Abstract
The present study documents the changes in the γ-aminobutyric acid (GABA), the total phenolic (TP), total flavonoid (TF), and isoflavone contents, the antioxidant activities and the digestive enzyme inhibition during the solid lactic acid fermentation of isoflavone-enriched soybean leaves (IESLs) with starters Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184. The contents of glutamic acid (GA) and GABA remained almost unchanged during fermentation with P1201. In contrast, the contents of GABA increased from 144.24 to 173.09 and 175.59 mg/100 g, and the contents of GA decreased from 43.68 to 18.26 and 11.25 mg/100 g during the IESLs’ fermentation with BMK184 and the combined use of P1201 + BMK184, respectively. The total isoflavone content decreased during fermentation, but the isoflavone aglycone levels increased; in fact, the levels of daidzein and genistein were the highest after the use of P1201 + BMK184 (2265.57 μg/g) and BMK184 (1055.27 μg/g), respectively, at 72 h of fermentation. Correspondingly, the DPPH (90.90%), the ABTS (91.09%), and the hydroxyl (74.88%) radical scavenging activities, the ferric reducing/antioxidant power (2.45), as well as the α-glucosidase (49.86%) and pancreatic lipase (37.30%) inhibition activities exhibited their highest levels after fragmentation with P1201 + BMK184.
1. Introduction
Soybean (Glycine max Merr. L.) leaves are a by-product of soybeans and are mainly used as Jangajji (Korean pickle) and Ssam in Gyeongsangnam-do and Jeju-do in Korea. Soybean leaves contain at least a dozen physiologically active substances such as isoflavones, flavones, flavonols, terokapanes, phenolic compounds, and sugar alcohols [1,2,3]. Several studies have reported changes in the isoflavone contents and the physicochemical properties of soybean leaf extracts as a result of the extraction conditions employed [4], as well as an increase in the isoflavone aglycone content in soybean leaf extracts by lactic acid fermentation [5]. Moreover, a study has been conducted on the production of soybean leaves containing a 5-fold higher amount of isoflavone glycosides (daidzin, genistein, malonyldaidzin, and malonylgenistein) as a result of treating soybean leaves with plant growth regulators (such as ethylene and ethephon) [3].
Isoflavones are phytoestrogens in plant resources and play a similar role to estrogen; they are present in most foods in the form of glycosides. Intestinal microbes in the body metabolize them after ingestion. They are absorbed in the form of aglycones, so that only a portion of the intake is absorbed by the body [6,7,8,9]. On the other hand, the aglycone form has been reported to have higher digestive stability, bioactivity, and bioavailability than the glycoside [10]. It has health benefits such as antioxidant, anti-inflammation, anti-cancer, anti-obesity, anti-osteoporosis, and gut biota regulation [11,12,13,14].
Gamma-aminobutyric acid (GABA) is a non-protein amino acid neurotransmitter that suppresses excitement and is one of the most commonly used neurotransmitters in the central nervous system of mammals [15,16]. It is known that it is effective in reducing blood pressure and improving memory, but it is not easy to expect such physiological effects only by ingesting natural food containing GABA [17,18]. For this reason, studies were conducted in order to enhance GABA production by using a mechanism in which glutamic acid (GA) is converted to GABA by causing a decarboxylation reaction led by glutamate decarboxylase (GADase) [19].
In order to increase the utilization of bioactive substances in isoflavone-enriched soybean leaves (IESLs), studies on the conversion of isoflavone aglycones and GABA by food processing (such as heat treatment and fermentation) are required. In this study, we assessed the changes in the levels of bioactive substances (GABA, total phenols (TP), total flavonoids (TF), and isoflavones) and biological activities (antioxidant and digestive enzyme inhibition) during the solid lactic acid fermentation of IESLs by using the starters Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184.
2. Materials and Methods
2.1. Preparation of IESLs and Starters
In order to harvest the IESLs, soybean seeds (Daewon seeds) were sown in the research fields and cultivated for about 60 days so as to maximize the growth of leaves before soybean pods emerged at JC & Farm Corporation (Namhae-gun, Gyeongsangnam-do, Korea) between April and June 2018. The ethephon (0.2 mg/mL) was sprayed on the soybean leaves twice every 24 h, until the solution dripped [3]. Subsequently, 96 h after the first spraying, the soybean leaves were harvested, washed with water, and dried in a food dryer at 45 °C The supplied dried soybean leaves were crushed and stored at −40 °C for further study. Lactic acid bacteria (LAB), such as Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184, were used as starters for the IESLs’ fermentation. Two LABs were previously isolated from kimchi and fermented plant [9,16].
2.2. Culture Medium and Chemical Regents
The LABs were precultured in a Lactobacilli MRS broth and agar (MRSB and MRSA, Difco, Becton Dickinson Co., Spark, MD, USA) media. In order to measure the antioxidants and the enzyme activities, the reagents 2,2-diphenyl-1-picrydrazyl (DPPH), 2,4,6-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 2,4,5-tri(2-pyridyl)-1,3,5-triazine (TPTZ), thiobarbituric acid (TBA), trichloroacetic acid (TCA), p-nitrophenyl-α-D-glucopyranoside (p-NPG), and p-nitrophenyl-butyrate (p-NPB), as well as the enzymes α-glucosidase and pancreatic lipase were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Chemicals for measuring TP and TF contents (such as the Folin-Ciocalteu’s reagent and diethylene glycol) were also purchased from Sigma-Aldrich (St. Louis, MO, USA). Amongst the six isoflavone standards, daidzin, genistin, daidzein, genistein, malonyldaidzin, and malonylgenistin were purchased from Sigma-Aldrich (St. Louis, MO, USA) and the LC Laboratories (Woburn, MA, USA). For the analysis, the reagents and solvents (such as HPLC-grade water, methanol, acetonitrile, glacial acetic acid, etc.) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fisher Scientific International, Inc. (Fairlawn, NJ, USA), respectively.
2.3. Solid Lactic Acid Fermentation of IESLs
A mixture of 2 g of sucrose, 100 g of IESLs, and 400 mL of distilled water was kept in a glass bottle (1-L volume) and was sterilized at 121 °C for 15 min. The seed culture solution was prepared as follows: the single colony was transferred to MRS broth media and cultured at 30 °C for 48 h in a shaking incubator. The sterilized IESLs were cooled at room temperature and were inoculated with (i) Lactiplantibacillus plantarum P1201, (ii) Levilactobacillus brevis BMK184, or (iii) a mixture of Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184, at seed culture solutions of 5% (v/v), 5% (v/v), and 2.5% (v/v), respectively. Subsequently, the samples were fermented at 37 °C for 0, 12, 24, 48, and 72 h, respectively. The fermented samples were then collected and preserved for experimental analysis.
2.4. Determination of pH, Acidity, and Viable Cell Numbers
The sample pH was measured by using a pH meter (MP220 pH meter, London, UK), and the acidity was calculated by mixing distilled water (50 mL) and sample (1 mL) in order to obtain a neutralization titration (pH 8.2 ± 0.1) consumption of a 0.1 N NaOH solution, that was converted to lactic acid and expressed as a percentage (%). In order to measure the number of viable cells, after decimal dilutions by using sterile water, was spread on a MRSA medium, and was cultured at 30 °C for 48 h. The resulting colonies were recorded as log cfu/g.
2.5. Determination of Enzymatic Activity
The activities of glutamate decarboxylase and β-glycosidase were determined as described by Hwang et al. [16]. For the glutamate decarboxylase assay, the standard reactant in each sample was composed of 200 µL of 20 mM acetate buffer (pH 5.5) containing 50 µmol of bromocresol green, 10 mM of PLP, 10 µL of 1% GA, and crude enzyme extract (2.5 units). After a 30-min incubation at 48 °C, the absorbance of the mixture was measured at 620 nm (UV-1800 240 V). In order to determine the β-glycosidase activity, the standard reaction medium in each well consisted of 250 μL of 50 mM sodium phosphate buffer (pH 7.0) with 5 mM p-NPG and crude enzyme extract (2.5 units). After reacting at 37 °C for 30 min, the enzymatic reaction was stopped by adding 500 mL of 0.2 M glycine-NaOH (pH 10.5), and the contents were immediately measured with a spectrophotometer (UV-1800 240V, Shimadzu Corp., Kyoto, Japan) at 405 nm.
2.6. Determination of GA and GABA
The free amino acids of the fermented samples were determined according to Hwang et al. [20]. Firstly, 0.1 g of fermented soybean leaves samples were kept in a test tube containing 5 mL of distilled water. Subsequently, the sample was homogenized and hydrolyzed at 60 °C for 1 h by using a heating block (HB-48P, DAIHAN Scientific, Wonju, Korea). After hydrolysis, 1 mL of 10% 5-sulfosalicylic acid dehydrate was added, and the mixture was allowed to stand at 4 °C for 2 h, followed by centrifugation at 15,000 rpm for 3 min. The filtrate was then collected and concentrated under reduced pressure at 50 °C, followed by the addition of 2 mL of lithium citrate buffer (pH 2.2) so as to dissolve it. The solution was further filtered through a 0.45-μm membrane filter, and the filtrate was used for the amino acid profiling through an automatic amino acid analyzer (L-8900, Hitachi High-Technologies Corp., Tokyo, Japan).
