Effect of Biochar Addition on Mechanism of Heavy Metal Migration and Transformation in Biogas Residue Aerobic Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composting Process
2.2. Analytical Methods
2.3. Determination of Heavy Metals
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Activity Changes of Heavy Metals Cu, Zn, Cd, and Pb
3.2. Environmental Risk Assessment of Heavy Metals
3.3. Formatting of Mathematical Components
4. Effect Mechanism of Biochar on Composting of Biogas Residue
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, X.; Xi, B.D.; He, X.S. Hydrophobicity-dependent electron transfer capacities of dissolved organic matter derived from chicken manure compost. Chemosphere 2019, 222, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Tan, W.B. Biowaste-source-dependent synthetic pathways of redox functional groups within humic acids favoring pentachlorophenol dechlorination in composting process. Environ. Int. 2020, 135, 105380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Q.; Ma, J. Fungal community composition change and heavy metal accumulation in response to the long-term application of anaerobically digested slurry in a paddy soil. Ecotoxicol. Environ. Saf. 2020, 196, 110453. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Zuo, B.; Wang, Y.; Yuan, X.; Cui, Z. Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef]
- Lei, C.; Yongcui, W.; Bin, H.; Jian, M.; Xin, C. Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting. Molecules 2021, 26, 5225. [Google Scholar] [CrossRef]
