Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains
2.2. Cultivation Media
2.3. Experimental Design: Cultivation of High-Lipid Content Microalgae under Nutrient Limitation
2.4. Growth Parameters
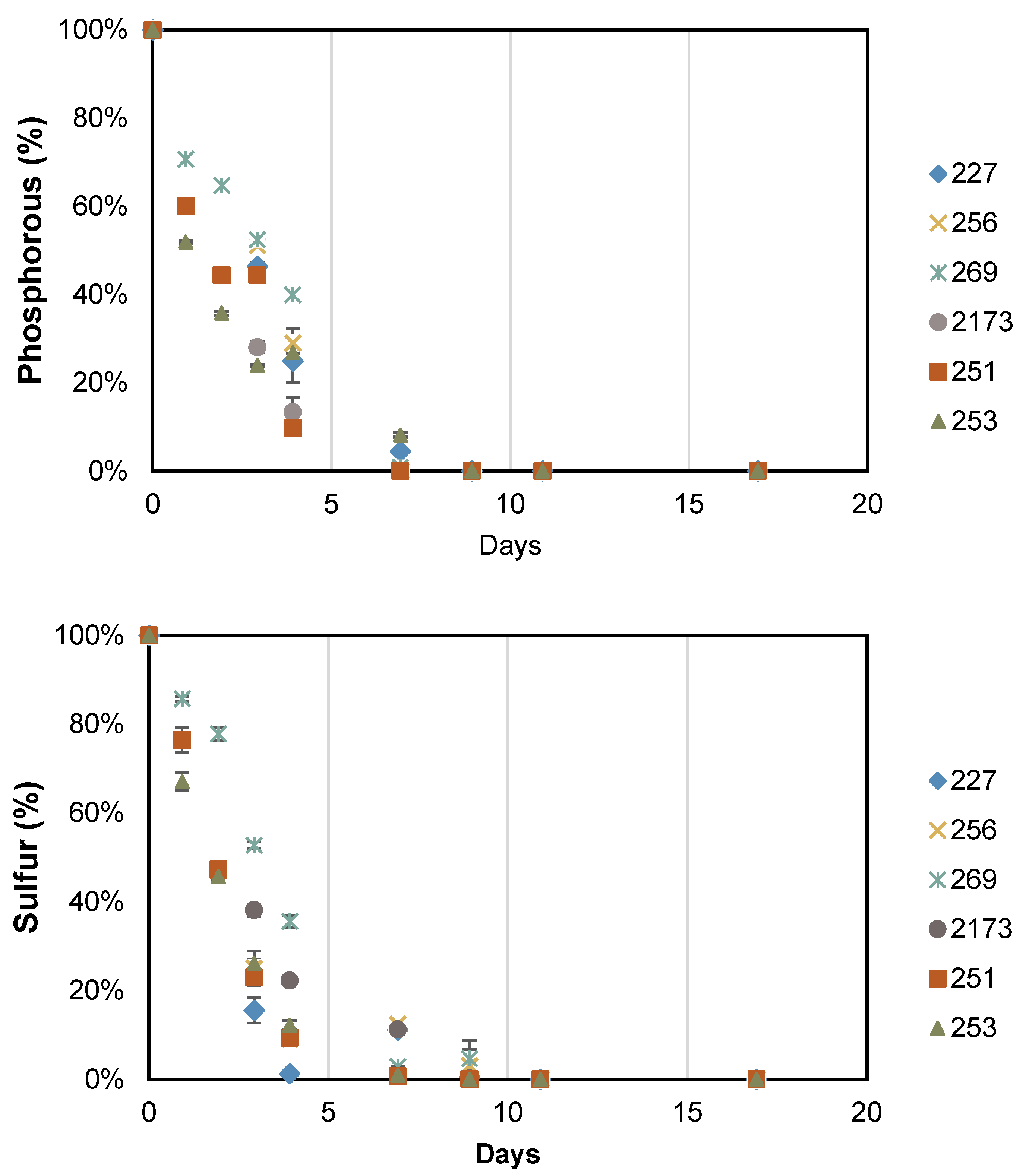
2.5. Intracellular Nutrient Content
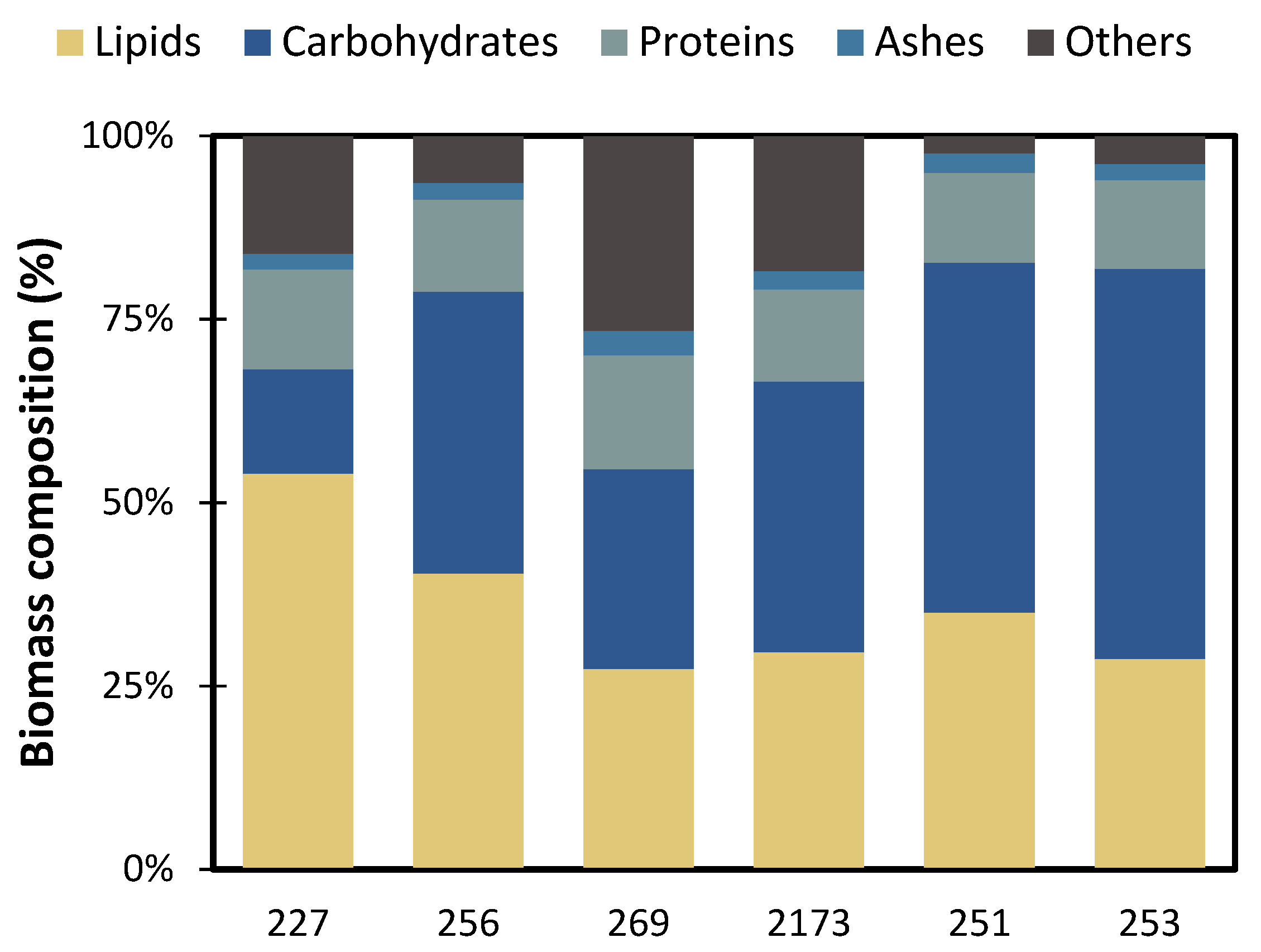
2.6. Biomass Characterization
2.7. Fatty Acid Profile
2.8. Biodiesel Calculations
3. Results and Discussion
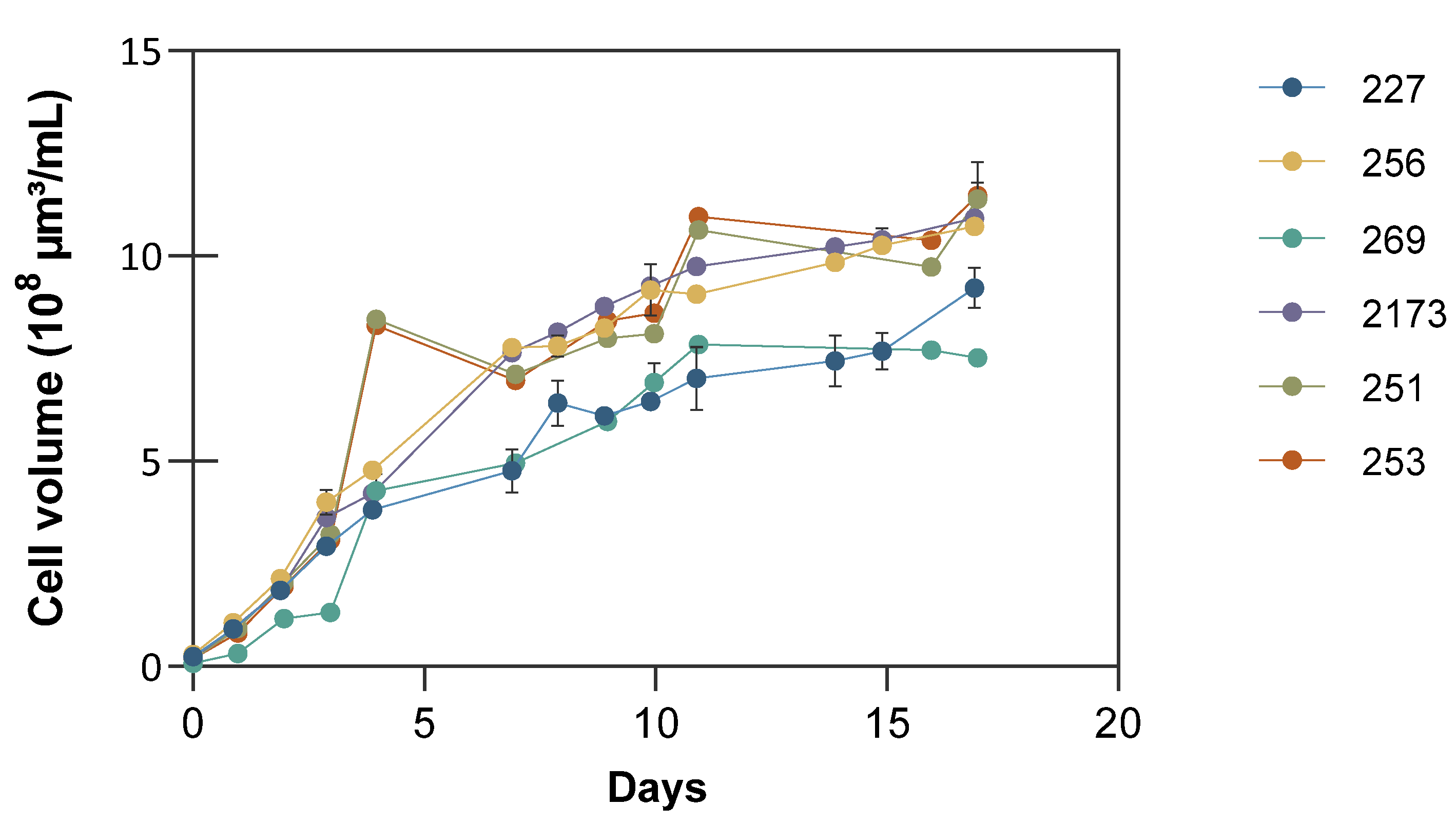
3.1. Growth and Biomass Accumulation under Nutrient Deficient Conditions
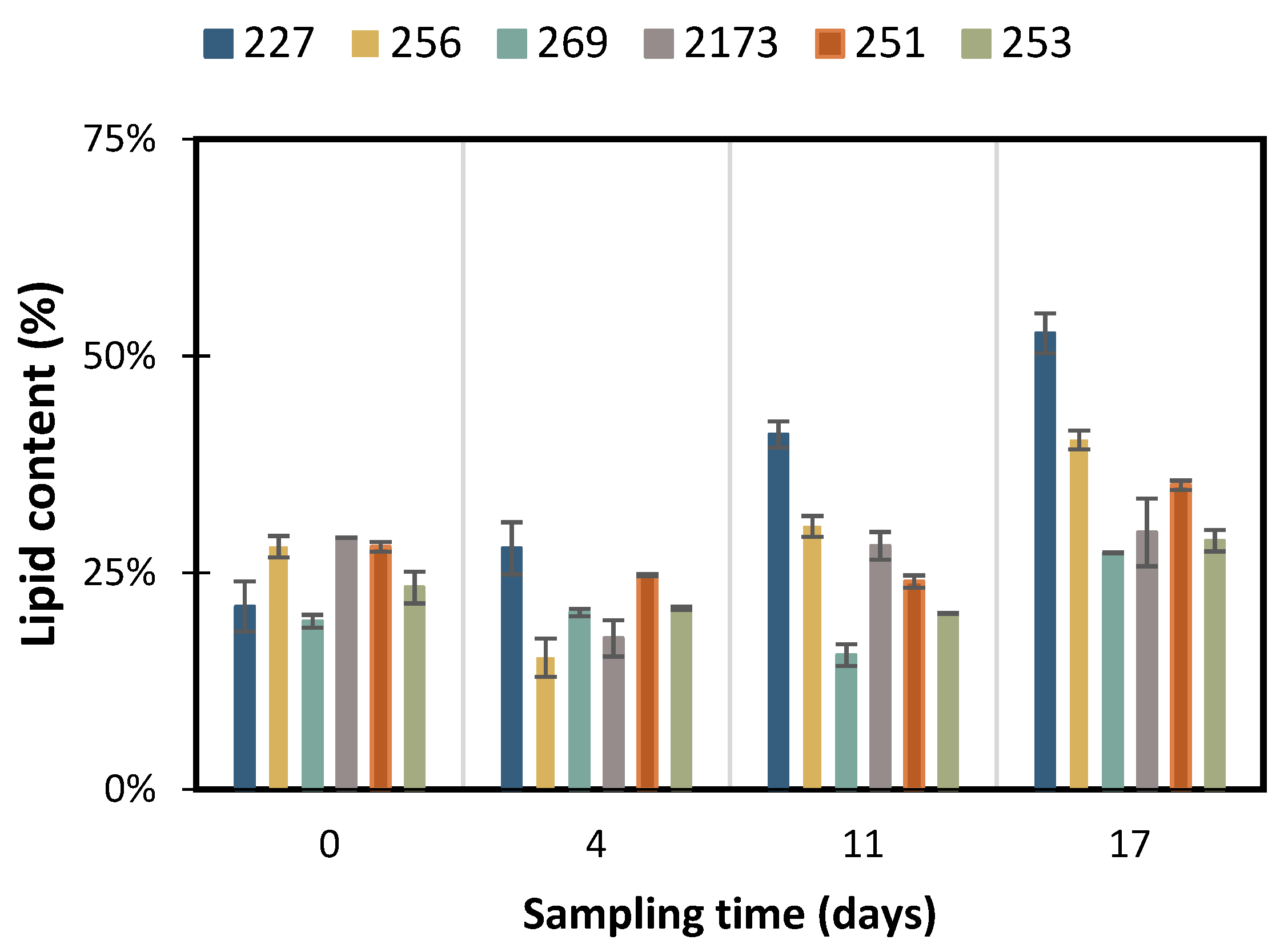
3.2. Accumulation of Storage Compounds under Nutrient Deficient Conditions
3.3. Impact of the Biomass Composition as a Biofuel Feedstock
3.4. Fatty Acid Profile for Biodiesel Applications
3.5. Chlorellaceae as a Source of Biomass for Biofuel Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of Biodiesel from Microalgae through Biological Carbon Capture: A Review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R.; Mallick, N. Microalgal Biodiesel Production: Realizing the Sustainability Index. Front. Bioeng. Biotechnol. 2021, 9, 620777. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater Treatment by Microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-Cycle Analysis on Biodiesel Production from Microalgae: Water Footprint and Nutrients Balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Feng, P.; Feng, J.