β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plasmid Construction
2.2. Fermentation Conditions
2.3. Pretreatment and Enzymatic Hydrolysis
2.4. Fed-Batch Fermentation
2.5. Analytical Methods
2.6. Statistics
3. Results and Discussion
3.1. Construction of β-Farnesene Biosynthesis Pathway in Yarrowia lipolytica
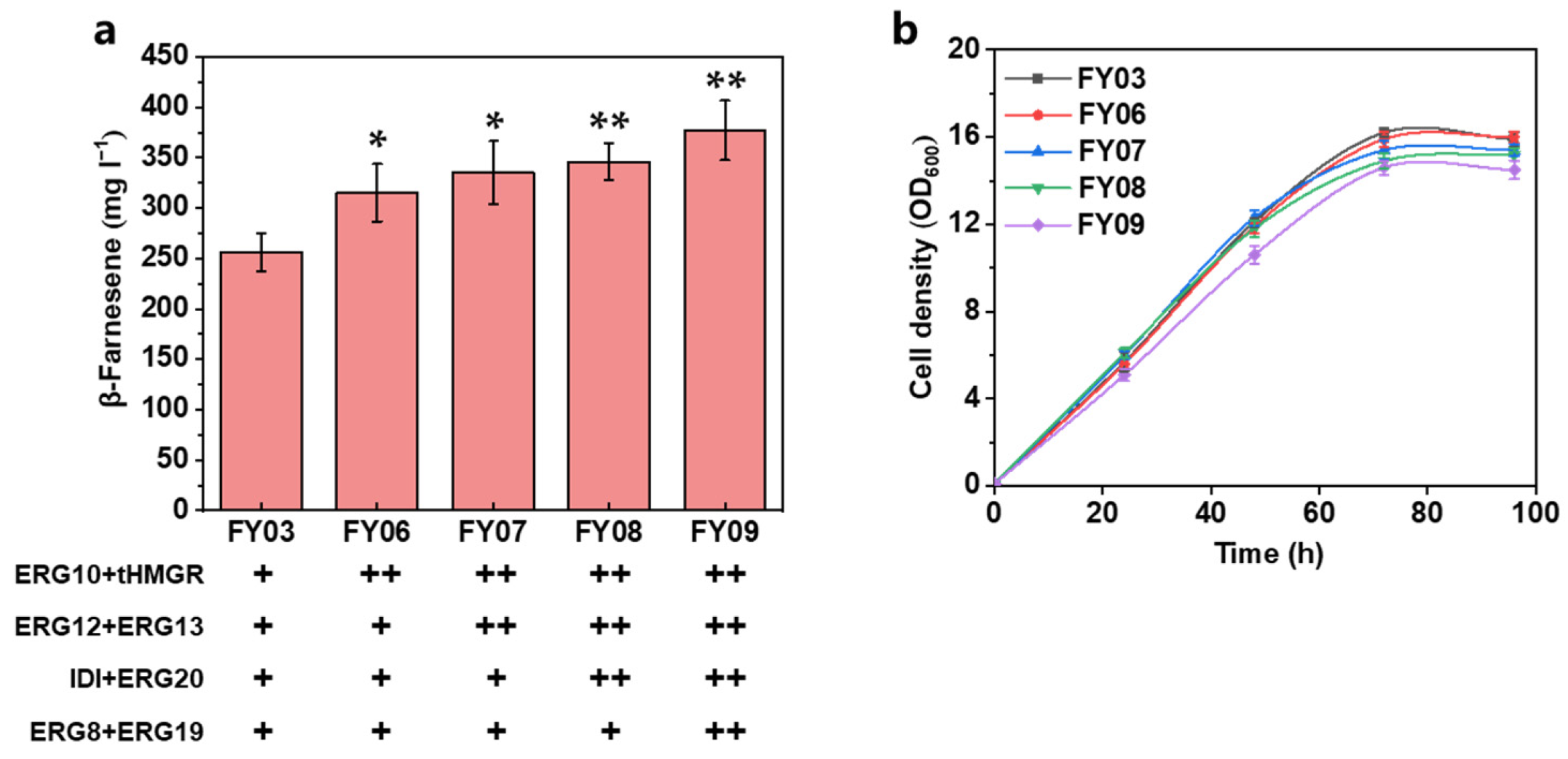
3.2. Further Increasing the Carbon Flux of the MVA Pathway
3.3. Establishing Lignocellulosic Hydrolysate Utilization Strategy in Fermentation Media
3.4. Upscaling Fermentation in 2 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huelin, F.E.; Murray, K.E. α-Farnesene in the natural coating of apples. Nature 1966, 210, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, X.; Pan, G.; Hou, X.; Zhang, H.; Yuan, Y. In vitro characterization of a (E)-beta-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene 2015, 571, 58–64. [Google Scholar] [CrossRef]
- Yu, X.; Jones, H.D.; Ma, Y.; Wang, G.; Xu, Z.; Zhang, B.; Zhang, Y.; Ren, G.; Pickett, J.A.; Xia, L. (E)-beta-Farnesene synthase genes affect aphid (Myzus persicae) infestation in tobacco (Nicotiana tabacum). Funct. Integr. Genom. 2012, 12, 207–213. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Zhan, Y.; Wang, K.; Francis, F.; Liu, Y. New slow release mixture of (E)-β-farnesene with methyl salicylate to enhance aphid biocontrol efficacy in wheat ecosystem. Pest Manag. Sci. 2021, 77, 3341–3348. [Google Scholar] [CrossRef]
- You, S.; Yin, Q.; Zhang, J.; Zhang, C.; Qi, W.; Gao, L.; Tao, Z.; Su, R.; He, Z. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 355–389. [Google Scholar]
- Akutagawa, S.; Taketomi, T.; Kumobayashi, H.; Takayama, K.; Someya, T.; Otsuka, S. Metal-Assisted Terpenoid Synthesis. V. The Catalytic Trimerization of Isoprene to trans-β-Farnesene and Its Synthetic Applications for Terpenoids. Bull. Chem. Soc. Jpn. 1978, 51, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Arkoudis, E.; Stratakis, M. Synthesis of Cordiaquinones B, C, J, and K on the Basis of a Bioinspired Approach and the Revision of the Relative Stereochemistry of Cordiaquinone C. J. Org. Chem. 2008, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Silver, P.A. Synthetic biology expands chemical control of microorganisms. Curr. Opin. Chem. Biol. 2015, 28, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolston, B.M.; Edgar, S.; Stephanopoulos, G. Metabolic Engineering, Past and Future. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 259–288. [Google Scholar] [CrossRef]
- Yao, P.; You, S.; Qi, W.; Su, R.; He, Z. Investigation of fermentation conditions of biodiesel by-products for high production of β-farnesene by an engineered Escherichia coli. Environ. Sci. Pollut. R 2020, 27, 22758–22769. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Chang, H.; Zhang, C.; Gao, L.; Qi, W.; Tao, Z.; Su, R.; He, Z. Recycling strategy and repression elimination for lignocellulosic-based farnesene production with an engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 9858–9867. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlén, M.; Nielsen, J.; Hudson, E.P. Affibody scaffolds improve sesquiterpene production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the oleaginous yeast Yarrowia lipolytica for β-farnesene overproduction. Biotechnol. J. 2021, 16, 2100097. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef]