- Knoop, C.; Tietze, M.; Dornack, C.; Raab, T. Fate of nutrients and heavy metals during two-stage digestion and aerobic post-treatment of municipal organic waste. Bioresour. Technol. 2018, 251, 238–248. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 58, 2282–2287. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, H.; Sun, L.; Cui, J.; Liu, J.; Tang, Q.; Du, F.; Liu, X.; Yao, D. Resource Utilization of Biogas Waste as Fertilizer in China Needs More Inspections Due to the Risk of Heavy Metals. Agriculture 2022, 12, 72. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Fu, Q. Effects of four additives in pig manure composting on greenhouse gas emission reduction and bacterial community change. Bioresour. Technol. 2019, 292, 121896. [Google Scholar] [CrossRef]
- He, X.; Yin, H.; Han, L. Effects of biochar size and type on gaseous emissions during pig manure/wheat stalk aerobic composting: Insights into multivariate-microscale characterization and microbial mechanism. Bioresour. Technol. 2019, 271, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, B.; Damasceno, F.A.; Andrade, R.R.; Saraz, J.A.O.; Barbari, M.; Vega, F.A.O.; Nascimento, J.A.C. Comparison of airflow homogeneity in Compost Dairy Barns with different ventilation systems using the CFD model. Agron. Res. 2020, 18, 788–796. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Xu, X.; Liu, Z.; Meng, X. Evaluation of the effects of different fertilization modes on black soil fertility in China based on principal component and cluster analysis. Agrochimica 2020, 64, 149–166. [Google Scholar] [CrossRef]
- Qu, J.; Sun, Y.; Awasthi, M.K.; Liu, Y.; Xu, X.; Meng, X.; Zhang, H. Effect of different aerobic hydrolysis time on the anaerobic digestion characteristics and energy consumption analysis. Bioresour. Technol. 2021, 320, 124332. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Xiong, T.; Wang, H.; Wu, Z.; Jiang, L.; Zeng, G.; Li, Y. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer. Environ. Sci. Pollut. Res. 2018, 25, 32562–32571. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Dong, Y. Stabilization and passivation of multiple heavy metals in soil facilitating by pinecone-based biochar: Mechanisms and microbial community evolution. J. Hazard. Mater. 2021, 420, 126588. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, Z.; Guo, K. Speciation analysis and speciation transformation of heavy metal ions in passivation process with thiol-functionalized nano-silica. Chem. Eng. J. 2019, 369, 979–987. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, R.; Li, G. Impact of biochar, calcium magnesium phosphate fertilizer and spent mushroom substrate on humification and heavy metal passivation during composting. Sci. Total Environ. 2022, 824, 153755. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Z.M.; Li, X.; Liu, H.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qiu, L.; Zheng, S.A. Kinetic approach for assessing the effect of flooding on the redistribution of heavy metals in paddy soil. Fresenius Environ. Bull. 2014, 23, 113–121. [Google Scholar]
- Shen, Z.; Hou, D.; Jin, F. Effect of production temperature on lead removal mechanisms by rice stalk biochars. Sci. Total Environ. 2019, 655, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Cai, Q.; Mo, C.; Wu, Q. Concentration and speciation of heavy metals in six different sewage sludge-composts. J. Hazard. Mater. 2007, 147, 1063–1072. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zeng, G.; Dong, H.; Chen, Y.; Huang, C. Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 2018, 261, 10–18. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Chen, H.; Wang, M.; Zhang, Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245, 300–308. [Google Scholar] [CrossRef]