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. The Roles of Starch and Lipid in Chlorella Sp. during Cell Recovery from Nitrogen Starvation. Bioresour. Technol. 2018, 247, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Lam, M.K.; Uemura, Y.; Mansor, N.; Lim, J.W.; Show, P.L.; Tan, I.S.; Lim, S. High Biodiesel Yield from Wet Microalgae Paste via In-Situ Transesterification: Effect of Reaction Parameters towards the Selectivity of Fatty Acid Esters. Fuel 2020, 272, 117718. [Google Scholar] [CrossRef]
- Ratha, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal Liquefaction of Algal Feedstocks: The Effect of Biomass Characteristics and Extraction Solvents. Renew. Sustain. Energy Rev. 2022, 156, 111973. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for High-Value Compounds and Biofuels Production: A Review with Focus on Cultivation under Stress Conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yusof, N.; Maeda, T.; Mustapha, N.A.; Mohd Yusoff, M.Z.; Raja Khairuddin, R.F. Mechanism of Carbon Partitioning towards Starch and Triacylglycerol in Chlorella Vulgaris under Nitrogen Stress through Whole-Transcriptome Analysis. Biomass Bioenergy 2020, 138, 105600. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel Production from Microalgae: A Review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- López Barreiro, D.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal Liquefaction (HTL) of Microalgae for Biofuel Production: State of the Art Review and Future Prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.; Rosendahl, L. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Silvello, M.A.; Severo Gonçalves, I.; Patrícia Held Azambuja, S.; Silva Costa, S.; Garcia Pereira Silva, P.; Oliveira Santos, L.; Goldbeck, R. Microalgae-Based Carbohydrates: A Green Innovative Source of Bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef]
- Hu, R.; Cao, Y.; Chen, X.; Zhan, J.; Luo, G.; Hao Ngo, H.; Zhang, S. Progress on Microalgae Biomass Production from Wastewater Phycoremediation: Metabolic Mechanism, Response Behavior, Improvement Strategy and Principle. Chem. Eng. J. 2022, 137187, in press. [Google Scholar] [CrossRef]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohr, T.; Wolf, M. Phylogenetic Relationship of Chlorella and Parachlorella Gen. Nov. (Chlorophyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Bioremediation and Biofuel Production from Chlorella Sp.: A Comprehensive Review. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 635–655. ISBN 9789811322648. [Google Scholar]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of Lipids in 10 Strains of Chlorella and Parachlorella, and Enhanced Lipid Productivity in Chlorella Vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. High Productivity Cultivation of a Heat-Resistant Microalga Chlorella Sorokiniana for Biofuel Production. Bioresour. Technol. 2013, 131, 60–67. [Google Scholar] [CrossRef]
- Shen, X.-F.; Chu, F.-F.; Lam, P.K.S.; Zeng, R.J. Biosynthesis of High Yield Fatty Acids from Chlorella Vulgaris NIES-227 under Nitrogen Starvation Stress during Heterotrophic Cultivation. Water Res. 2015, 81, 294–300. [Google Scholar] [CrossRef]
- Hutner, S.H.; Provasoli, L.; Schatz, A.; Haskins, C.P. Some Approaches to the Study of the Role of Metals in the Metabolism of Microorganisms. Proc. Am. Philos. Soc. 1950, 94, 152–170. [Google Scholar]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-Protein Conversion Factors Revisited for Applications of Microalgal Biomass Conversion to Food, Feed and Fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R.; Martinez-Goss, M.R. Lipid Accumulation and Profiling in Microalgae Chlorolobion Sp. (BIOTECH 4031) and Chlorella Sp. (BIOTECH 4026) during Nitrogen Starvation for Biodiesel Production. J. Appl. Phycol. 2021, 33, 1–11. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel Highly Efficient Starch Producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Kinetics of Growth and Lipids Accumulation in Chlorella Vulgaris during Batch Heterotrophic Cultivation: Effect of Different Nutrient Limitation Strategies. Bioresour. Technol. 2017, 243, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and Cyanobacterial Cultivation: The Supply of Nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef]
- Di Caprio, F. A Fattening Factor to Quantify the Accumulation Ability of Microorganisms under N-Starvation. New Biotechnol. 2021, 66, 70–78. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of Nutrient Supply Status on Biomass Composition of Eukaryotic Green Microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Li, T.; Gargouri, M.; Feng, J.; Park, J.-J.; Gao, D.; Miao, C.; Dong, T.; Gang, D.R.; Chen, S. Regulation of Starch and Lipid Accumulation in a Microalga Chlorella Sorokiniana. Bioresour. Technol. 2015, 180, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Dao, L.H.T.; Muylaert, K.; Beardall, J. Influence of Different Degrees of N Limitation on Photosystem II Performance and Heterogeneity of Chlorella Vulgaris. Algal Res. 2017, 26, 84–92. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, Morphological and Thermal Analysis of Mixed Microalgae Species from Drain Water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Yin, X.; Wu, C. Insights into the Evolution of Fuel-N to NOx Precursors during Pyrolysis of N-Rich Nonlignocellulosic Biomass. Appl. Energy 2018, 219, 20–33. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Algal Nutrition—Mineral Nutrition. In Handbook of Microalgal Culture; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 95–115. ISBN 978-0-470-99528-0. [Google Scholar]
- Lababpour, A. Continuous Hydrothermal Liquefaction for Biofuel and Biocrude Production from Microalgal Feedstock. ChemBioEng. Rev. 2018, 5, 90–103. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xie, J.; Liu, H.; Yin, X.; Wu, C. Bio-Oil Production from Hydrothermal Liquefaction of High-Protein High-Ash Microalgae Including Wild Cyanobacteria Sp. and Cultivated bacillariophyta Sp. Fuel 2016, 183, 9–19. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Soderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal Treatment of High Ash Microalgae: Focusing on the Physicochemical and Combustion Properties of Hydrochars. Energy Fuels 2020, 34, 1929–1939. [Google Scholar] [CrossRef]
- EN 14214:2008; Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirement Methods. European Committee for Standardization: Brussels, Belgium, 2008.