- Liu, H.-H.; Ji, X.-J.; Huang, H. Biotechnological applications of Yarrowia lipolytica, Past, present and future. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef]
- Gao, S.; Tong, Y.; Zhu, L.; Ge, M.; Zhang, Y.; Chen, D.; Jiang, Y.; Yang, S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017, 41, 192–201. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Wang, D.-P.; Tian, Y.; Fan, X.; Wang, C.; Lu, X.-Y.; Li, P.-W.; Ji, X.-J.; Liu, H.-H. Metabolic engineering of Yarrowia lipolytica for improving squalene production. Bioresour. Technol. 2021, 323, 124652. [Google Scholar] [CrossRef]
- Shaikh, K.M.; Odaneth, A.A. Metabolic engineering of Yarrowia lipolytica for the production of isoprene. Biotechnol. Prog. 2021, 37, e3201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liwei, M.; Park, J.-B.; Jeong, S.-H.; Wei, G.; Wang, Y.; Kim, S.-W. Microbial Platform for Terpenoid Production, Escherichia coli and Yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Guo, Q.; Wang, P.; Qian, H.; Gao, X.; Zhang, Y. Kinetics study of levulinic acid production from corncobs by tin tetrachloride as catalyst. Bioresour. Technol. 2018, 260, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Ma, L.; Dong, S.; Liu, L. Corn stalk as starting material to prepare a novel adsorbent via SET-LRP and its adsorption performance for Pb(II) and Cu(II). R Soc. Open Sci. 2020, 7, 191811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Gao, F.; Yin, D.M.; Luo, Q.; Fu, Z.Q.; Zhou, Y.G. Processing of Superfine Grinding Corn Straw Fiber-Reinforced Starch Film and the Enhancement on Its Mechanical Properties. Polymers 2018, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Fu, S.; Yang, Z.; Lu, J.; Guo, R. Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour. Technol. 2018, 259, 18–23. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, L.; Yin, C.; Lebailly, P.; Azadi, H. Urban residents’ willingness to pay for corn straw burning ban in Henan, China, Application of payment card. J. Clean. Prod. 2018, 193, 471–478. [Google Scholar] [CrossRef]
- Jia, F.; Liu, H.-J.; Zhang, G.-G. Preparation of carboxymethyl cellulose from corncob. Procedia Environ. Sci. 2016, 31, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Kohlhaas, M.; Enoki, J.; Meier, R.; Schönenberger, B.; Wohlgemuth, R.; Kourist, R.; Niemeyer, F.; van Niekerk, D.; Bräsen, C.; et al. A combined experimental and modelling approach for the Weimberg pathway optimization. Nat. Commun. 2020, 11, 1098. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L. Bioconversion of corn stover hydrolysate to ethanol by a recombinant yeast strain. Fuel Process. Technol. 2010, 91, 1807–1811. [Google Scholar] [CrossRef]
- Qureshi, N.; Cotta, M.A.; Saha, B.C. Bioconversion of barley straw and corn stover to butanol (a biofuel) in integrated fermentation and simultaneous product recovery bioreactors. Food Bioprod. Process. 2014, 92, 298–308. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Kenealy, W.R.; Jeffries, T.W. Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J. Ind. Microbiol. Biotechnol. 2011, 38, 1649–1655. [Google Scholar] [CrossRef]
