The Biorefinery of the Marine Microalga Crypthecodinium cohnii as a Strategy to Valorize Microalgal Oil Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. cohnii Starter Cultures
2.2. Bioreactor Cultivations
2.2.1. Inoculum
2.2.2. Bioreactor Experiments
2.3. Analytical Methods
2.3.1. FAEE Quantification
2.3.2. Flow Cytometry
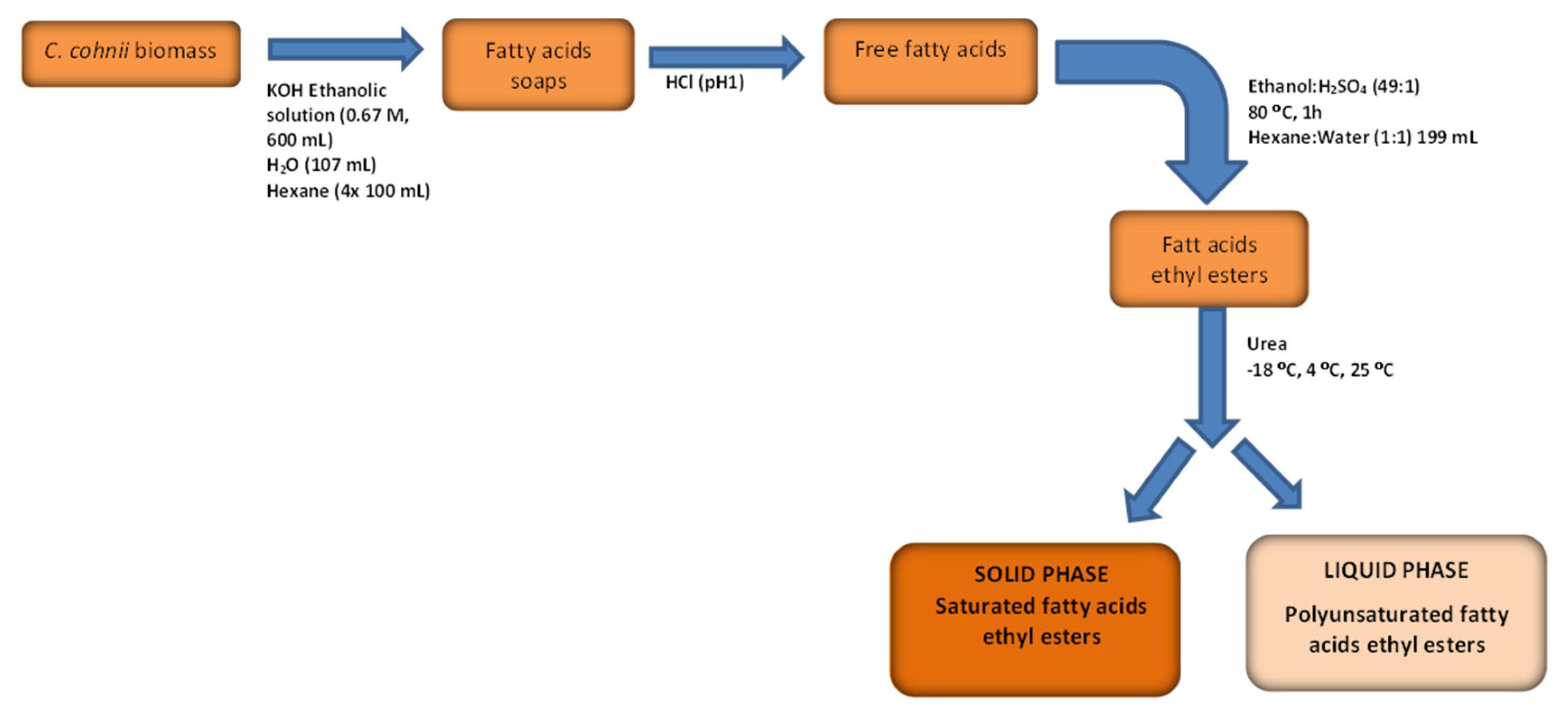
2.3.3. C. cohnii Biomass Saponification and EE Fractionation
Biomass Saponification
Ethylation
Urea Crystallization and Fractionation
2.3.4. Estimation of Solid Phase Properties Based on its FA EE Profile
3. Results and Discussion
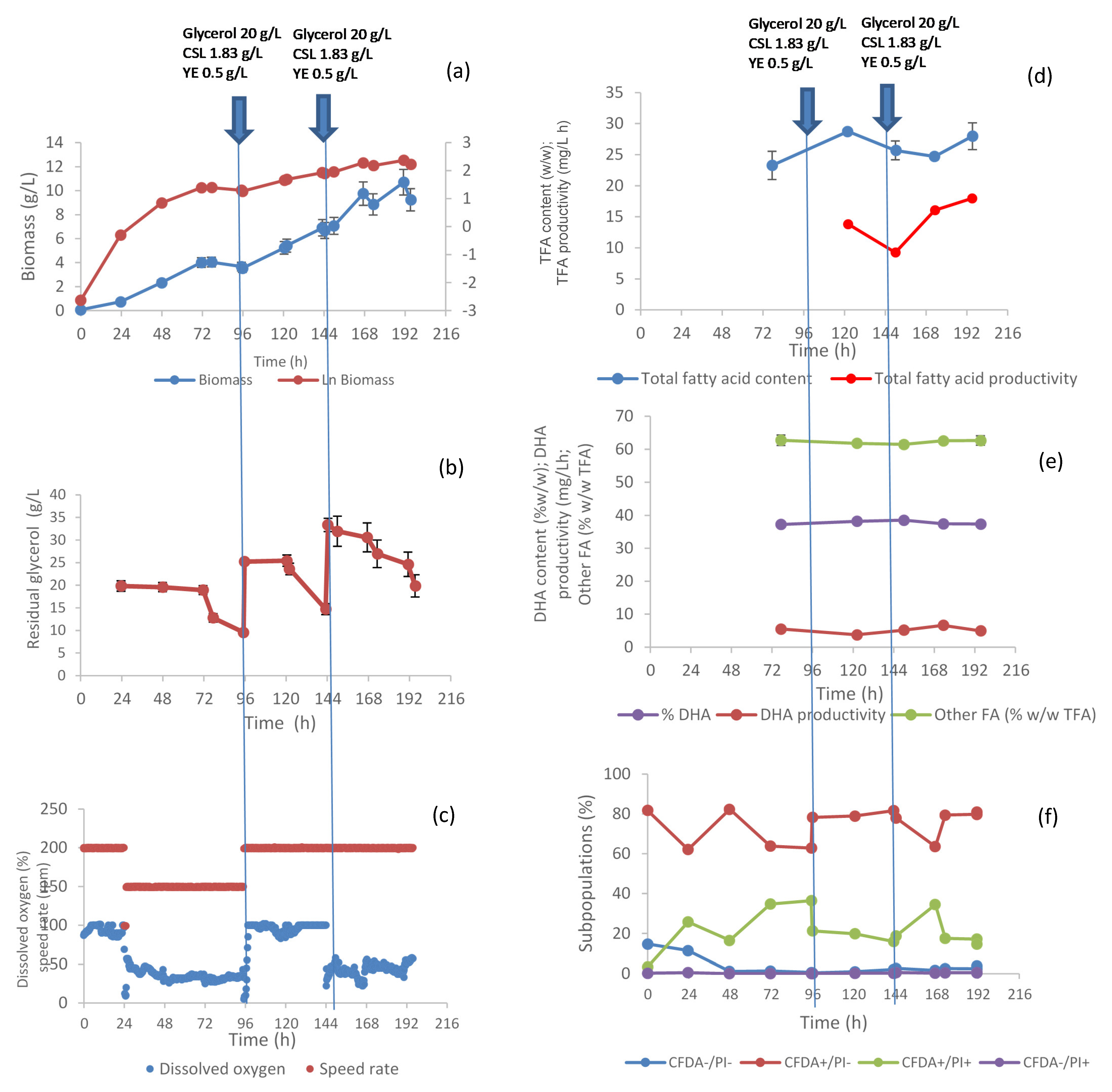
3.1. C. cohnii Fed-Batch Fermentation
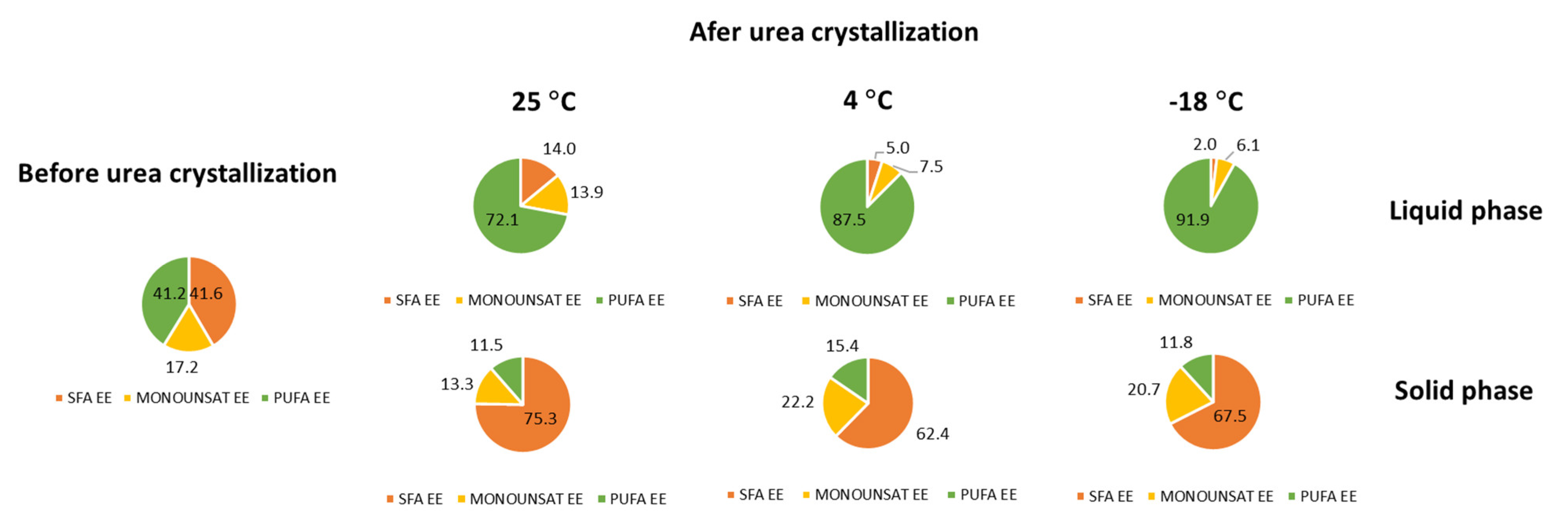
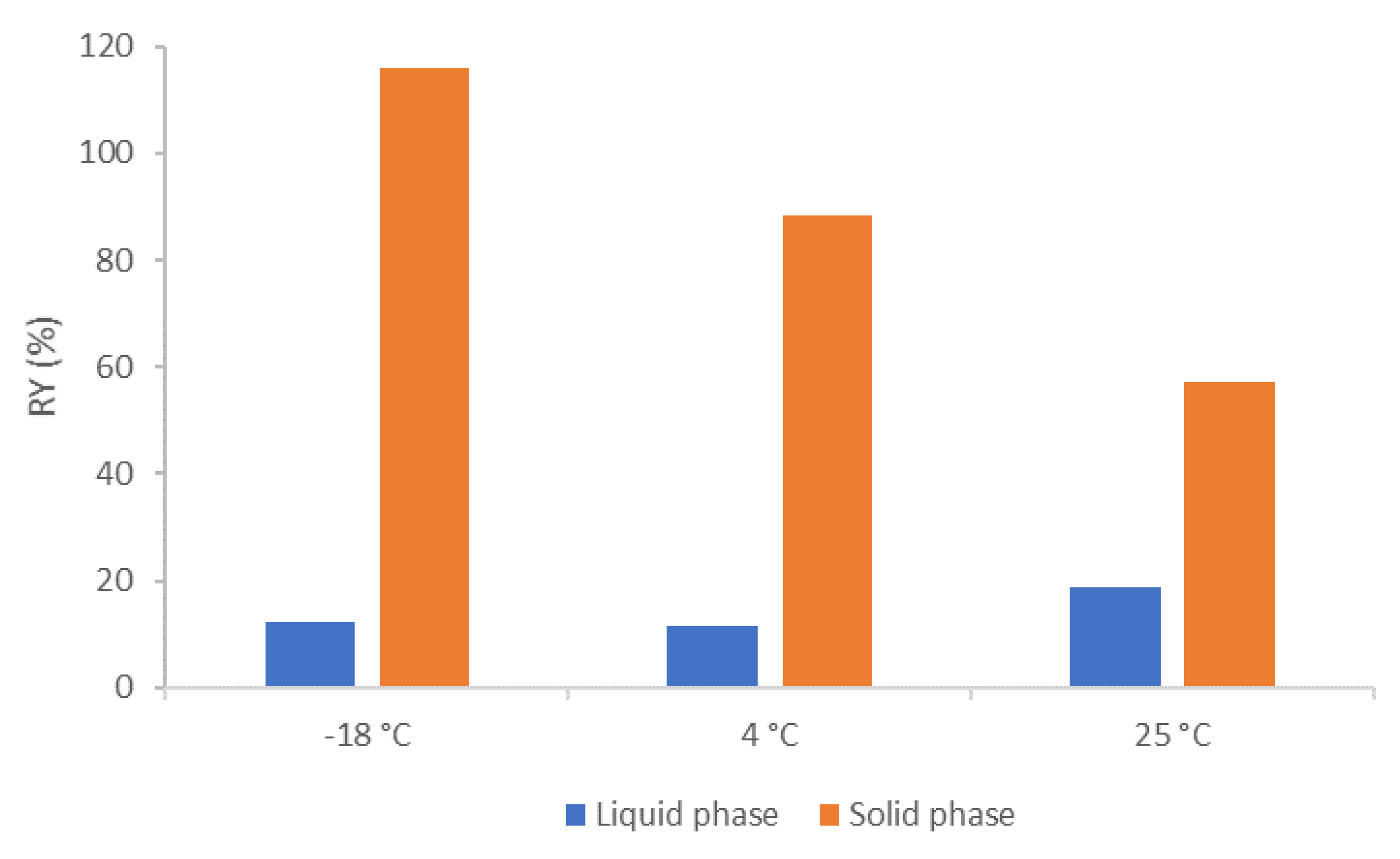
3.2. C. cohnii FAEE Fractionation
3.3. Theoretical Estimation of FAEE Solid Phase Quality, as Biofuel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, P.; Kuryatov, A.; Axelsen, P. A new synthetic medium for the optimziation of Docosahexaenoic Acid production in Crypthecodinium cohnii. PLoS ONE 2020, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.; Lim, D.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P. Microalgal biofactories. A promising approach towards sustainable ómega-3 fatt acid production. Microb Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The dark side of microalgae biotechnology: A heterotrophic biorefinery platform directed to ω-3 rich lipid production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Chénais, B.; Fournière, M.; Bourgougnon, N.; Dedoux, G. Algal derived functional lipids and their role in promoting health. In Recent Advances in Micro and Microalgal Processing: Food and Health Perspectives; Gaurav, R., Yan, Y., Eds.