Abstract
Oil palm frond as an abundant and inexpensive lignocellulosic waste was used to optimize alkaline pretreatment for ethanol production. The studied lignocellulosic waste is one of the largest biomasses (47%) in oil palm waste. Oil palm frond fibers were processed by steam explosion, hot water extraction, and alkaline extraction pretreatment, followed by simultaneous saccharification and fermentation (SSF), for ethanol production as an alternative energy resource. To optimize alkaline extraction for oil palm frond, a Taguchi method with a three-factor design constituted a concentration of NaOH (15%, 20%, and 25%), time (30, 60, and 90 min), and temperature (70, 80, and 90 °C). An optimum alkaline extraction condition of 15% NaOH at 90 °C for 60 min gave the highest percentage of α-cellulose (80.74%) and the lowest percentages of lignin (15.99%), ash (1.05%), and pentosan (2.09%). In addition, the optimized pretreatment condition significantly improved α-cellulose to 52.65% and removed lignin up to 51.78%. Simultaneous saccharification and fermentation (SSF) was carried out with 10% (dry weight) alkaline pretreated OPF fibers, Celluclast 1.5 L (15 FU/gram substrate), Novozyme 188 (15 IU/gram substrate), and Saccharomyces cerevisiae SC90 at 40 and 45 °C. The highest ethanol concentration, theoretical ethanol yield, and ethanol productivity observed at 40 °C were 33.15 g/L, 72.54%, and 0.55 g/L/h, respectively. The results suggest that an optimized alkaline pretreatment process using palm frond as a lignocellulosic waste is a sustainable approach to produce efficient ethanol production.
1. Introduction
Increasing demand for energy worldwide owing to depleting naturally occurring fuels (fossil fuels) has diverted the interest of scientists to alternative renewable energy means. The consumption of lignocellulosic biomass, for instance, agricultural wastes/byproducts and forest/crop residues, for producing ethanol is one way to reduce our reliance on crude oil as a source of energy [1]. In addition, lignocellulosic biomass is taken into account to be lower priced than fossil fuel because of its abundant accessibility [2].
Oil palm trees (Elaeis guineensis Jacq.) are fit to grow in tropical areas predominantly, in Southeast Asian countries, such as Indonesia, Malaysia, and Thailand [3]. The foremost byproduct of palm trees is palm oil [4], which is consumed as edible oil and energy manufactured goods [5], which account for more than USD 50 billion per annum [6].
In Thailand, oil palm plantation is strengthened by the government, and its growth has doubled in the last two decades [3]. Furthermore, such intensification in oil palm plantation has significantly contributed to change in climate by discharging carbon from woods to the atmosphere, and a gigantic amount of waste is being produced from the industry [7]. Oil palm frond (OPF) as lignocellulosic waste is readily available in Thailand because 96% of oil palm waste is above the ground (frond, trunk, and bunches) [8]. Typically, two fronds of mature oil palm plantation are chopped once in a month, which approximately produces 12 tons of dry weight of OPF/ha/yr [9]. Around 75% of the frond waste is left on the ground as agricultural waste [10], while the rest is burned to prevent insect breeding, causing environmental pollution [11]. OPF as a lignocellulosic waste is highly composed of cellulose, hemicellulose, and lignin. In comparison with other oil palm wastes, OPF has the least amount of lignin and contains a high content of cellulose (40–56%) and hemicellulose (16–38%) [12], making it a desirable lignocellulosic material for microbial growth and ethanol production.
An efficient pretreatment is needed for breaking down the chemical and physical structures of lignocellulosic biomass to improve the accessibility of enzymes to disrupt the crystalline structure of cellulose [13]. Moreover, an effective pretreatment of lignocellulosic material depends on its economic viability, desired product, and the nature of biomass [14]. Numerous pretreatments have been categorized as physical, physiochemical, chemical, and biological pretreatments to upsurge the production of simple fermentable saccharides from lignocellulosic biomass [15,16].
Steam explosion as a physical pretreatment is commonly used to break down the internal cell structure of biomass through high temperature and pressure for a few minutes [17]. Likewise, during steam explosion, most of the hemicellulose is removed; on the other hand, for the elimination of lignin, a chemical pretreatment is needed to boost the hydrolysis of cellulose [18]. For the delignification of lignocellulosic material, especially oil palm trunk (OPT), different chemical pretreatments have been used, such as H2O2 pretreatment [15,19] and NaOH pretreatment [20,21]. In contrast with the rest of the chemical pretreatments, the alkaline pretreatment method is a promising way that requires less time, temperature, and pressure [22]. Similarly, it portentously progresses enzyme hydrolysis and produces less inhibitors [23]. The mechanism of alkaline pretreatment is by degrading the structure of lignin and causes swelling of fibers, which leads to improving the surface area, reduces cellulose crystallinity, decreases the degree of polymerization, and eliminates the acetyl group from hemicellulose [24,25]. Simultaneous saccharification and fermentation (SSF) is required for efficient ethanol production, consuming lignocellulosic material. However, SSF has many advantages over the hydrolysis and fermentation (SHF) process, such as both hydrolysis and fermentation steps coming about together in the same reactor [26], less production of inhibitors [27], a reduced amount of processing cost, and fewer chances of contamination [28]. The optimum temperature of most cellulase enzymes is usually above 45–55 °C, which is fairly high for the growth of S. cerevisiae and to produce efficient ethanol [29]. To overcome this problem, a thermotolerant S. cerevisiae SC 90 was used, which could produce ethanol at 40 °C [21]. To elevate various parameters and conditions, there are several optimizing methods, such as the response surface method, artificial neural network, and Taguchi method [30]. In the present study, the Taguchi method, which is a statistical method developed by Taguchi and Konishi [31], was used to optimize several experimental parameters with minimum numbers of experiments using an orthogonal array [31].