- Cao, G.; Ren, N.; Wang, A.; Lee, D.-J.; Guo, W.; Liu, B.; Feng, Y.; Zhao, Q. Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 2009, 34, 7182–7188. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Holkenbrink, C.; Dam, M.I.; Kildegaard, K.R.; Beder, J.; Dahlin, J.; Doménech Belda, D.; Borodina, I. EasyCloneYALI, CRISPR/Cas9-based synthetic toolbox for engineering of the yeast Yarrowia lipolytica. Biotechnol. J. 2018, 13, 1700543. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, Y.; Xu, G.; Chu, J.; Zhuang, Y.; Zhang, S. Influence of High Solid Concentration on Enzymatic Hydrolysis and Fermentation of Steam-Exploded Corn Stover Biomass. Appl. Biochem. Biotech. 2010, 160, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Frogue, K.; Ramesh, A.; Misa, J.; Wheeldon, I. CRISPRi repression of nonhomologous end-joining for enhanced genome engineering via homologous recombination in Yarrowia lipolytica. Biotechnol. Bioeng. 2017, 114, 2896–2906. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Blazeck, J.; Liu, L.; Redden, H.; Alper, H. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Appl. Environ. Microbiol. 2011, 77, 7905–7914. [Google Scholar] [CrossRef]
- Maruyama, T.; Ito, M.; Honda, G. Molecular Cloning, Functional Expression and Characterization of (E)-β-Farnesene Synthase from Citrus junos. Biol. Pharm. Bull. 2001, 24, 1171–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Ching, C.B. Mitochondrial acetyl-CoA utilization pathway for terpenoid productions. Metab. Eng. 2016, 38, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ye, L.; Lv, X.; Xu, H.; Yu, H. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 8–18. [Google Scholar] [CrossRef]
- Chandran, S.S.; Kealey, J.T.; Reeves, C.D. Microbial production of isoprenoids. Process Biochem. 2011, 46, 1703–1710. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, S.R.; Xu, H.; Zhang, G.-C.; Lane, S.; Kim, H.; Jin, Y.-S. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Xiao, F.; Gao, J.; Ye, C.; Jiang, L.; Dong, C.; Lian, J. Establishing Komagataella phaffii as a Cell Factory for Efficient Production of Sesquiterpenoid α-Santalene. J. Agric. Food Chem. 2022, 70, 8024–8031. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qiao, K.; Stephanopoulos, G. (13)C Metabolic Flux Analysis of acetate conversion to lipids by Yarrowia lipolytica. Metab. Eng. 2016, 38, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Nielsen, J.; Liu, Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017, 17, fox080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellou, S.; Triantaphyllidou, I.-E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- van Rossum, H.M.; Kozak, B.U.; Niemeijer, M.S.; Dykstra, J.C.; Luttik, M.A.; Daran, J.M.G.; Van Maris, A.J.; Pronk, J.T. Requirements for Carnitine Shuttle-Mediated Translocation of Mitochondrial Acetyl Moieties to the Yeast Cytosol. Mbio 2016, 7, e00520-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, G.M.; Hussain, M.S.; Gambill, L.; Gao, D.; Yaguchi, A.; Blenner, M. Engineering xylose utilization in Yarrowia lipolytica by understanding its cryptic xylose pathway. Biotechnol. Biofuels 2016, 9, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-K.; Bordes, F.; Letisse, F.; Nicaud, J.