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Diao, J.; Song, X.; Zhang, X.; Chen, L.; Zhang, W. Genetic Engineering of Crypthecodinium cohnii to increase growth and lipid accumulation. Front. Microbiol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S. Omega-e fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii growth and omega fatty acid production in mediums supplemented with extact from recycled biomass. Mar. Drugs 2022, 20, 68. [Google Scholar] [CrossRef]
- Karr, J.; Alexander, J.E.; Winningham, R. Omega-3polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef]
- Chalima, A.; Taxeidis, D.; Topakas, E. Optimization of the production of docosahexaenoic fatty acid by the heterotrophic microalga Crypthecodinium cohnii utilizing a dark fermentation effluent. Ren Energy 2020, 152, 102–109. [Google Scholar] [CrossRef]
- Ritter, J.; Budge, S.; Jovica, F.; Reis, A. Oxidation rates of triacylglycerol and ethyl ester fish oil. J. Am. Oil Soc. 2015, 92, 561–569. [Google Scholar] [CrossRef]
- Espinosa, A.; Ross, A.; Dovale-Rosabal, G.; Fuente, F.; Uribe-Oporto, E.; Sacristán, C.; Ruiz, P.; Valenzuela, R.; Romero, N.; Aubourg, S.; et al. EPA/DHA concentrate by urea complexation decreases hyperinsulinemia and increases Plin5 in the liver of mice fed a high-fat diet. Molecules 2020, 25, 3289. [Google Scholar] [CrossRef]
- Moreno-Perez, S.; Luna, P.; Señorans, F.J.; Guisan, J.M.; Fernandez-Lorente, G. Enzymatic synthesis of triacylglycerols of docosahexaenoid acid: Transesterification of its ethyl esters with glycerol. Food Chem. 2015, 187, 225–229. [Google Scholar] [CrossRef]