2.7. Preparation of Extracts
One gram of fermented soybean leaves was mixed with 40 mL ethanol (80%), and the mixture was kept at room temperature for 12 ± 2 h. Subsequently, the supernatant was collected by centrifugation followed by filtration with a 0.45 μm polyvinylidene difluoride membrane filter. The filtrate was used in order to measure the TP, TF, and isoflavone contents, the radical scavenging, and the digestive enzyme activities.
2.8. Determination of TP and TF Contents
The TP content of the extract was measured according to a slightly modified method, as described by Hwang et al. [16]. In brief, 0.5 mL of the sample extract and 0.5 mL of 25% Na2CO3 solution were added to the test tube, and were allowed to stand for 3 min; subsequently, 0.25 mL of the Folin-Ciocalteu phenol was added to that solution and the reaction solution was allowed to react at 30 °C for 1 h. The reaction solution’s absorbance was measured at 750 nm by using a spectrophotometer. The TP content was calculated from a standard calibration curve of gallic acid.
The TF content was measured according to the method adopted from Lee et al. [6]. After 0.5 mL of the extract was dispensed into a test tube, 1 mL of diethylene glycol and 0.01 mL of 1 N NaOH were added thereto, followed by a reaction in a constant temperature water bath, at 37 °C, for 1 h. The absorbance of the reaction solution was measured at 420 nm, and the TF content was calculated according to the standard calibration curve of rutin.
2.9. Determination of Isoflavones
The isoflavone content of the extract was analyzed by using high-performance liquid chromatography (HPLC; Agilent 1200 HPLC System, Agilent Technologies Inc., Waldbronn, Germany) according to Lee et al. [7]. The Lichrophore 100 RP C18 (LichroCART 125–4, 5 μm, 125 mm × 4 mm; Merck KGaA, Darmstadt, Germany) column was used for the stationary phase of the isoflavone binding, and the mobile phase solvent consisted of 0.2% acetic acid in water (solvent A) and 0.2% acetic acid in acetonitrile (solvent B). The condition of each mobile phase solvent was 0% (0 min), 10% (15 min), 20% (25 min), 25% (35 min), 35% (45 min), and 35% (50 min) based on solvent B. The sample injection volume and column temperature was set to 20 μL and 30 °C, respectively. The flow rate was maintained at 1 mL/min, and β-glycosides and aglycones were detected at 254 nm through a diode array detector. The content of each detected isoflavone peak was calculated by applying it to a standard calibration curve.
2.10. Determination of Antioxidant Activities
The DPPH, ABTS, and hydroxyl radical scavenging activities of the extract were measured according to Lee et al. [6]. In brief, 0.8 mL of a 1.5 × 10−4 M DPPH solution and 0.2 mL of the extract were mixed and were allowed to react for 30 min in the dark. Subsequently, the absorbance of the solution was measured at 525 nm with the use of a spectrophotometer.
The ABTS radical scavenging activity of the extract was obtained by mixing a 7 mM ABTS solution and 2.45 mM potassium persulfate at 1:1, and then leaving the mixture for 14 ± 2 h in a dark room so as to allow the formation of the ABTS cation. The ABTS radical stock solution was diluted in methanol to an absorbance of 0.7 ± 0.02 at 732 nm. Thereafter, 0.1 mL of the extract was added to 0.9 mL of the prepared ABTS radical solution, was allowed to react for 3 min, and its absorbance was measured at 732 nm.
The hydroxyl radical scavenging activity of the extract was observed by sequentially adding 0.2 mL of 10 mM FeSO4-EDTA, 0.2 mL of 10 mM 2-deoxyribose, 1.2 mL of extract, and 0.4 mL of 10 mM H2O2 to the test tube. The mixture was allowed to react at 37 °C for 4 h. Thereafter, 1 mL of 1% TBA and 1 mL of 2.8% TCA solutions were added to the mixture and were boiled in a water bath at 100 °C for 20 min. After cooling, the absorbance of the reaction mixture was measured at 525 nm. The DPPH and the ABTS hydroxyl radical scavenging activities were calculated as a percentage (%) as follows:
Radical scavenging activity (%) = [1 − (experiment sample absorbance/negative control absorbance)] × 100
The ferric reducing/antioxidant power (FRAP) was measured according to Hwang et al. [9]. Firstly, the acetate buffer, the TPTZ reagent, and the FeCl3 solution were mixed at a 10:1:1 (v/v/v) ratio, and were allowed to pre-react at 37 °C for 15 min. Subsequently, 0.95 mL of the pre-reacted FRAP reagent and 0.05 mL of the extract were added to a test tube, were left to react at 37 °C for 15 min, and the absorbance was measured at 590 nm.
2.11. Determination of Enzyme Inhibitory Effects
The extracts’ α-glucosidase and pancreatic lipase inhibitory activities were tested according to a method that was adopted from Lee et al. [7]. In brief, 30 μL of the extract, 50 μL of 1 U/mL α-glucosidase or pancreatic lipase, and 50 μL of 200 mM sodium phosphate buffer (pH 6.8) were added to the test tube and were allowed to pre-react at 37 °C for 10 min. Subsequently, 100 μL of 10 mM p-NPG or p-NPB dissolved in 200 mM sodium phosphate buffer (pH 6.8) were added and were allowed to react at 37 °C for 10 min. In order to terminate the reaction, 750 μL of 100 mM Na2CO3 were added, and the absorbance was measured at 420 nm. The extract-induced α-glucosidase and pancreatic lipase inhibitory activities were calculated as a percentage (%) by the following equation:
Inhibition activity (%) = [1 − (experiment sample absorbance/negative control absorbance)] × 100
2.12. Statistical Analysis
All analyses were expressed as the mean ± SD (standard derivation) with tree replicate measurements. The significant differences among samples were determined by Tukey’s multiple test (p < 0.05) using the Statistical Analysis System (SAS) software (ver. 9.4; SAS institute, Cary, NC, USA).
3. Results
3.1. pH, Acidity, Viable Cell Numbers, and Enzyme Activities during Fermentation
The alteration of pH, acidity, and viable cell numbers during the lactic acid fermentation of IESLs was observed (Table 1). During the fermentation of IESL, using pure or a mixture with the starter P1201 and BMK184, the pH gradually decreased from 0 h 6.19 (P1201), 6.19(BMK184), and 6.14 (P1201 + BMK184) to 72 h 3.97, 4.19, and 5.39. While the acidity and the viable cell numbers gradually increased from 0 h 0.32, 0.32, and 0.33% to 72 h 1.89, 0.93, and 1.80% and from 0 h 6.48, 6.60, and 6.35 log cfu/g to 72 h 9.61, 9.69, and 9.71 log cfu/g, respectively. In the case of the GADase, the activity of P1201 was weak to <0.01 unit/g. BMK184 and the mixture were gradually increased until 48 h, and then decreased slightly (0 h: 0.12 and 0.13: 48 h; 4.43 and 4.13; 72 h: 3.87 and 3.72 unit/g).

Table 1.
Changes of pH, acidity, and viable cell numbers during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters.
On the other hand, the β-glucosidase activities were found to be gradually increased until 48 h (0 h: 0.88, 0.11, and 0.11 → 48 h: 2.43, 2.54, and 2.66 unit/g), and then similar to (→ 72 h: 2.04, 2.30, and 2.42 unit/g). The GADase and β-glucosidase activities were highest at 48 h of fermentation when the BMK184 or the P1201 + BMK184 starters were used, respectively.
3.2. Measurement of GA and GABA Contents during the Fermentation of IESLs
The changes in the contents of GA and GABA during the fermentation of IESLs occurred, as seen in Table 2. The GA and GABA contents of the IESLs’ fermentation were 52.25 (P1201), 44.51(BMK184), and 43.68 (P1201 + BMK184) mg/100 g and 131.69, 141.63, and 144.24 mg/100 g at 0 h, respectively. In the case of the P1201 starter, the GABA content was slightly increased to 147.17 mg/100 g until 24 h of fermentation; however, no significant changes were observed during the rest of the following period of fermentation.

Table 2.
Changes of glutamic acid (GA) and γ-aminobutyric acid (GABA) contents during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters.
As the IESLs’ fermentation proceeds with the BMK184 or the P1201 + BMK184 starter, the GA contents were found to gradually decrease by more than two times (from 43.68 mg/100 g to 18.26 and 11.25 mg/100 g, respectively) until 72 h of fermentation; correspondingly, the GABA contents gradually increased to 20% (from 144.24 mg/100 g to 173.09 and 175.59 mg/100 g, respectively), and the content of GABA was highest when the mixture of starters (P1201 + BMK184) was used.
3.3. Enhancement of TP and TF Contents during the Fermentation of IESLs
Changes in the values of TP and TF during the fermentation of IELS are presented in Table 3. TP and TF contents at 0 h of fermentation were 9.55, 0.68, and 9.70 GAE mg/g and 15.04, 15.45, and 16.02 RE mg/g, respectively. As fermentation proceeded, all fermented IESL products using P1201, BMK184, and the combined P1201 + BMK184 strains increased, and both the contents of TP (10.71, 11.38, and 11.90 GAE mg/g) and TF (18.74, 19.33, and 21.48 RE mg/g) were found to be the highest at 72 h of fermentation. The P1201 + BMK184-fermented product (at 72 h) exhibited the highest TP and TF contents.

Table 3.