- Meng, J.; Wang, L.; Liu, X.; Wu, J.; Brookes, P.C.; Xu, J. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour. Technol. 2013, 142, 641–646. [Google Scholar] [CrossRef]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zou, X.; Shu, R.; Ding, L.; Yao, K. Impact of humin on soil adsorption and remediation of Cd (II), Pb (II), and Cu (II). Soil Sediment Contam. Int. J. 2016, 25, 700–715. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe−N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbial fuel cell. Int. J. Agric. Biol. Eng. 2020, 13, 207–214. [Google Scholar] [CrossRef]
- Barančíková, G.; Makovníková, J. The influence of humic acid quality on the sorption and mobility of heavy metals. Plant Soil Environ. 2003, 49, 565–571. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Biogas Residue | Pig Manure | Corn Stalk | Biochar |
|---|---|---|---|---|
| TOC (%) | 29.92 ± 0.58 | 8.52 ± 0.58 | 41.53 ± 2.18 | 46.14 ± 1.76 |
| TN (%) | 1.53 ± 0.03 | 0.54 ± 0.03 | 0.78 ± 0.06 | 0.94 ± 0.03 |
| Moisture content (%) | 68.20 ± 1.35 | 69.34 ± 2.86 | 10.44 ± 0.51 | 7.42 ± 0.17 |
| TP (g·kg−1) | 6.75 ± 0.04 | 21.76 ± 1.05 | 0.35 ± 0.02 | 5.25 ± 0.22 |
| TK (g·kg−1) | 5.94 ± 0.06 | 10.62 ± 0.82 | 1.13 ± 0.08 | 38.96 ± 1.18 |
| pH | 7.46 ± 0.37 | 7.48 ± 0.32 | 6.96 ± 0.35 | 9.90 ± 0.37 |
| EC (mS·cm−1) | 12.19 ± 0.48 | 5.26 ± 0.15 | 2.58 ± 0.20 | 12.14 ± 0.48 |
| OM (%) | 59.58 ± 1.26 | 46.05 ± 1.48 | 90.82 ± 3.56 | 64.23 ± 2.87 |
| Cu(mg·kg−1) | 78.04 ± 1.38 | 94.96 ± 4.68 | 13.44 ± 0.84 | 0.16 ± 0.01 |
| Zn (mg·kg−1) | 66.64 ± 2.31 | 185.83 ± 12.79 | 8.01 ± 0.63 | 0.29 ± 0.01 |
| Cd (mg·kg−1) | 0.69 ± 0.04 | 0.46 ± 0.02 | 0.14 ± 0.01 | 0.01 ± 0.01 |
| Pb (mg·kg−1) | 5.11 ± 0.17 | 3.82 ± 0.21 | 3.96 ± 0.22 | 0.18 ± 0.01 |
| Treatment | Biogas Residue (kg) | Corn Stalk (kg) | Pig Manure (kg) | Biochar (kg) | TC (db) | TN (db) |