- Ratledge, C.; Streekstra, H.; Cohen, Z.; Fichtali, J. Downstream Processing, Extraction, and Purification of Single Cell Oils. In Single Cell Oils: Microbial and Algal Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; Elsevier Inc.: San Diego, CA, USA, 2010; pp. 179–197. [Google Scholar]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Pei, G.; Li, X.; Liu, L.; Liu, J.; Wang, F.; Chen, L.; Zhang, W. De novo transcriptomic and metabolic analysis of docosahexaenoic acid (DHA)-producing Crypthecodinium cohnii during fed-batch fermentation. Algal. Res. 2017, 26, 380–391. [Google Scholar] [CrossRef]
- Mendes, A.; Lopes da Silva, T.; Reis, A. DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol. Biotechnol. 2007, 45, 38–44. [Google Scholar]
- Senanayake, S.P.J.; Shahidi, F. Concentration of docosahexaenoic acid (DHA) from algal oil via urea complexation. J. Food Lipids 2000, 7, 51–61. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of microalgal biorefinery. Tibtech 2019, 37, 3. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Liu, Y. Concentration of Omega-3 polyunsaturated fatty acids from oil of Schizochytrium limacinum by molecular distillation: Optimization of technological conditions. Ind. Eng. Chem. Res. 2013, 52, 3918–3925. [Google Scholar] [CrossRef]
- Chern, T.; Ju, Y. An improved fractional crystallization method for the enrichment of γ-linolenic acid in borage oil fatty acid. Ind. Eng. Chem. Res. 2001, 40, 3781–3784. [Google Scholar]
- Cheong, L.; Guo, Z.; Yang, Z.; Chua, S.; Xu, X. Extraction nd enrichment of n-3 polyunsaturated fatty acids and ethyl esters through reversible π-π complexation with aromatic rings containing ionic liquids. J. Agric. Food. Chem. 2011, 59, 8961–8967. [Google Scholar] [CrossRef]
- Dong, Q.; Li, M.; Yang, Y.; Bao, Z. Separation of eicosapentaenoic acid ethyl ester and docosahexaenoic acid ethyl ester by simulating moving bed chromatography. Chin. J. Chromat. 2018, 36, 858. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Wang, H.; Li, S.; Tian, S. Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J. Supercrit. Fluids 2011, 57, 44–49. [Google Scholar] [CrossRef]