Changes of total phenolic (TP) and total flavonoid (TF) contents during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters.
3.4. Changes in Isoflavone Contents during the Fermentation of IESLs
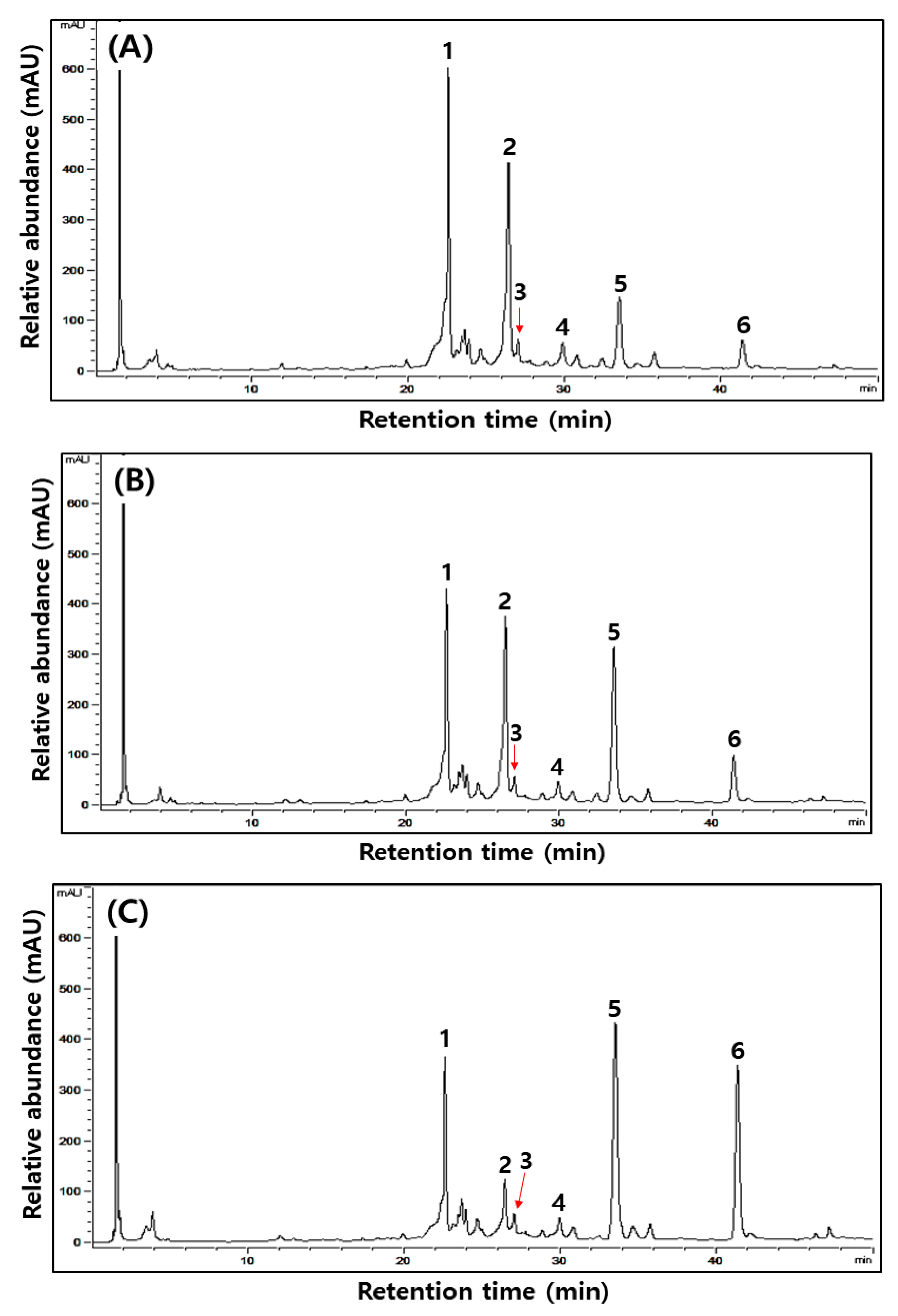
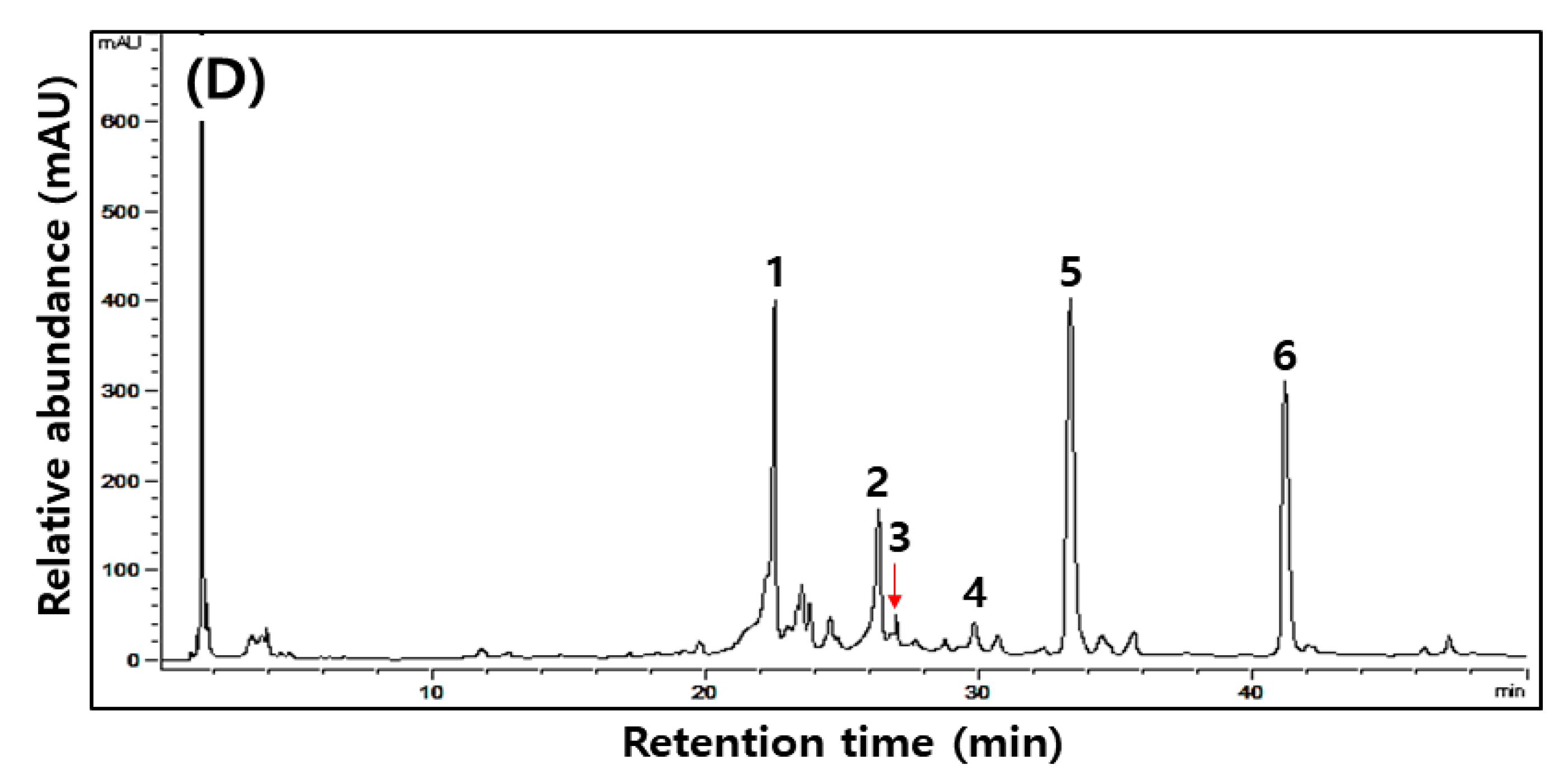
Changes in six kinds of isoflavone contents identified during the fermentation of IESLs are presented in Table 4 and Figure 1. The daidzin, genistin, malonyldaidzin, malonylgenistin, daidzein, and genistein contents were all detected at 0 to 72 h of fermentation, while the aglycone forms of daidzein and genistein were detected as major compounds. Amongst the isoflavones, glycosides (daidzin and genistin) and malonyglycosides (malonyldaidzin and malonylgenistin) tended to decrease as the fermentation proceeded, and while the fermentations using starters resulted in low contents of glycosides and malonylglycosides, respectively, after 72 h of fermentation. On the other hand, in the form of aglycones, daidzein (754.11, 247.54, and 747.54 μg/g at 0 h) and genistein (240.38, 236.93, and 217.93 μg/g at 0 h) gradually increased as the fermentation proceeded, thereby reaching 1460.02, 2008.11, and 2265.57 μg/g of daidzein and 316.55, 1055.27, and 987.65 μg/g of genistein, respectively, at 72 h of fermentation. Both daidzein (1460.02–2265.57 μg/g) and genistein (316.55–1055.27 μg/g) at 72 h of fermentation were confirmed to be at least 1.5-times higher than what they were at the initial stage of the fermentation. Amongst them, daidzein exhibited the highest content (2265.57 μg/g) in the fermented extract of the IESLs with the use of P1201 + BMK184 as starters, and so did genistein (1055.27 μg/g) with the use of BMK184 as a starter.

Table 4.