|---|---|---|---|---|---|---|
| T1 | 8.00 | 1.05 | 0.85 | 0.10 | 1.22 | 0.049 |
| T2 | 8.00 | 0.93 | 0.87 | 0.20 | 1.22 | 0.049 |
| T3 | 8.00 | 0.83 | 0.88 | 0.29 | 1.22 | 0.049 |
| T4 | 8.00 | 0.74 | 0.88 | 0.38 | 1.22 | 0.049 |
| CK | 8.00 | 1.17 | 0.83 | - | 1.22 | 0.049 |
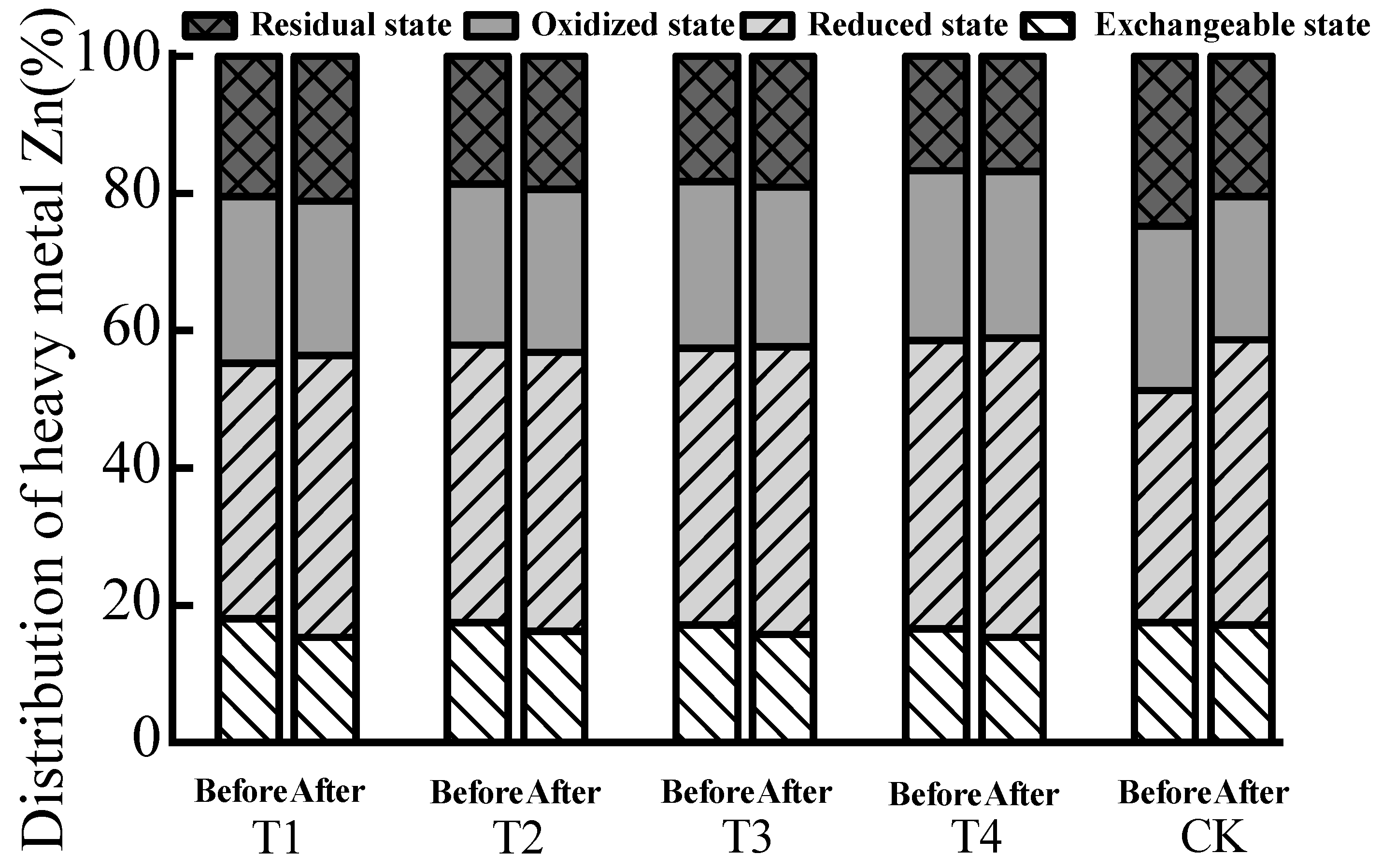
| Treatment | Sample | Exchangeable State | Reduced State | Oxidized State | Residual State | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/kg) | Allocation Rate (%) | Passivation Effect (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | ||
| T1 | Before | 4.73 | 7.69 | 7.41 b | 3.03 | 4.93 | 46.53 | 75.65 | 7.22 | 11.74 |
| After | 4.24 | 7.12 | 2.09 | 3.51 | 45.43 | 76.29 | 7.79 | 13.08 | ||
| T2 | Before | 4.46 | 7.26 | 8.84 b | 2.21 | 3.60 | 47.09 | 76.61 | 7.71 | 12.54 |
| After | 4.02 | 6.61 | 2.89 | 4.75 | 42.90 | 70.58 | 10.97 | 18.05 | ||
| T3 | Before | 4.22 | 6.88 | 10.16 a | 2.64 | 4.31 | 46.28 | 75.49 | 8.17 | 13.33 |
| After | 3.80 | 6.18 | 2.58 | 4.20 | 46.18 | 75.15 | 8.89 | 14.47 | ||
| T4 | Before | 4.36 | 7.15 | 11.75 a | 2.65 | 4.34 | 43.02 | 70.50 | 10.99 | 18.01 |
| After | 3.62 | 6.31 | 2.00 | 3.48 | 40.91 | 71.26 | 10.88 | 18.95 | ||
| CK | Before | 3.75 | 6.09 | 3.68 c | 2.89 | 4.69 | 48.14 | 78.20 | 6.78 | 11.01 |
| After | 3.64 | 5.87 | 2.79 | 4.50 | 48.67 | 78.45 | 6.94 | 11.19 | ||
| Treatment | Sample | Exchangeable State | Reduced State | Oxidized State | Residual State | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/kg) | Allocation Rate (%) | Passivation Effect (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | ||
| T1 | Before | 10.62 | 18.07 | 15.26 a | 21.83 | 37.14 | 14.32 | 24.36 | 12.01 | 20.43 |
| After | 9.18 | 15.31 | 24.64 | 41.09 | 13.48 | 22.48 | 12.66 | 21.11 | ||