- Oh, C.; Kim, G.; Park, S.; Choi, S.; Park, M.; Lee, O.; Seo, J.; Son, H. Purification of high purity docosahexaenoic acid from Schizochytrium sp. SH 103 using preparative-scale HPLC. Appl. Biol. Chem. 2020, 63, 56. [Google Scholar] [CrossRef]
- Abu-Nasr, A.; Potts, W.; Holman, R. Highly unsaturated fatty acids. II. Fractionation by urea inclusion compounds. J. Am. Oil Chem. Soc. 1954, 31, 16–20. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Rodríguez, A.; Contreras, E.; Ortiz-Viedma, J.; Muñoz, M.; Trigo, M.; Aubourg, S.; Espinosa, A. Concentration of EPA and DHA from refined salmon oil by optimizing the urea-fatty acid adduction reaction conditions using response surface methodology. Molecules 2019, 24, 1642. [Google Scholar] [CrossRef]
- Toumi, A.; Politaeva, N.; Ðurović, S.; Mukhametova, L.; Ilyashenko, S. Obtaining DHA–EPA oil concentrates from the biomass of microalga Chlorella sorokiniana. Resources 2022, 11, 20. [Google Scholar] [CrossRef]
- Setyawardhani, D.; Sulistyo, H.; Sediawan, B.; Fahrurrozi, M. Separating poly-unsaturated fatty acids from vegetable oil using urea complexation. The crystallization temperature effect. J. Eng. Sci. Technol. 2015, 10, 41–49. [Google Scholar]
- Arous, F.; Atitallah, I.; Nasri, M.; Mechici, T. A sustainable use of low-cost raw substrates for biodiesel production by the oleaginous yeast Wickerhamomyces anomalus. 3 Biotech 2017, 7, 268. [Google Scholar] [CrossRef]