Changes of isoflavone contents during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters.
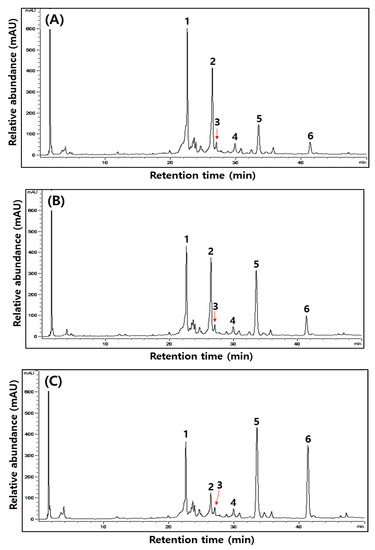
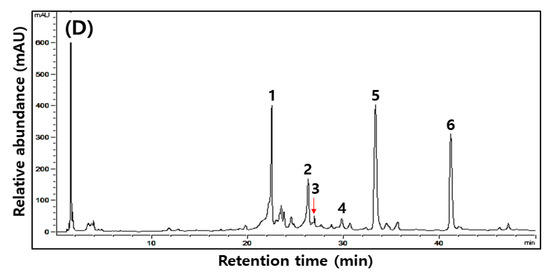
Figure 1.
Typical HPLC chromatograms of isoflavones during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. Samples: The IESLs were fermented at 35 °C for 72 h, with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. (A) 0 h, (B) P1201 for 72 h, (C) BMK for 72 h, and (D) P1201 and BMK184 for 72 h. Chromatogram: 1, daidzin; 2, genistin; 3, malonyldidzin; 4, malonylgenistin; 5, dadzein; 6, genistein.
3.5. Increased Antioxidant Activity in the Fermented IESLs
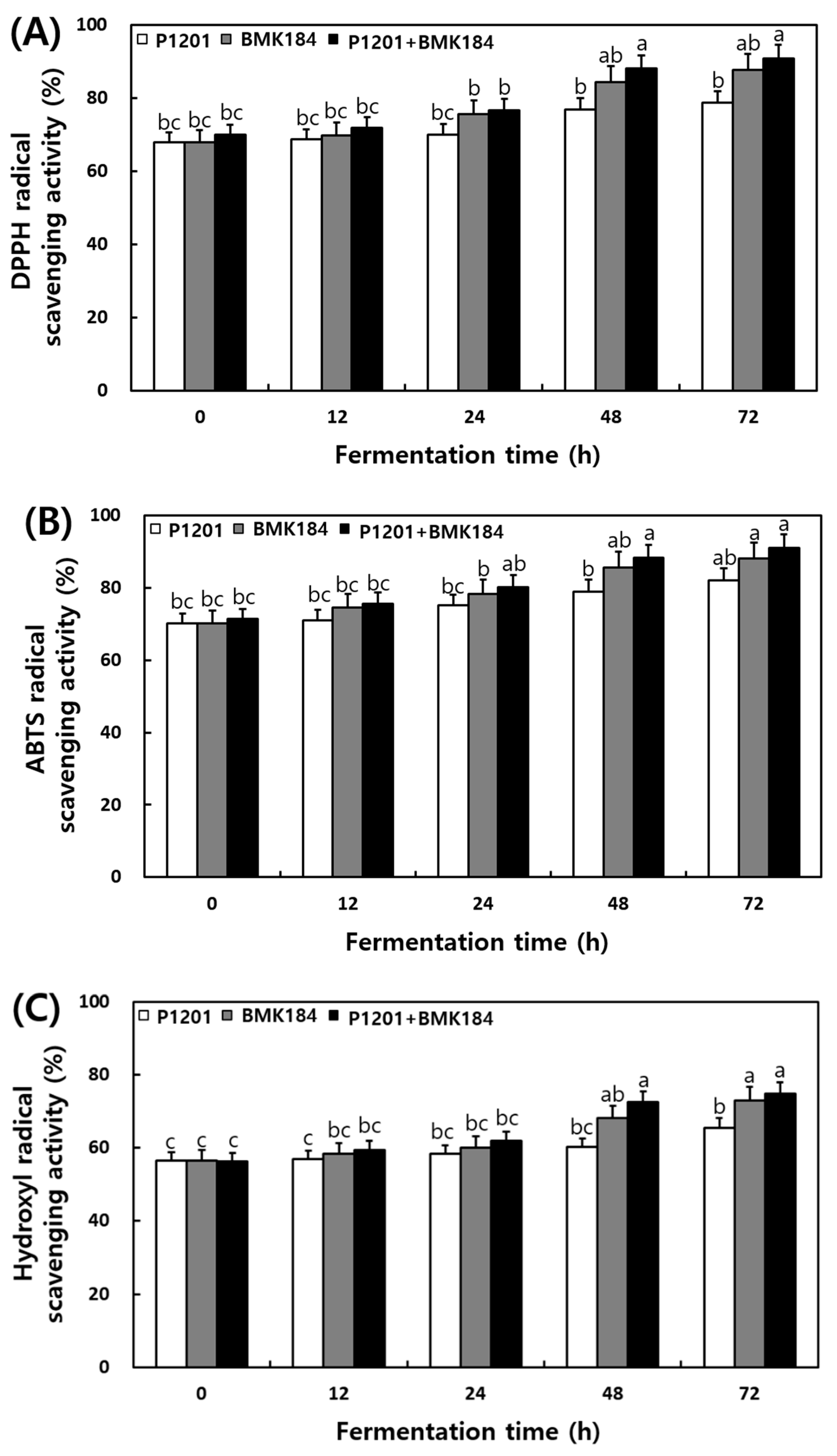
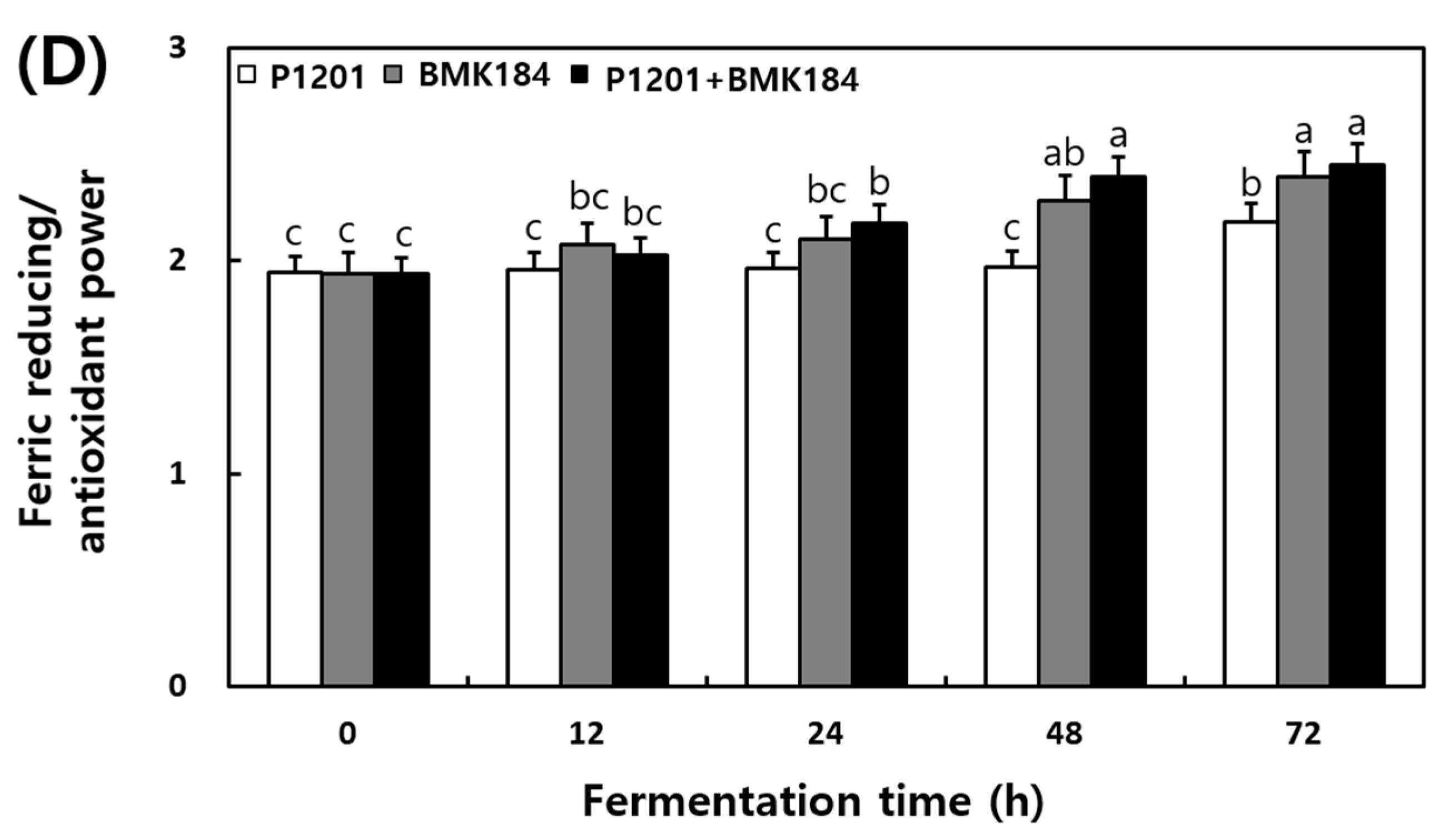
The changes observed in the antioxidant activities during the fermentation of IESLs are presented in Figure 2. The DPPH radical scavenging activity of soybean leaf fermentation was 67.98%, 67.88%, and 69.97% at 0 h of fermentation with the use of P1201, BMK184, and P1201 + BMK184 as starters, respectively; these activities were similar to those observed at the initial stage of the fermentation. Interestingly, it increased up to 90%; in particular, the BMK184 starter induced the highest activity (90.98%) in the fermentation products. The ABTS radical scavenging activity was 70.09–71.32% at 0 h of the fermentation. Notably, the highest content (91.09%) was shown in the fermented product after using P1201 + BMK184 as starters, at 72 h of fermentation. The hydroxyl radical scavenging activity was about 56% at the beginning of the fermentation (0 h), but the activity increased as the fermentation proceeded, and at the end of the fermentation (72 h), the P1201 (65.48%), BMK184 (72.96%), and BMK184 + P1201 (74.88%) starters showed the highest activity in that order. The FRAP was found to be within the range of 1.939 to 1.944 after 0 h of fermentation, and after 12 h fermentation, the FRAP was in the following order: P1201 < P1201 + BMK184 < BMK184. After 72 h of fermentation, the FRAP values obtained were 2.18, 2.39, and 2.45, with the use of P1201, BMK184, and P1201 + BMK184, respectively.