| T2 | Before | 10.33 | 17.50 | 7.48 b | 23.85 | 40.41 | 13.83 | 23.43 | 11.01 | 18.65 |
| After | 9.97 | 16.19 | 25.03 | 40.64 | 14.62 | 23.74 | 11.96 | 19.43 | ||
| T3 | Before | 10.10 | 17.10 | 7.71 b | 23.79 | 40.27 | 14.41 | 24.39 | 10.78 | 18.25 |
| After | 9.34 | 15.78 | 24.79 | 41.87 | 13.80 | 23.31 | 11.27 | 19.04 | ||
| T4 | Before | 9.72 | 16.51 | 7.45 b | 24.75 | 42.02 | 14.62 | 24.82 | 9.81 | 16.66 |
| After | 9.02 | 15.28 | 25.75 | 43.63 | 14.32 | 24.26 | 9.93 | 16.82 | ||
| CK | Before | 10.22 | 17.46 | 1.76 c | 19.76 | 33.75 | 14.06 | 24.01 | 14.50 | 24.77 |
| After | 10.44 | 17.15 | 25.28 | 41.54 | 12.66 | 20.80 | 12.48 | 20.51 | ||
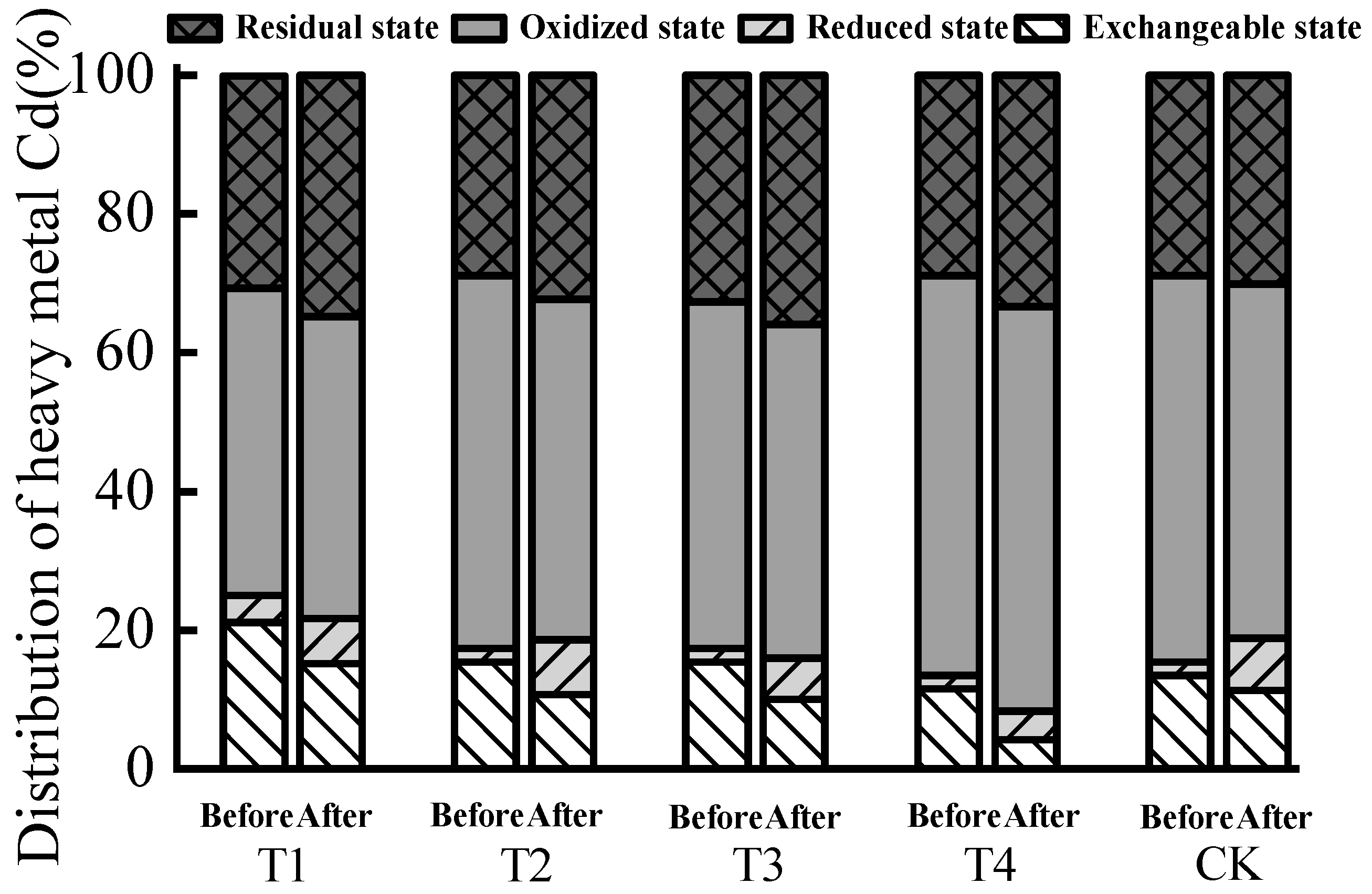
| Treatment | Sample | Exchangeable State | Reduced state | Oxidized State | Residual State | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/kg) | Allocation Rate (%) | Passivation Effect (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | ||
| T1 | Before | 0.11 | 21.15 | 28.06 b | 0.02 | 3.85 | 0.23 | 44.23 | 0.16 | 30.58 |
| After | 0.07 | 15.22 | 0.03 | 6.52 | 0.20 | 43.48 | 0.16 | 34.78 | ||
| T2 | Before | 0.08 | 15.38 | 29.90 b | 0.01 | 1.92 | 0.28 | 53.85 | 0.15 | 28.85 |
| After | 0.06 | 10.78 | 0.04 | 7.84 | 0.25 | 49.02 | 0.17 | 32.36 | ||
| T3 | Before | 0.08 | 15.38 | 35.00 b | 0.01 | 1.92 | 0.26 | 50.00 | 0.17 | 32.69 |
| After | 0.05 | 10.00 | 0.03 | 6.00 | 0.24 | 48.00 | 0.18 | 36.00 | ||
| T4 | Before | 0.06 | 11.54 | 63.89 a | 0.01 | 1.92 | 0.30 | 57.69 | 0.15 | 28.85 |
| After | 0.02 | 4.17 | 0.02 | 4.17 | 0.28 | 58.33 | 0.16 | 33.33 | ||
| CK | Before | 0.07 | 13.46 | 15.90 c | 0.01 | 1.92 | 0.29 | 55.77 | 0.15 | 28.85 |
| After | 0.06 | 11.32 | 0.04 | 7.55 | 0.27 | 50.94 | 0.16 | 30.19 | ||
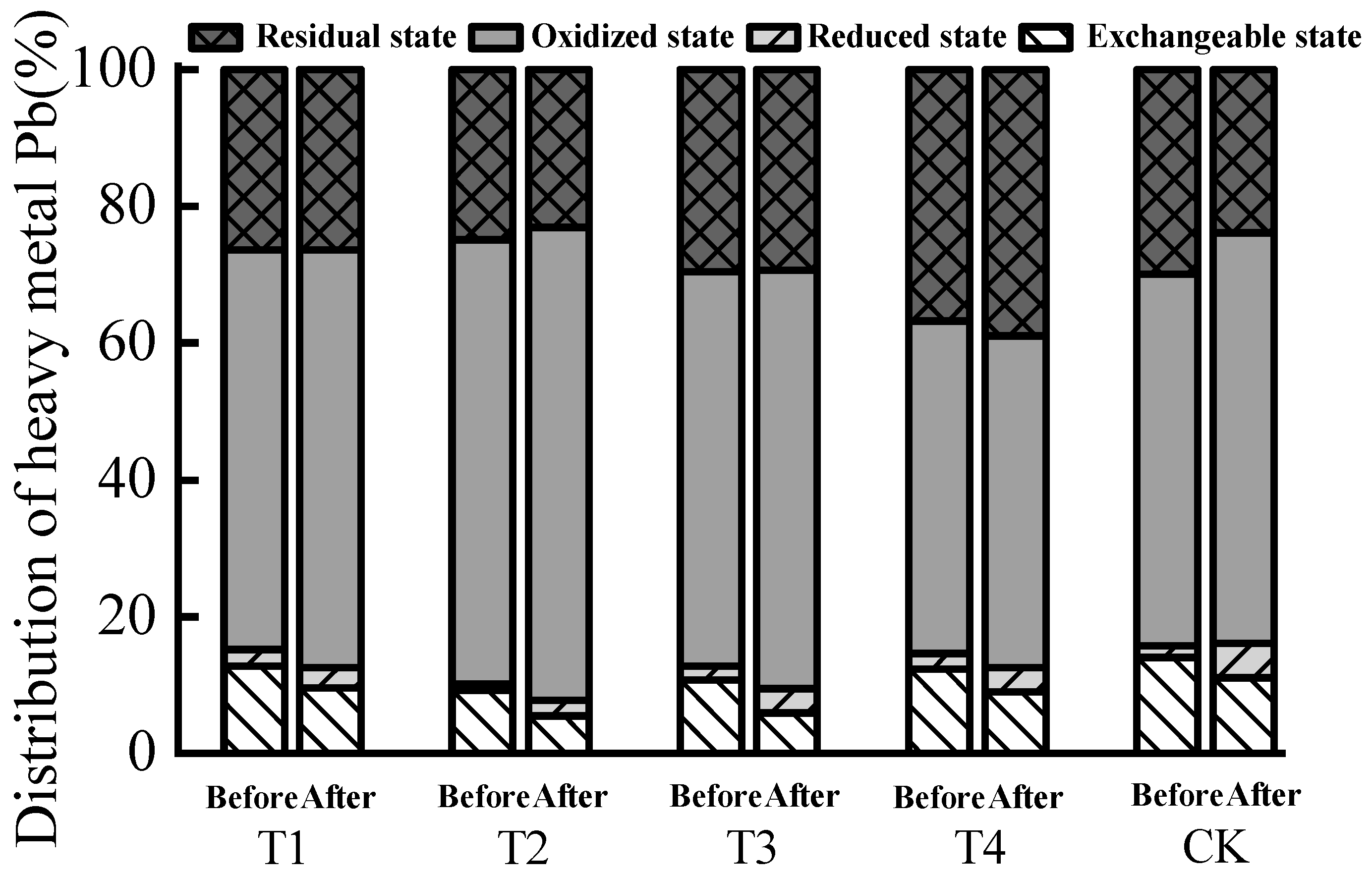