- Safdar, W.; Shamoon, M.; Zan, X.; Haider, J.; Sharif, H.; Shoaib, M.; Song, Y. Growth kinetics, fatty acid composition and metabolic activity changes of Crypthecodinium cohnii under different nitrogen source and concentration. AMB Expr. 2017, 7, 85. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Pei, G.; Cui, J.; Diao, J.; Lv, M.; Chen, L.; Zhang, W. Repeated fed-batch strategy and metabolism analysis to achieve high docosahexaenoic acid productivity in Crypthecodinium cohnii. Microb. Cell Fact. 2020, 19, 9. [Google Scholar] [CrossRef]
- Moniz, P.; Silva, C.; Oliveira, A.C.; Reis, A.; Lopes da Silva, T. Raw glycerol-based medium for DHA and Lipids production using the marine heterotrophic microalga Crypthecodinium cohnii. Process 2021, 9, 2005. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Santos, A.R.; Reis, A. Valorizing fish canning industry by-products to produce ω-3 compounds and biodiesel. Env. Technol Innov. 2018, 9, 74–81. [Google Scholar] [CrossRef]
- Krisnangkura, K. A Simple Method for Estimation of Cetane Index of Vegetable Oil Methyl Esters. J. Am. Oil Soc. 1986, 63, 552–553. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Costa, J.A.V.; Assis, D.J.; Lemos, P.V.F.; Druzian, J.I.; de Souza, C.O.; Nunes, I.L.; Chinalia, F.A. Spirulina sp. as a bioremediation agent for aquaculture wastewater: Production of high added value compounds and estimation of theoretical biodiesel. BioEnergy Res. 2021, 14, 254–264. [Google Scholar] [CrossRef]
- Swaaf, M.; Rijk, T.; Eggink, G.; Sijtsma, L. Optimization of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J. Biotechnol. 1999, 70, 185–192. [Google Scholar] [CrossRef]
- Cui, J.; Diao, J.; Sun, T.; Shi, M.; Liu, L.; Wang, F.; Chen, L.; Zhang, W. 13C Metabolic Flux Analysis of Enhanced Lipid Accumulation Modulated by Ethanolamine in Crypthecodinium cohnii. Front. Microbiol. 