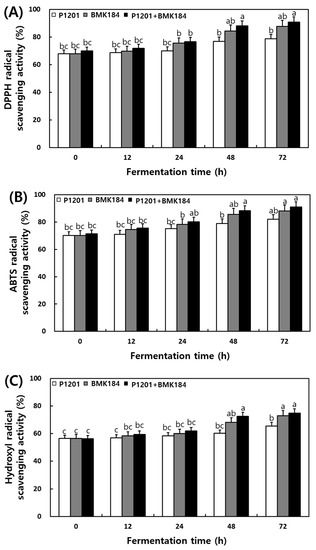
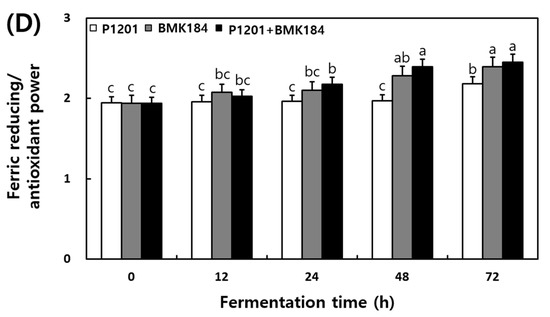
Figure 2.
Changes of antioxidant effects during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. Samples: The IESLs were fermented at 35 °C for 72 h, with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. (A) DPPH radical scavenging activity, (B) ABTS radical scavenging activity, (C) Hydroxyl radical scavenging activity, and (D) Ferric reducing/antioxidant power. All values represent the means of the determinations performed in three independent experiments. Different small letters correspond to the significant differences relating to the fermentation time and starter using Tukey’s multiple test (p < 0.05).
3.6. Changes in Enzymatic Inhibitory Activity during the Fermentation of IESLs
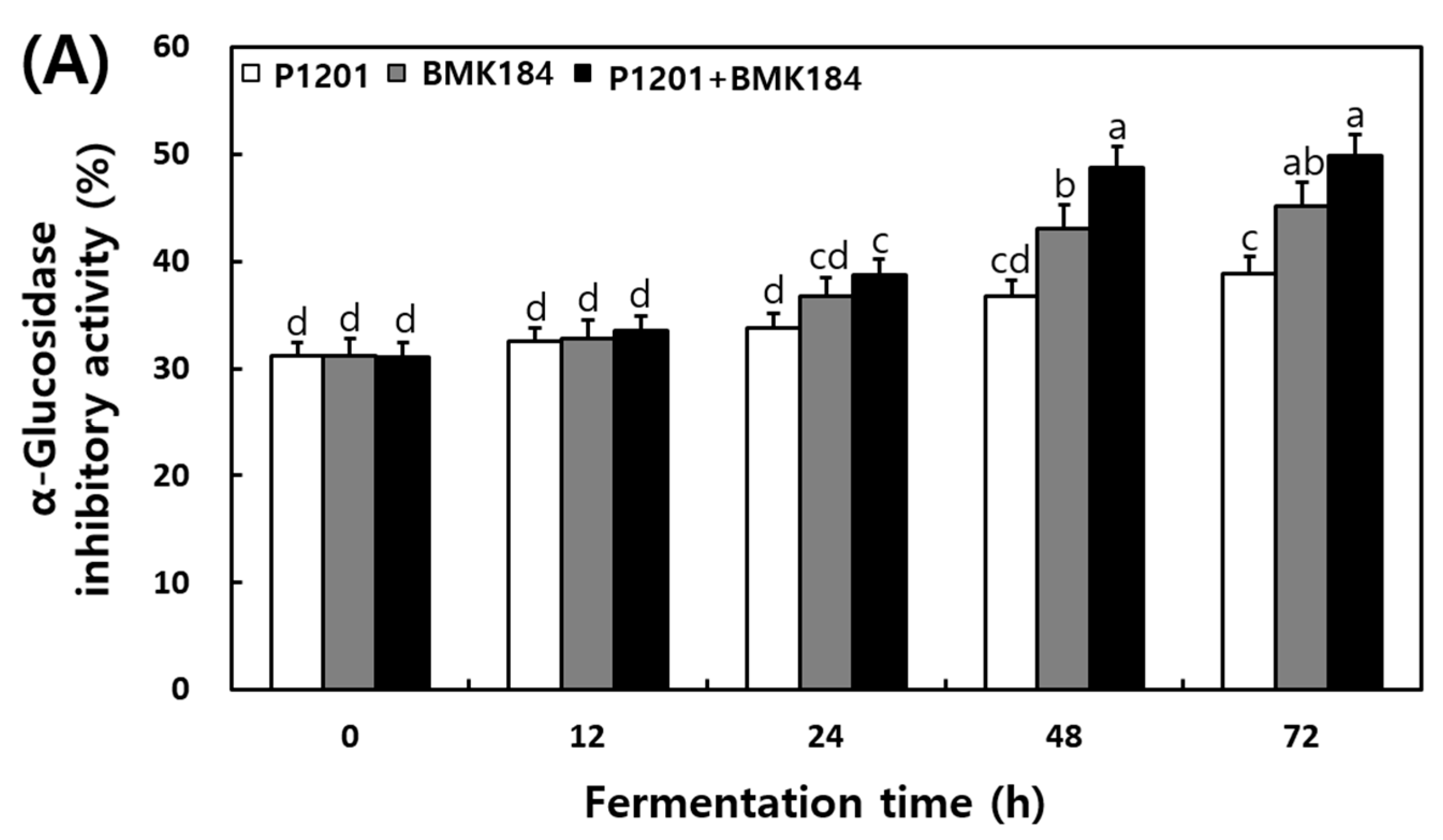
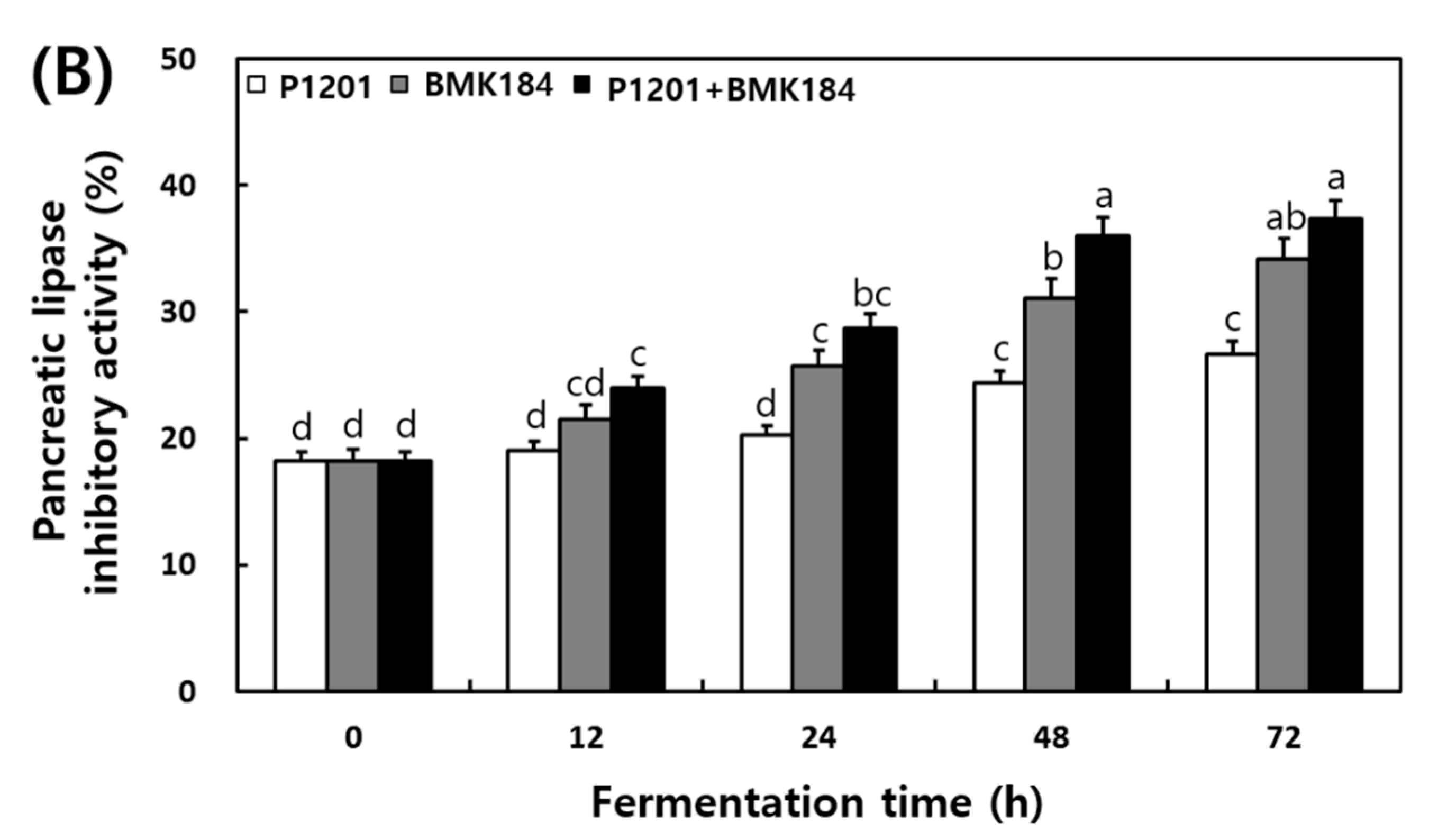
The changes in the α-glucosidase and pancreatic lipase inhibitory activities that were observed in the extracts of the fermented IESLs, are presented in Figure 3. The α-glucosidase inhibitory activity of the fermented IESLs was maintained as the fermentation proceeded in the following order: P1201 < BMK184 < P1201 + BMK184; in fact, the inhibitory activity gradually increased until 72 h. The mixture of starters (P1201 + BMK184) exhibited the highest inhibitory activity at 49.86%. On the other hand, the pancreatic lipase inhibitory activity was estimated at 18.2% at the beginning of the fermentation process, and its activity gradually increased in a time-dependent manner. At 72 h of fermentation, the levels of P1201, BMK184, and P1201 + BMK184 were 26.64%, 34.11%, and 37.30%, respectively.