-M. Engineering precursor pools for increasing production of odd-chain fatty acids in Yarrowia lipolytica. Metab. Eng. Commun. 2021, 12, e00158. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, S.; Zhang, Y.; Lu, M.; Sha, Y.; Zhai, R.; Xu, Z.; Jin, M. A novel fermentation strategy for efficient xylose utilization and microbial lipid production in lignocellulosic hydrolysate. Bioresour. Technol. 2022, 361, 127624. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, Y.; Wang, K.; Ledesma-Amaro, R.; Ji, X.-J. Engineering Yarrowia lipolytica to produce fuels and chemicals from xylose: A review. Bioresour. Technol. 2021, 337, 125484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Cui, Z.; Qi, Q.; Hou, J. α-Farnesene production from lipid by engineered Yarrowia lipolytica. Bioresour. Bioprocess 2021, 8, 78. [Google Scholar] [CrossRef]
- Picaud, S.; Brodelius, M.; Brodelius, P. Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry 2005, 66, 961–967. [Google Scholar] [CrossRef] [PubMed]







| Strain | Characteristics | Source |
|---|---|---|
| MYA2613 | matA, Ura3-302, Leu2-270, XPR2-322, Axp2-delta NU49,XPR2::SUC2 | ATCC |
| FY01 | MYA2613 Δku70, Δku80:: Leu2 | Lab storage |
| FY02 | FY01 intA1:: aaBFS | This work |
| FY03 | FY02 intC1:: ERG10, tHMGR, intC2:: ERG12, ERG13, intC3:: IDI, ERG20, intE1:: ERG8, ERG19 | This work |
| FY04 | FY03 ΔaaBFS:: cjBFS | This work |
| FY05 | FY03 ΔaaBFS:: mcBFS | This work |
| FY06 | FY03 intE2:: ERG10, tHMGR | This work |
| FY07 | FY03 intE3:: ERG12, ERG13 | This work |
| FY08 | FY03 intE4:: IDI, ERG20 | This work |
| FY09 | FY03 intF1:: ERG8, ERG19 | This work |
| FY10 | FY09 intF2:: tHMGR, tHMGR | This work |
| FY11 | FY09 intF2:: nadh-HMGR, nadh-HMGR | This work |
| FY12 | FY09 intF2:: nadh-HMGR, tHMGR | This work |
| FY13 | FY12 intF2:: aaBFS | This work |
| FY14 | FY12 intF2:: aaBFS, aaBFS | This work |
| Sample | Glucose (g L−1) | Xylose (g L−1) | Acetic Acid (g L−1) | Furfural (g L−1) | 5-Hydroxymethylfurfurals (g L−1) | Soluble Lignin (g L−1) |
|---|---|---|---|---|---|---|
| Hydrolysate | 40.3 ± 0.4 | 14.7 ± 0.3 | 1.4 ± 0.04 | 1.30 ± 0.07 | 0.70 ± 0.02 | 0.31 ± 0.02 |
| Concentrated hydrolysate | 107.4 ± 0.6 | 39.17 ± 0.5 | 3.73 ± 0.06 | 3.46 ± 0.09 | 1.86 ± 0.03 | 0.83 ± 0.01 |
| Detoxified hydrolysate | 102.3 ± 0.6 | 37.6 ± 0.3 | 3.62 ± 0.05 | 0 | 0 | 0.12 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, H.; Xv, C.; Su, C.; Feng, P.; Zhang, C.; Wang, M.; Fang, Y.; Tan, T. β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation 2022, 8, 532. https://doi.org/10.3390/fermentation8100532
Bi H, Xv C, Su C, Feng P, Zhang C, Wang M, Fang Y, Tan T. β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation. 2022; 8(10):532. https://doi.org/10.3390/fermentation8100532
Chicago/Turabian StyleBi, Haoran, Chenchen Xv, Changsheng Su, Pan Feng, Changwei Zhang, Meng Wang, Yunming Fang, and Tianwei Tan. 2022. "β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica" Fermentation 8, no. 10: 532. https://doi.org/10.3390/fermentation8100532
APA StyleBi, H., Xv, C., Su, C., Feng, P., Zhang, C., Wang, M., Fang, Y., & Tan, T. (2022). β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation, 8(10), 532. https://doi.org/10.3390/fermentation8100532