2018, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Berzins, K.; Muiznieks, R.; Baumanis, M.R.; Strazdina, I.; Shvirksts, K.; Prikule, S.; Galvanauskas, V.; Pleissner, D.; Pentjuss, A.; Grube, M.; et al. Kinetic and stoichiometric modeling-based analysis of docosahexaenoic acid (DHA) production Potential by Crypthecodinium cohnii from glycerol, glucose and ethanol. Mar. Drugs 2022, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ji, X.; Ren, L.; Li, G.; Huang, H. Improving docosahexaenoic acid production by Schizochytrium sp. Using a newly designed high-oxygen-supply bioreactor. AIChE J. 2017, 63, 4279–4286. [Google Scholar] [CrossRef]
- De La Jara, A.; Mendonza, H.; Martel, A.; Molina, C.; Nordströn, R.V.; Díaz, R. Flow cytometric determination of lipid content in a marine dinoflagellate, Crypthecodinium cohnii. J. Appl. Phycol. 2003, 15, 433–438. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Reis, A. The use of multi-parameter flow cytometry to study the impact of n-dodecane additions to marine dinoflagellate microalga Crypthecodinium cohnii bath fermentation and DHA production. J. Ind. Microbiol. Biotechnol. 2008, 35, 875–887. [Google Scholar] [CrossRef]
- Taborda, T.; Moniz, P.; Reis, A.; Lopes da Silva, T. Evaluating low-cost substrates for Crypthecodinium cohnii lipids and DHA production, by flow cytometry. J. Appl. Phycol. 2021, 33, 263–274. [Google Scholar] [CrossRef]
- Isleten-Hosoglu, M.; Elibol, M. Improvement of medium composition and cultivation conditions for growth and lipid production by Crypthecodinium cohnii. Rom. Biotechnol. Lett. 2017, 22, 13086–23095. [Google Scholar]
- Rivas, B.; Moldes, A.; Domínguez, J.; Parajó, J. Development of culture media containing spent yeast cells of Debaryomyces hansenii and corn steep liquor for lactic acid production with Lact. Rhamnosus. Int. J. Food Microbiol. 2004, 97, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 11 August 2022).