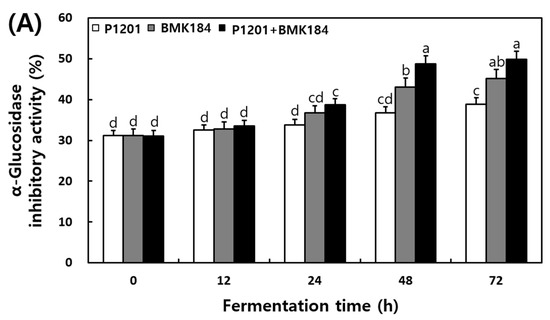
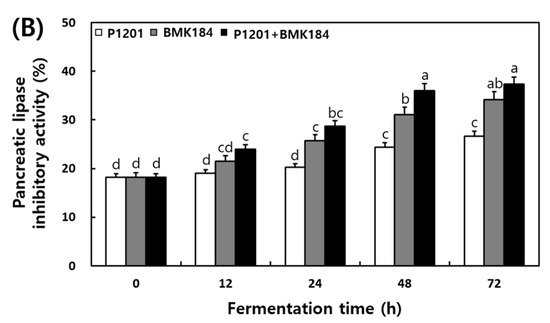
Figure 3.
Changes of enzymatic inhibitory effects during the fermentation of isoflavone-enriched soybean leaves (IESLs) with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. Samples: The IESLs were fermented at 35 °C for 72 h, with the use of Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 as starters. (A) α–Glucosidase inhibitory activity and (B) Pancreatic lipase inhibitory activity. All values represent the means of the determinations performed in three independent experiments. Different small letters correspond to the significant differences relating to the fermentation time and starter using Tukey’s multiple test (p < 0.05).
4. Discussion
The solid lactic acid fermentation of soybean powder with Lactobacillus starters for the bioconversion of GABA and isoflavone aglycone contents has been well-characterized. However, the bioconversion of these parameters of soybean leaves has not been well documented yet. Notably, the IESLs’ bioconversion to GABA, aglycone, as well as their antioxidant activities have not been reported yet either. In this study, we first studied the ethephon-induced higher isoflavone-glycone enriched soybean leaves bioconversion of GABA and isoflavone aglycone with the well-defined starters Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184. After fermentation, the starters successfully converted the significant amounts of GABA, TP, and TF contents, as well as isoflavone aglycone.
In particular, the pH value of the fermented IESLs decreased during lactic acid fermentation, but the acidity increased. Very similar studies [6,7,9,16,20,21] on soybean powder fermentation with the use of various Lactobacillus starters have reported the acidity to increase; consequently, the pH of the fermented products decreased. Previously, Lactiplantibacillus plantarum has been shown to exert an average final pH of 4.0 at 72 h of soy milk fermentation [6], and Levilactobacillus brevis has been shown to exert an average pH of 5.0 at 72 h [16], both being in agreements with the findings of the present study. Mixtures of bacteria or consortia applications on substrates have consistently demonstrated the maximum wanted outcomes, as observed in the cases of lignocellulose degradation [22] and eggplant growth promotion [23]. In a related study, Hwang et al. [9] have reported that a mixed culture of Lactiplantibacillus plantarum and Levilactobacillus brevis during soybean fermentation improved the growth of viable cell numbers when compared to the effects of these same starters when applied individually. Apparently, the changes in pH, acidity, and viable cell numbers during the solid lactic acid fermentation of IESLs were similar to those observed by previous studies [6,7,9,16,20]. GADase acts as a single step in the biosynthesis of GABA and can be found in various organisms [24,25,26]. It has been reported that GADase exhibits optimum activity at pH 5.9 [27], which slightly deviates from the present study’s findings. While the characteristics of GADase varied by microbial species [28,29], the GADase activity of this study also varied according to species. A similar result was observed for the β-glucosidase activity which was found to be higher with the use of the BMK184 and the mixture of P1201 + BMK184 as starters, than with the use of P1201 as a starter. The increased β-glucosidase activity of soy milk was increased by a fermentation using Lactobacillus acidophilus [9]. Moreover, the use of Bifidobacterium lactis and Lacticaseibacillus casei as starters affected the activity of β-glucosidase in a manner that also varied according to the fermentation time and the growth of the microorganisms [30]. In general, the β-glucosidase produced by LAB hydrolyzes the β-1-6 glucoside bond of the substrates to the biofunctional isoflavone aglycone [31]. These findings lead us to conclude that the mixture of starters has efficiently worked for the bioconversion of the IESLs and is suitable for the enhancement of the GABA and isoflavone aglycone production.
GABA is converted by the action of the enzyme GADase on GA. In the results of lactic acid fermentation with milk, mulberry leaf powder, soybean powder, etc., GABA increased as GA decreased [9,32,33,34]. Likewise, this study also showed that GA decreased as GABA increased. In the study conducted by Park and Oh [35], Levilactobacillus brevis bacteria exhibited a better GABA production ability than Lactiplantibacillus plantarum. In this study, the Levilactobacillus brevis BMK184 produced GABA better than the Lactiplantibacillus plantarum P1201 and showed similar results. Streeter and Thompson [27] have reported that the GADase activity can increase under slightly acidic (pH 5 to 6) conditions, thereby resulting in a high GABA production, while Kook and Cho [36] have reported that the GADase activity increases as the pH decreases. Oh and Cha [37] have also reported that GADase activity increased with acidic conditions. However, in the case of P1201 fermentation, acid concentrations increase as the fermentation proceeds, but no significant differences were identified by previous studies. On the other hand, in the fermented products of BMK184 and of P1201 + BMK184, the GABA content increased as the acidity increased during the fermentation period. Finally, the fermented product using P1201 + BMK184 as a starter exhibited a slightly higher GABA content than that generated by the use of P1201, at 72 h of fermentation. It is believed that this result was affected by the acid produced by P1201. Therefore, the LAB that produce a large amount of lactic acid increase the activity of GADase, so one cannot consider that their ability to produce GABA is excellent. It is believed that the GABA-producing ability can be increased when the excellent GABA production of the BMK184 starter and the acid production of the P1201 starter are combined and used for fermentation.
Han and Chung [38] have previously reported that the TP and TF contents of the fermented mulberry leaves after using the Leuconostoc mesenteroides, Lactiplantibacillus plantarum, and Latilactobacillus sakei strains, increased compared to before fermentation. Studies on the lactic acid fermentation of medicinal leafy plants such as Houttuynia cordata Thunb [39] and Gynura procumbens [40] have also reported that their TP and TP contents increased after fermentation as compared to those detected before the undertaking of the fermentation. Phenolic and flavonoid compounds are present in natural plants and are known to be involved in various antioxidant activities in the body when ingested [8,9,21,41]. In this study, the TP and FP contents were increased as a result of the fermentation delivered by LAB. This result is due to the fact that the plant cell walls are destroyed by enzymes such as β-glucosidase, decarboxylase, esterase, and relevant hydrolase enzymes produced by LAB. Accordingly, the glycosides form phenol and flavonoid compounds that are bound to the plant cell walls and are hydrolyzed and converted into active aglycone forms [42,43,44].