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal Fatty Acid Composition: Implications for Biodiesel Quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Species and Strain Selection. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Developments in Applied Phycology; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 77–89. ISBN 978-94-007-5479-9. [Google Scholar]
- Obeid, F.; Chu Van, T.; Brown, R.; Rainey, T. Nitrogen and Sulphur in Algal Biocrude: A Review of the HTL Process, Upgrading, Engine Performance and Emissions. Energy Convers. Manag. 2019, 181, 105–119. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, Z.; Chen, L.; Jarvis, J.; Paz, N.; Schaub, T.; Nirmalakhandan, N.; Brewer, C.E. Hydrothermal Liquefaction of High- and Low-Lipid Algae: Bio-Crude Oil Chemistry. Appl. Energy 2017, 206, 278–292. [Google Scholar] [CrossRef]


| Microalgae Strain | Growth Rate (µ/d) | Biomass | Lipids | Carbohydrates | ||||
|---|---|---|---|---|---|---|---|---|
| (g/L) | Overall P (g/L/d) | Max P (g/L/d) | (%) | Max P (mg/L/d) | (%) | Max P (mg/L/d) | ||
| C. vulgaris NIES 227 | 1.67 ± 0.1 | 5.31 ± 0.1 | 0.31 ± 0.00 | 0.48 ± 0.01 | 54 ± 2% | 212 ± 3 | 14 ± 1% | 92 ± 04 |
| C. vulgaris CCALA 256 | 1.55 ± 0.2 | 5.62 ± 0.1 | 0.32 ± 0.01 | 0.58 ± 0.08 | 40 ± 1% | 156 ± 4 | 38 ± 5% | 220 ± 24 |
| C. vulgaris CCALA 269 | 1.20 ± 0.1 | 3.91 ± 0.0 | 0.23 ± 0.00 | 0.58 ± 0.03 | 27 ± 1% | 91 ± 3 | 27 ± 3% | 131 ± 16 |
| C. sorokiniana NIES 2173 | 0.90 ± 0.1 | 5.63 ± 0.1 | 0.33 ± 0.01 | 0.70 ± 0.01 | 30 ± 4% | 149 ± 1 | 37 ± 6% | 244 ± 33 |
| P. kessleri CCALA 251 | 1.60 ± 0.1 | 5.00 ± 0.0 | 0.29 ± 0.00 | 0.50 ± 0.00 | 35 ± 1% | 124 ± 3 | 48 ± 1% | 236 ± 19 |
| P. kessleri CCALA 253 | 1.25 ± 0.1 | 5.16 ± 0.1 | 0.30 ± 0.00 | 0.47 ± 0.02 | 29 ± 1% | 108 ± 2 | 53 ± 1% | 199 ± 30 |
| Intracellular N Quota | |||||||
|---|---|---|---|---|---|---|---|
| Day | NIES 227 | CCALA 256 | CCALA 269 | NIES 2173 | CCALA 251 | CCALA 253 | |
| (A) pg of N per cell | 4 | 1.29 ± 0.05 | 1.62 ± 0.03 | 1.88 ± 0.22 | 2.01 ± 0.18 | 1.41 ± 0.03 | 1.15 ± 0.01 |
| 17 | 0.28 ± 0.02 | 0.33 ± 0.01 | 0.62 ± 0.08 | 0.51 ± 0.01 | 0.45 ± 0.01 | 0.52 ± 0.01 | |
| (B) mg of N per g of biomass | 4 | 85.4 ± 1.9 | 88.1 ± 3.7 | 183.8 ± 8.9 | 89.9 ± 2.8 | 98.6 ± 2.9 | 98.7 ± 1.7 |
| 17 | 26.0 ± 0.3 | 24.9 ± 0.6 | 40.0 ± 0.6 | 24.7 ± 0.4 | 29.2 ± 0.1 | 29.1 ± 0.3 | |
| Microalgae Strain | C (wt. %) | H (wt. %) | O (wt. %) | N (wt. %) | S (wt. %) | HHV (MJ/kg) |
|---|---|---|---|---|---|---|