- Mendes, A.; Guerra, P.; Madeira, V.; Ruano, F.; Lopes da Silva, T.; Reis, A. Study of docosahexaenoic acid production by the heterotrophic microalga Crypthecodinium cohnii CCMP 316 using carob pulp as a promising carbon source. World J. Microbiol. Biotechnol. 2007, 23, 1209–1215. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of omega-e docosahexaenoic acid production by marine dinoflagellate Crypthecodinium cohnii using rapeseed meal hydrolysate and waste molasses as feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isleten-Hosoglu, M.; Elibol, M. Bioutilization of cheese whey and corn steep liquor by heterotrophic microalgae Crypthecodinium cohnii for biomass and lipid production. Akad. Gida 2017, 15, 233–241. [Google Scholar]
- Available online: https://ftloscience.com/methanol-toxic-ethanol/ (accessed on 11 August 2022).
- Lin, W.; Wu, F.; Yue, L.; Du, G.; Tian, L.; Wang, Z. Combination of urea complexation and molecular distillation to purify DHA and EPA from sardine oil ethyl esters. J. Am. Chem. Soc. 2014, 91, 687–695. [Google Scholar] [CrossRef]
- Magallanes, L.; Tarditto, L.; Grosso, N.; Pamparo, M.; Gayol, M. Highly concentrated omega-3 fatty acid ethyl esters by urea complexation and molceular distillation. J. Sci. Food. Agric. 2019, 99, 877–884. [Google Scholar] [CrossRef]
- Wanasundara, U.; Shahidi, F. Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation; optimization of reaction conditions. Food Chem. 1999, 65, 41–49. [Google Scholar] [CrossRef]
- Kaliban, C.; Roy, R.; Chadha, A. Docosahexaenoic acid production by a novel high yielding strain of Thraustochytrium sp. of Indian origin: Isolation and bioprocess optimization studies. Algal Res. 2018, 32, 93–100. [Google Scholar]
- Ramos, M.; Fernández, C.; Casas, A.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Biores. Technol. 2009, 100, 261–286. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
| Strain | Low-Cost Substrate | Cultivation System | Biomass Concentration (g/L) | Lipid Content (% w/w) | Lipid Productivity (mg/L h) | DHA Productivity (mg/L h) | Reference |
|---|---|---|---|---|---|---|---|
| C. cohnii CCMP 316 | Carob pulp syrup | 2L-bioreactor | 42.0 | 9.2 | 38.5 | 18.5 | [39] |
| C. cohnii ATCC 30772 | Rapeseed meal hydrolysate + waste molasses | 250 mL shake flasks | 2.9 | 27.7 | 4.7 | 0.5 | [40] |
| C. cohnii CCMP 316 | Cheese whey + CSL | 250 mL shake flaks | - | 28.7 | - | - | [41] |
| C. cohnii ATCC 30772 | Raw glycerol + CSL | 7L-biorector/batch | 5.3 | 11.0 | 4.0 | 1.6 | [25] |
| C. cohnii ATCC 30772 | Raw glycerol + CSL | 7L-bioreactor/fed-batch | 9.2 | 28.0 | 13.1 | 5.1 | This work |
| EE Profile before Urea Crystallization | EE Profile after Urea Crystallization | ||||||
|---|---|---|---|---|---|---|---|
| Liquid Phase | Solid Phase | ||||||
| FAEE | 25 °C | +4 °C | −18 °C | 25 °C | +4 °C | −18 °C | |
| 10:0 | 0.3 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 |
