample | 0 h | 12 h | 24 h |
|---|---|---|---|
| Control | 0.152 ± 0.015 a | 0.175 ± 0.007 a | 0.202 ± 0.011 a |
| 1.3 g/L | 0.152 ± 0.020 a | 0.170 ± 0.010 a | 0.211 ± 0.011 a |
| 2.6 g/L | 0.153 ± 0.017 a | 0.185 ± 0.015 a | 0.217 ± 0.014 ab |
| 5.3 g/L | 0.154 ± 0.023 a | 0.186 ± 0.027 a | 0.232 ± 0.008 b |
| Sample | 0 h | 12 h | 24 h |
|---|---|---|---|
| Control | 0.161 ± 0.005 a | 0.313 ± 0.007 b | 0.417 ± 0.017 b |
| 1.3 g/L | 0.163 ± 0.005 a | 0.320 ± 0.015 b | 0.410 ± 0.025 b |
| 2.6 g/L | 0.162 ± 0.005 a | 0.357 ± 0.017 ab | 0.455 ± 0.019 ab |
| 5.3 g/L | 0.163 ± 0.007 a | 0.377 ± 0.013 a | 0.475 ± 0.013 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, D.; Aleman, R.S.; Cedillos, R.; Olson, D.W.; Aryana, K.; Marcia, J.; Boeneke, C. Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis). Fermentation 2022, 8, 499. https://doi.org/10.3390/fermentation8100499
Paz D, Aleman RS, Cedillos R, Olson DW, Aryana K, Marcia J, Boeneke C. Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis). Fermentation. 2022; 8(10):499. https://doi.org/10.3390/fermentation8100499
Chicago/Turabian StylePaz, David, Ricardo S. Aleman, Roberto Cedillos, Douglas W. Olson, Kayanush Aryana, Jhunior Marcia, and Charles Boeneke. 2022. "Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis)" Fermentation 8, no. 10: 499. https://doi.org/10.3390/fermentation8100499
APA StylePaz, D., Aleman, R. S., Cedillos, R., Olson, D. W., Aryana, K., Marcia, J., & Boeneke, C. (2022). Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis). Fermentation, 8(10), 499. https://doi.org/10.3390/fermentation8100499