| Treatment | Sample | Exchangeable State | Reduced State | Oxidized State | Residual State | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/kg) | Allocation Rate (%) | Passivation Effect (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | Content (mg/kg) | Allocation Rate (%) | ||
| T1 | Before | 0.59 | 12.77 | 25.16 b | 0.11 | 2.38 | 2.70 | 58.44 | 1.22 | 26.41 |
| After | 0.54 | 9.56 | 0.17 | 3.01 | 3.45 | 61.06 | 1.49 | 26.37 | ||
| T2 | Before | 0.42 | 9.25 | 40.33 a | 0.04 | 0.88 | 2.95 | 64.98 | 1.13 | 24.89 |
| After | 0.35 | 5.52 | 0.14 | 2.21 | 4.39 | 69.24 | 1.46 | 23.03 | ||
| T3 | Before | 0.48 | 10.76 | 45.02 a | 0.09 | 2.02 | 2.57 | 57.62 | 1.32 | 29.60 |
| After | 0.30 | 5.92 | 0.18 | 3.55 | 3.10 | 61.14 | 1.49 | 29.39 | ||
| T4 | Before | 0.54 | 12.33 | 27.21 b | 0.10 | 2.28 | 2.13 | 48.63 | 1.61 | 36.76 |
| After | 0.35 | 8.97 | 0.14 | 3.59 | 1.89 | 48.46 | 1.52 | 38.97 | ||
| CK | Before | 0.66 | 14.01 | 20.84 c | 0.08 | 1.70 | 2.56 | 54.35 | 1.41 | 29.94 |
| After | 0.64 | 11.09 | 0.29 | 5.03 | 3.46 | 59.97 | 1.38 | 23.92 | ||
| Treatment | Single Potential Ecological Risk Coefficient ER | Potential Ecological Risk Index RI | Potential Ecological Risk Index | ||||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Cd | Pb | ||||
| T1 | Before | 37.60 | 3.89 | 67.92 | 13.93 | 123.34 | Minor |
| After | 33.22 | 3.74 | 56.25 | 13.96 | 107.17 | Minor | |
| T2 | Before | 34.86 | 4.36 | 74.00 | 15.09 | 128.31 | Minor |
| After | 22.70 | 4.15 | 60.88 | 16.71 | 104.44 | Minor | |
| T3 | Before | 32.52 | 4.48 | 61.76 | 11.89 | 110.65 | Minor |
| After | 29.56 | 4.25 | 53.33 | 12.01 | 99.15 | Minor | |
| T4 | Before | 22.76 | 5.00 | 74.00 | 8.60 | 110.36 | Minor |
| After | 21.38 | 5.11 | 60.00 | 7.83 | 94.32 | Minor | |
| CK | Before | 40.40 | 3.04 | 74.00 | 11.70 | 129.14 | Minor |