The isoflavone glycoside derivatives are converted into isoflavone aglycone derivatives by the microbial acids or β-glucosidase. They are known to increase the aglycone contents, thereby decreasing glycoside contents during the fermentation of IESLs [45,46]. Fermentation of soybean foods by using Lactiplantibacillus plantarum, Bifidobacterium longum, and Levilactobacillus brevis, corresponded to a decrease in the values of glycosides as the fermentation progressed [7,9,16,20]. Similarly to previous reports, this study also showed that the aglycone contents increased as the glycoside contents decreased during the fermentation process. Therefore, the metabolites (lactic acid) and the β-glucosidase produced during the fermentation of the lactic acid bacteria act in order to separate the sugars from the glycosides; consequently, the progress of the IESLs’ fermentation leads to an increment of aglycone compounds [7,16,45,46,47].
Hwang et al. [9] have reported that the antioxidant abilities against radicals were elevated as follows: ABTS > DPPH > hydroxyl (from 63.5% to 86.5%, from 50.2% to 70.3%, and from 39.3% to 55.2%, respectively) during the fermentation of soy milk by a two-LAB mixture (such as Levilactobacillus brevis and Lactiplantibacillus plantarum), which is consistent with the IESLs’ fermented products in the present study. Kim et al. [48] have reported that phenolic compounds such as flavonoids correlate with radical scavenging activity. The increased antioxidant activity during fermentation was due to an increment of the phenol and flavonoid compounds’ hydrolysis by microorganisms [43,49]. Aglycone was found to be superior to the glycoside form when measuring the antioxidant activity [50], and the antioxidant activity increased in accordance with the TP and isoflavone aglycones’ levels [7,16]. Jin et al. [51] have reported that the DPPH scavenging activity of the fermented green tea by Levilactobacillus brevis was associated with the detected GABA content. Therefore, the increase in the antioxidant activity during the fermentation of IESLs was judged to be affected by the contents of GABA, TP, TF, and isoflavone aglycones. Even with the same soybean leaf substrate, it is believed that the antioxidant activity was also different due to a difference in the metabolites produced by the enzyme activity of each LAB, which is in agreements with the findings of Hur et al. [43].
Alpha-glucosidase is a crucial enzyme that acts at the final stage of carbohydrate digestion in the small intestine’s mucous membrane and hydrolyzes the carbohydrates into monosaccharides [52]. The inhibitors inhibit this action, thereby delaying the digestion and absorption of glucose (an essential factor in managing type-2 diabetes) and lowering the blood sugar levels after a meal [53]. Pancreatic lipase is a fat digestive enzyme that helps the absorption of dietary triglycerides. Pancreatic lipase inhibitors help prevent obesity and adult diseases by inhibiting the absorption of lipids in the body [54]. Stern et al. [55] have reported that the hydroxyl group of polyphenols plays a vital role in enzyme inhibition. Tan et al. [56] have reported that soybeans’ TP and TF contents affect α-glucosidase and pancreatic lipase. The soy milk-fermented product using Lactiplantibacillus plantarum had a higher inhibitory effect on α-glucosidase and pancreatic lipase than before the fermentation, in a manner similar to the present results regarding the TP, TF, and isoflavone aglycone contents [7]. Moreover, the digestive enzyme inhibition, such as that of α-glucosidase and pancreatic lipase, has been shown to display high activities (from 50.6% to 67.2% and from 10.6% to 51.4%, respectively) during the fermentation of soy milk to soy-yogurt with the use of a mixture of starters (including Levilactobacillus brevis and Lactiplantibacillus plantarum) [9]. Therefore, the observed increased TP, TF, and isoflavone aglycone contents of the fermented IESLs’ products with the use of a mixture of starters (including Levilactobacillus brevis and Lactiplantibacillus plantarum) was attributed to an increment in the α-glucosidase and pancreatic lipase inhibitory activities of the fermented IESLs.
5. Conclusions
This study ensured that the levels of GABA and phytoestrogen, the antioxidant activities, and the digestive enzyme inhibition activities of fermented IESLs’ extracts were increased during fermentation with Lactiplantibacillus plantarum P1201 and/or Levilactobacillus brevis BMK184 starters. In particular, the contents of GABA, TP, TF, and isoflavone aglycones (such as daidzein and genistein) were the highest and corresponded to excellent antioxidants (as evidenced through the assessment of DPPH, ABTS, hydroxyl, and FRAP) and digestive enzyme (e.g., α-glucosidase and pancreatic lipase) activity inhibition effects, in the IESLs fermented with a mixture of the assessed starters (Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184). Therefore, the fermented IESLs with the mixture of strains P1201 and BMK184 can be used as a base material in order to enhance the complex functionalities of GABA, total phenolic, and isoflavone aglycone contents, which can be used as a natural component of functional foods.
Author Contributions
H.Y.L.: Investigation, Methodology, Software, Validation, Visualization, Writing—original draft. D.Y.C.: Data curation, Formal analysis, Methodology, Validation, Visualization. K.J.J.: Investigation, Methodology, Software, Validation, Visualization. J.H.L.: Methodology, Software, Validation, Visualization, Writing—review and editing. J.G.J.: Investigation, Methodology, Software, Validation, Visualization. M.J.K.: Investigation, Methodology, Validation, Visualization. J.B.J.: Investigation, Methodology, Validation, Visualization. M.A.H.: Conceptualization, Data curation, Validation, Writing—review and editing. K.M.C.: Conceptualization, Investigation, Data curation, Funding acquisition, Project administration, Supervision, Validation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the ‘2021 Post-Doc. fellowship Program’ of Gyeongsang National University and by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (Grant number 2016R1D1A1B01009898), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data reported in this study is contained within the article. The underlying raw data is available on request from the corresponding author.
Conflicts of Interest
The authors declare that there are no conflict of interests regarding the publication of this paper.
References
- Wang, H.J.; Muphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Baiseitova, A.; Lee, K.W.; Kim, K.D.; Park, K.H. Comparative investigation on metabolites changes in soybean leaves by ethylene and activation of collagen synthesis. Ind. Crops Prod. 2020, 154, 112743. [Google Scholar] [CrossRef]
- Yuk, H.J.; Song, Y.H.; Curtis-Long, M.J.; Kim, D.W.; Woo, S.G.; Lee, Y.B.; Uddin, Z.; Kim, C.Y.; Park, K.H. Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in soybean (Glycine max) leaves. J. Agric. Food Chem. 2016, 64, 7315–7324. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kwun, S.Y.; Park, E.H.; Kim, M.D. Changes in isoflavone contents and physicochemical properties of soybean leaf extract by extraction conditions. Microbiol. Biotechnol. Lett. 2019, 47, 64–68. [Google Scholar] [CrossRef]
- Kwun, S.Y.; Yoon, J.A.; Park, E.H.; Kim, M.D. Improving the aglycon isoflavone content in soybean leaf extracts by lactic acid fermentation. J. Agri. Life Environ. Sci. 2019, 31, 160–170. [Google Scholar]
- Lee, J.H.; Hwang, C.E.; Cho, E.J.; Song, Y.H.; Kim, S.C.; Cho, K.M. Improvement of nutritional components and in vitro antioxidative properties of soy-powder yogurts using Lactobacillus plantarum. J. Food Drug Anal. 2018, 26, 1054–1065. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Hwang, C.E.; Haque, M.A.; Kim, S.C.; Lee, C.S.; Kang, S.S.; Cho, K.M.; Lee, D.H. Changes in conjugated linoleic acid and isoflavone contents from fermented soymilks using Lactobacillus plantarum P1201 and screening for their digestive enzyme inhibition and antioxidant properties. J. Funct. Foods 2018, 43, 17–28. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.E.; Kim, S.C.; Kim, D.H.; Lee, H.Y.; Shu, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Jun, Y.S.; Jang, D.; Cho, C.H.; Lee, S.-H.; Han, N.S.; Kim, D.-O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.S.; Qi, W.T.; Guo, W.; Wang, C.L.; Hu, Z.B.; Li, A.K. Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr. Res. 2018, 62, 1384. [Google Scholar] [CrossRef]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T.; et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Yu, M.; Wang, X.; Deng, M.; Zhai, X.; Li, R.; et al. Dietary genistein could modulate hypothalamic circadian entrainment, reduce body weight, and improve glucose and lipid metabolism in female mice. Int. J. Endocrinol. 2019, 2019, 2163838. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Liang, Z.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res. Int. 2022, 152, 110868. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, S.B. High production of GABA in Pyrus usuriensis Maximowicz fruit extract by mixed fermentation of lactic acid bacteria. Koran J. Food Preserv. 2019, 26, 642–649. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Lee, J.H.; Hong, S.Y.; Cho, K.M. Enhanced biological effect of fermented soy-powder milk with Lactobacillus brevis increasing in γ-aminobutyric acid and isoflavone aglycone contents. J. Appl. Biol. Chem. 2018, 61, 245–255. [Google Scholar] [CrossRef]