| 12:0 | 4.0 ± 0.2 | 3.0 ± 0.2 | 1.8 ± 0.1 | 1.2 ± 0.3 | 3. ± 0.18 | 4.8 ± 0.2 | 4.6 ± 0.2 |
| 14:0 | 17.4 ± 0.5 | 7.6 ± 0.4 | 2.4 ± 0.1 | 0.7 ± 0.0 | 27.7 ± 1.9 | 25.4 ± 1.3 | 27.2 ± 1.4 |
| 14:1ω5 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.4 | 0.0 ± 0.0 |
| 16:0 | 19.2 ± 0.0 | 3.4 ± 0.2 | 0.8 ± 0.0 | 0.0 ± 0.0 | 41.9 ± 2.1 | 30.5 ± 1.5 | 34.3 ± 1.7 |
| 16:1ω9 | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 | 1.7 ± 0.8 | 3.0 ± 1.0 | 2.6 ± 0.0 |
| 18:0 | 0.7 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.5 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0 |
| 18:1ω9 | 13.8 ± 0.3 | 10.5 ± 0.5 | 5.3 ± 0.03 | 3.2 ± 0.2 | 11.6 ± 0.6 | 19.0 ± 0.9 | 18.0 ± 0.9 |
| 18:2ω6 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| 22:5ω3 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 22:6ω3 | 40.6 ± 0.3 | 71.8 ± 3.6 | 87.2 ± 4.4 | 91.6 ± 4.6 | 11.4 ± 0.6 | 15.2 ± 0.8 | 11.8 ± 1.1 |
| SAT | 41.6 ± 0.5 | 14.0 ± 0.4 | 5.0 ± 0.2 | 2.0 ± 0.1o | 75.1 ± 2.5 | 61.7 ± 2.0 | 67.31 ± 2.2 |
| MONOUNSAT | 17.2 ± 0.3 | 13.9 ± 0.5 | 7.5 ± 0.3 | 6.1 ± 0.2 | 13.3 ± 0.6 | 22.65 ± 1.0 | 20.65 ± 0.9 |
| PUFA | 41.0 ± 0.3 | 72.1 ± 3.6 | 87.5 ± 4.4 | 91.9 ± 4.6 | 11.4 ± 0.6 | 15.94 ± 0.8 | 11.82 ± 0.6 |
| Estimated Values Solid Phase | |||||
|---|---|---|---|---|---|
| Temperature | Limits EN 14214 (Europe) | Limits ASTM D6751 (USA) | |||
| Parameter | 25 °C | 4 °C | −18 °C | ||
| Iodine value, IV (g I2/100 g) | 59.8 | 85.6 | 69.4 | <120 | - |
| Saponification value, SV (mg KOH/g) | 189 | 201 | 202 | - | <370 |
| Cetane number, CN | 61 | 55 | 58 | >51 | >47 |
| LCSF (% w/w) | 5 | 4 | 4 | - | - |
| CFPP (°C) | −0.9 | −5.1 | −4.0 | (Class C) | <5 |
| Polyunsaturated (≥4 double bonds) alkyl esters (% w/w) | 11.4 | 15.2 | 11.8 | <1 | - |
| C18:3 (% w/w) | Not det. | Not det. | Not det. | <12 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniz, P.; Martins, D.; Oliveira, A.C.; Reis, A.; Lopes da Silva, T. The Biorefinery of the Marine Microalga Crypthecodinium cohnii as a Strategy to Valorize Microalgal Oil Fractions. Fermentation 2022, 8, 502. https://doi.org/10.3390/fermentation8100502
Moniz P, Martins D, Oliveira AC, Reis A, Lopes da Silva T. The Biorefinery of the Marine Microalga Crypthecodinium cohnii as a Strategy to Valorize Microalgal Oil Fractions. Fermentation. 2022; 8(10):502. https://doi.org/10.3390/fermentation8100502
Chicago/Turabian StyleMoniz, Patrícia, Daniela Martins, Ana Cristina Oliveira, Alberto Reis, and Teresa Lopes da Silva. 2022. "The Biorefinery of the Marine Microalga Crypthecodinium cohnii as a Strategy to Valorize Microalgal Oil Fractions" Fermentation 8, no. 10: 502. https://doi.org/10.3390/fermentation8100502
APA StyleMoniz, P., Martins, D., Oliveira, A. C., Reis, A., & Lopes da Silva, T. (2022). The Biorefinery of the Marine Microalga Crypthecodinium cohnii as a Strategy to Valorize Microalgal Oil Fractions. Fermentation, 8(10), 502. https://doi.org/10.3390/fermentation8100502