| After | 39.70 | 3.88 | 69.38 | 15.91 | 128.87 | Minor | |
| Single potential ecological risk coefficient ER | Potential ecological risk index RI | Potential ecological risk index | |||||
| ER ≤ 40 | RI ≤ 150 | Minor ecological hazard | |||||
| 40 < ER ≤ 80 | 150 < RI ≤ 300 | Moderate ecological hazard | |||||
| 80 < ER ≤ 160 | 300 < RI ≤ 600 | Strong and high ecological harm | |||||
| 160 < ER ≤ 320 | RI > 600 | Extreme ecological hazard | |||||
| ER > 320 | |||||||
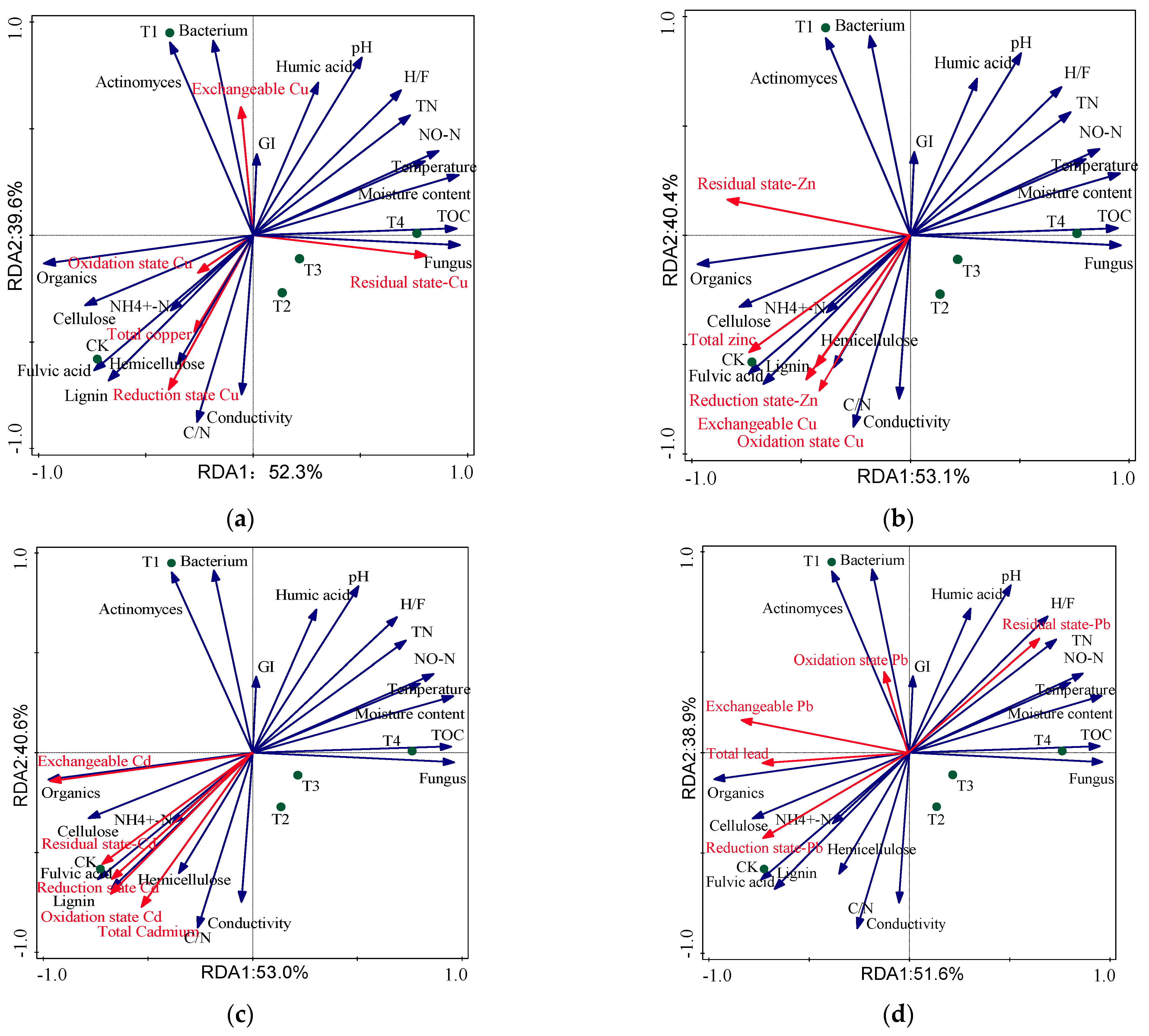
| Index | Cu (%) | Zn (%) | Cd (%) | Pb (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A1 | A2 | A3 | A4 | A1 | A2 | A3 | A4 | A1 | A2 | A3 | A4 | |
| Eigenvalue | 52.3 | 39.6 | 7.4 | 0.7 | 53.1 | 40.4 | 6.3 | 0.2 | 53.0 | 40.6 | 6.2 | 0.2 | 51.6 | 38.9 | 8.9 | 0.6 |
| Explanatory variable | 52.3 | 91.9 | 99.3 | 100 | 53.1 | 93.5 | 99.8 | 100 | 53.0 | 93.6 | 99.8 | 100 | 51.6 | 90.5 | 99.4 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Qu, J.; Qu, Y.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Effect of Biochar Addition on Mechanism of Heavy Metal Migration and Transformation in Biogas Residue Aerobic Compost. Fermentation 2022, 8, 523. https://doi.org/10.3390/fermentation8100523
Yan W, Qu J, Qu Y, Yue T, Zhang Q, Yi W, Liu X, Sun Y. Effect of Biochar Addition on Mechanism of Heavy Metal Migration and Transformation in Biogas Residue Aerobic Compost. Fermentation. 2022; 8(10):523. https://doi.org/10.3390/fermentation8100523
Chicago/Turabian StyleYan, Wencong, Jingbo Qu, Youpei Qu, Tian Yue, Quanguo Zhang, Weiming Yi, Xiaofeng Liu, and Yong Sun. 2022. "Effect of Biochar Addition on Mechanism of Heavy Metal Migration and Transformation in Biogas Residue Aerobic Compost" Fermentation 8, no. 10: 523. https://doi.org/10.3390/fermentation8100523