- Nikmaram, N.; Dar, B.N.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Greiner, R.; Johnson, S.K. Recent advances in γ-aminobutyric acid (GABA) properties in pulses: An overview. J. Sci. Food Agric. 2017, 97, 2681–2689. [Google Scholar] [CrossRef]
- Smart, T.G.; Stephenson, F.A. A half century of γ-aminobutyric acid. Brain Neurosci. Adv. 2019, 3, 2398212819858249. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villegas, J.M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef]
- Hwang, C.E.; Haque, M.A.; Lee, J.H.; Joo, O.S.; Kim, S.C.; Lee, H.Y.; Um, B.S.; Park, K.S.; Cho, K.M. Comparison of γ-aminobutyric acid and isoflavone aglycone contents, to radical scavenging activities of high-protein soybean sprouting by lactic acid fermentation with Lactobacillus brevis. Korean J. Food Preserv. 2018, 25, 7–18. [Google Scholar] [CrossRef]
- Hwang, C.E.; An, M.J.; Lee, H.Y.; Lee, B.W.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Seo, W.T.; Cho, K.M. Potential probiotic Lactobacillus plantarum P1201 to produce soy-yogurt with enhanced antioxidant activity. Korean J. Food Sci. Technol. 2014, 46, 556–565. [Google Scholar] [CrossRef]
- Haque, M.A.; Ashik, M.A.; Aktar, S.; Akter, M.S.; Halilu, A.; Haque, M.A.; Islam, M.R.; Abdullah-Al-Mamun, M.; Nahar, M.N.; Das, S.R.; et al. Rapid deconstruction of cotton, coir, areca, and banana fibers recalcitrant structure using a bacterial consortium with enhanced saccharification. Waste Biomass Valorization 2021, 12, 4001–4018. [Google Scholar] [CrossRef]
- Das, S.R.; Haque, M.A.; Akbor, M.A.; Abdullah-Al-Mamun, M.; Debnath, G.C.; Hossain, M.S.; Hasan, Z.; Rahman, A.; Islam, M.A.; Hossain, M.A.; et al. Organophosphorus insecticides mineralizing endophytic and rhizospheric soil bacterial consortium influence eggplant growth promotion. Arch. Microbiol. 2022, 204, 199. [Google Scholar] [CrossRef]
- Ham, S.; Bhatia, S.K.; Gurav, R.; Choi, Y.-K.; Jeon, J.-M.; Yoon, J.-J.; Choi, K.-Y.; Ahn, j.; Kim, H.T.; Yang, Y.-H. Gamma aminobutyric acid (GABA) production in Escherichia coli with pyridoxal kinase (pdxY) based regeneration system. Enzym. Microb. Technol. 2022, 155, 10994. [Google Scholar] [CrossRef]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A brief review on the non-protein amino acid, gamma-amino butyric acid (GABA): Its production and role in microbes. Curr. Microbiol. 2019, 77, 534–544. [Google Scholar] [CrossRef]
- Diana, M.; Tres, A.; Quílez, J.; Llombart, M.; Rfecas, M. Spanish cheese screening and selection of lactic acid bacteria with high gamma-aminobutyric acid production. LWT 2014, 56, 351–355. [Google Scholar] [CrossRef]
- Streeter, J.G.; Thompson, J.F. In vivo and in vitro studies on γ-aminobutyric acid metabolism with the radish plant (Raphanus sativus, L.). Plant Physiol. 1972, 49, 579–584. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Gao, D.; Cao, Y.; Xu, H. A high γ-aminobutyric acid producing Lactobacillus brevis isolated from Chinese traditional paocai. Ann. Microbiol. 2008, 58, 649–653. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lu, F.X.; Lu, Z.X.; Bie, X.M.; Jiao, Y.; Sun, L.J.; Yu, B. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp thermophilus Y2 under submerged fermentation. Amino Acids 2008, 34, 473–478. [Google Scholar] [CrossRef]
- Donkor, O.N.; Shah, N.P. Production of β-glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soymilk. J. Food Sci. 2008, 73, M15–M20. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.E. Stability of β-glucosidase activity produced by Bifidobacterium and Lactobacillus spp. in fermented soymilk during processing and storage. J. Food Sci. 2005, 70, M236–M241. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, S.; Chen, F.; He, M.; Lin, J. Isolation of high γ-aminobutyric acid-producing lactic acid bacteria and fermentation in mulberry leaf powders. Exp. Ther. Med. 2019, 18, 147–153. [Google Scholar] [CrossRef]
- Han, M.; Liao, W.; Wu, S.; Gong, X.; Bai, C. Use of Streptococcus thermophilus for the in situ production of γ-aminobutyric acid-enriched fermented milk. J. Dairy Sci. 2020, 103, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.F.; Vivier, R.D.; Johanningsmeier, S.D. Formation of γ-aminobutyric acid (GABA) during natural lactic acid fermentation of cucumber. J. Food Comp. Anal. 2021, 96, 103711. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H. Production and characterization of GABA rice yogurt. Korean J. Food Sci. Technol. 2005, 14, 518–522. [Google Scholar]
- Kook, M.C.; Cho, S.C. Production of GABA (gamma amino butyric acid) by lactic acid bacteria. Korean J. Food Sci. Anim. Resour. 2013, 33, 377–389. [Google Scholar] [CrossRef]
- Oh, S.H.; Cha, Y.S. Regulation of γ-aminobutyric acid production in tobacco plants by expressing a mutant calmodulin gene. Agric. Chem. Biotechnol. 2000, 42, 69–73. [Google Scholar]
- Han, K.H.; Chung, H.Y. Quality characteristics of the fermented mulberry leaves. Food Eng. Prog. 2019, 23, 251–257. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, H.j.; Chung, H.S.; Seong, J.H.; Kim, H.S.; Kim, D.S.; Lee, Y.G. Anti-oxidative activity of the extracts from Houttuynia cordata Thunb. fermented by lactic acid bacteria. J. Life. Sci. 2016, 26, 468–474. [Google Scholar] [CrossRef]
- Bae, D.B.; Kim, K.H.; Yook, H.S. Antioxidant and tyrosinase inhibitory activities of fermented Gynura procumbens. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1214–1222. [Google Scholar] [CrossRef]
- Cho, K.M.; Cho, H.K.; Lee, J.Y.; Seo, W.T.; Kim, M.K. Quality characteristics and antioxidant effects during makgeolli fermentation by purple sweet potato-rice nuruk. Korean J. Food Sci. Technol. 2012, 44, 728–735. [Google Scholar] [CrossRef]
- Chien, H.L.; Huang, H.Y.; Chou, C.C. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Park, G.; Park, J.Y.; Chang, Y.H. Changes in flavonoid aglycone contents and antioxidant activities of citrus peel depending on enzyme treatment times. J. Korean Soc. Food Sci. Nutr. 2019, 48, 542–550. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Cho, E.K.; Cho, H.Y.; Kim, B.C.; Shin, H.H.; Cho, S.C.; Kook, M.C.; Pyun, Y.R. Development of Pretreatment and Mixed Culture Processes for Plant Originated Lactic Acid to Produce a Functional. Korean J. Food Nutr. 2011, 24, 117–123. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Hwang, C.E.; Lee, C.K.; Lee, J.H.; Kim, G.M.; Jeong, S.H.; Cho, K.M. Characteristics and antioxidant effect of garlic in the fermentation of Cheonggukjang by Bacillus amyloliquefaciens MJ1-4. J. Microbiol. Biotechnol. 2014, 24, 959–968. [Google Scholar] [CrossRef]
- Kim, G.D.; Lee, Y.S.; Cho, J.Y.; Lee, Y.H.; Choi, K.J.; Lee, Y.; Han, T.H.; Lee, S.H.; Park, K.H.; Moon, J.H. Comparison of the content of bioactive substances and the inhibitory effects against rat plasma oxidation of conventional and organic hot peppers (Capsicum annuum L.). J. Agric. Food Chem. 2010, 58, 12300–12306. [Google Scholar] [CrossRef]
- Myo, H.; Nantarat, N.; Khat-Udomkiri, N. Changes in bioactive compounds of coffee pulp though fermentation-based biotransformation using Lactobacillus plantarum TISTR 543 and its antioxidant activities. Fermentation 2021, 7, 292. [Google Scholar] [CrossRef]
- Jeong, E.J.; Kim, J.Y.; Moon, S.H.; Park, G.Y. Characteristics, antioxidative activities and growth inhibitory effects in AGS human gastric adenocarcinoma cells of soymilk fermented by Bacillus subtilis KC-3 during fermentation. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1113–1118. [Google Scholar]
- Jin, Y.H.; Hong, J.H.; Lee, J.-H.; Yoon, H.; Pawluk, A.M.; Yun, S.J.; Mah, J.-H. Lactic acid fermented green tea with Levilactobacillus brevis capable of producing γ-aminobutyric acid. Fermentation 2021, 7, 110. [Google Scholar] [CrossRef]
- Chang, Y.; Fan, W.; Shi, H.; Feng, X.; Zhang, D.; Wang, L.; Zheng, Y.; Guo, L. Characterization of phenolics and discovery of α-glucosidase inhibitors in Artemisia argyi leaves based on ultra-performance liquid chromatography-tandem mass spectrometry and relevance analysis. J. Pharm. Biomed. Anal. 2022, 2020, 114982. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Mustar, S.; Khalid, N.M.; Rashed, A.A.; Noh, M.F.M.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against α-glucosidase and pancreatic lipase. LWT 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Mason, P.K. Phlorotannins-protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).