| C. vulgaris NIES 227 | 62.2 ± 0.1 | 9.3 ± 0.1 | 27.0 ± 2.2 | 2.6 ± 0.0 | 0.101 ± 0.01 | 29.8 |
| C. vulgaris CCALA 256 | 56.3 ± 2.6 | 8.2 ± 0.3 | 35.1 ± 0.6 | 2.4 ± 0.1 | 0.097 ± 0.03 | 25.7 |
| C. vulgaris CCALA 269 | 55.7 ± 2.1 | 8.1 ± 0.5 | 33.4 ± 0.3 | 3.0 ± 0.1 | 0.099 ± 0.01 | 25.4 |
| C. sorokiniana NIES 2173 | 53.5 ± 0.3 | 7.9 ± 0.1 | 40.6 ± 0.8 | 2.4 ± 0.0 | 0.102 ± 0.01 | 23.7 |
| P. kessleri CCALA 251 | 54.7 ± 0.4 | 7.9 ± 0.1 | 35.3 ± 1.2 | 2.4 ± 0.1 | 0.104 ± 0.01 | 24.7 |
| P. kessleri CCALA 253 | 52.0 ± 0.2 | 7.6 ± 0.1 | 39.3 ± 1.5 | 2.4 ± 0.0 | 0.100 ± 0.01 | 22.9 |
| Microalgae Strain | CN (min) | HHV (MJ/kg) | C18:3 (wt. %) | IV (g I2/ 100 g of fat) | SV (mg/ KOH g) | OS (h) | CFPP (°C) |
|---|---|---|---|---|---|---|---|
| C. vulgaris NIES 227 | 51.6 ± 0.2 | 46.5 ± 0.1 | 9% | 99.9 ± 0.6 | 196.7 ± 0.2 | 7.7 ± 0.1 | −1.5 ± 0.1 |
| C. vulgaris CCALA 256 | 42.5 ± 0.0 | 45.3 ± 0.0 | 24% | 138.9 ± 0.0 | 198.7 ± 0.1 | 4.7 ± 0.1 | 0.9 ± 0.0 |
| C. vulgaris CCALA 269 | 50.4 ± 0.1 | 46.3 ± 0.2 | 19% | 108.8 ± 0.4 | 191.1 ± 0.2 | 6.2 ± 0.1 | −5.5 ± 0.7 |
| C. sorokiniana NIES 2173 | 43.7 ± 0.7 | 45.4 ± 0.4 | 15% | 133.8 ± 1.57 | 198.8 ± 2.8 | 4.7 ± 0.1 | −2.3 ± 1.7 |
| P. kessleri CCALA 251 | 42.6 ± 0.2 | 45.4 ± 0.1 | 25% | 138.9 ± 0.6 | 198.4 ± 0.2 | 4.7 ± 0.1 | 0.5 ± 0.1 |
| P. kessleri CCALA 253 | 41.8 ± 0.1 | 45.2 ± 0.0 | 21% | 140.6 ± 0.3 | 201.1 ± 0.3 | 4.6 ± 0.1 | 3.1 ± 0.0 |
| European Standard EN 14214 [42] | ≥51 | - | <12% | ≤120 | - | ≥8 | ≤5/≤−20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Romero, A.; Da Costa Magalhães, B.; Dimitriades-Lemaire, A.; Sassi, J.-F.; Delrue, F.; Steyer, J.-P. Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation. Fermentation 2022, 8, 554. https://doi.org/10.3390/fermentation8100554
Ramírez-Romero A, Da Costa Magalhães B, Dimitriades-Lemaire A, Sassi J-F, Delrue F, Steyer J-P. Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation. Fermentation. 2022; 8(10):554. https://doi.org/10.3390/fermentation8100554
Chicago/Turabian StyleRamírez-Romero, Adriana, Bruno Da Costa Magalhães, Alexandra Dimitriades-Lemaire, Jean-François Sassi, Florian Delrue, and Jean-Philippe Steyer. 2022. "Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation" Fermentation 8, no. 10: 554. https://doi.org/10.3390/fermentation8100554
APA StyleRamírez-Romero, A., Da Costa Magalhães, B., Dimitriades-Lemaire, A., Sassi, J.-F., Delrue, F., & Steyer, J.-P. (2022). Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation. Fermentation, 8(10), 554. https://doi.org/10.3390/fermentation8100554