To the best of the author’s scientific capacity, the optimization of the alkaline pretreatment condition using the Taguchi method on oil palm frond (OPF) as waste is very limited in the literature. The current study optimized the alkaline pretreatment condition to eliminate lignin from OPF with different studied parameters (NaOH concentration, time, and temperature) by using the Taguchi method.
2. Materials and Methods
2.1. Materials
The equipment used in this study are a steam explosion machine (Kumakai Nitto, Osaka, Japan), crucible pores, different-sized sieves (425−250 μm), water bath (VS-1205S2W1, Vision Scientific Co. LTD., Westland, MI, USA), and high performance liquid chromatography (HPLC A5333, KNAUER, Berlin, Germany). The analytical-grade chemicals purchased from Sigma-Aldrich comprised cellobiose (99%), ethanol (98%), glucose (99%), acetic acid, sulfuric acid (98%), sodium carbonate, sodium chloride, sodium hydroxide (98%).
2.2. Raw Materials
Oil palm fronds (OPF) were collected from an agriculture farm located in Krabi Province, Thailand. The raw OPF was chopped using a disc wood chipper machine (SL-420) into smaller pieces (0.2–2 mm), dried in sunlight for 7 days, and used as raw material. The conditions of steam explosion with severity logRo 3.84 (210 °C; 4 min), hot water extraction (80 °C; 30 min), and fiber-to-liquid ratio (1:8 g/mL) were used according to [20].
2.3. Pretreatment
Optimization of Alkali Extraction by the Taguchi Method
Alkali extraction was performed in 2000 mL beakers containing different concentrations of NaOH solution with 1:8 solid-to-liquid ratio [5]. Three factors, concentration of sodium hydroxide (NaOH), extraction time, and temperature, were observed for their influence on optimized steam-exploded fibers (Table 1). The alkali extraction conditions were augmented by a Taguchi experimental design, and the three factors of an L9 (33) orthogonal array were selected (Table 2). In this study, the optimum conditions were expressed in terms of “the larger-the-better” for cellulose and “the smaller-the-better” for lignin, and the S/N for these attributes were assessed by using Equations (1) and (2), respectively:
where Yi is a variable used for either comparison or combination, i defines combinations of controlled factor levels, and n is the number of experiments accompanied.
S/N = log 10 (∑(1/Yi)2/n)
S/N = log 10 (∑(Yi)2/n)

Table 1.
Factors and their levels assigned to different columns and the orthogonal array of an L9 (33) design for optimizing the alkaline extraction conditions by the Taguchi method.

Table 2.
Factor levels in the experimental design for the optimization of alkaline extraction.
The steam exploded fibers were placed in a beaker with a total solid-to-liquor ratio of 1:8, and the sodium hydroxide concentrations in the extraction were 15%, 20%, and 25%. The mixture was stirred with a stirring rod. In a water bath, the beaker was heated to 70, 80, and 90 °C for 30, 60, and 90 min. The slurry was periodically agitated after every 5 min and left at room temperature to cool down. The fibers were squeezed, filtered, and washed by tap water to neutralize its pH (called alkaline extracted fibers (AEF)). They were collected, weighed, placed into plastic sealed bags, and stored at 4 °C for downstream processes.
2.4. Enzyme Hydrolysis
The raw material of oil palm frond and pretreated oil palm fronds (OPF) were subjected to enzyme hydrolysis at 50 °C and 150 rpm using a water bath shaker for 96 h. The 10% substrate on dry weight basis (raw material and alkaline pretreated oil palm frond fibers) was placed separately in Erlenmeyer flasks (500 mL) using 270 mL sodium citrate (0.05 M, pH 4.8), Celluclast 1.5 L (15 FPU/g substrate), and Novozyme 188 (15 IU/g substrate) [32]. Enzyme activity was evaluated by using the National Renewable Energy Laboratory’s (NREL, 2008) protocol LAP-006 (Measurement of Cellulase Activities) through filter paper unit (FPU) activity [33].
2.5. Fermentation
2.5.1. Preparation of Yeast Growth and Preinoculums
The industrial yeast strain Saccharomyces cerevisiae SC90 used in the fermentation part was purchased from Liquor Distillery Organization Excise Department, Thailand. This strain was grown on yeast peptone and dextrose (YPD agar) medium, containing 20 g/L glucose, 10 g/L yeast extract, 20 g/L peptone, and 15 g/L agar. Preinoculums were prepared by growing 2-day cultures on solid medium. A single yeast colony, transferred to a 125 mL shaking flask, comprising 30 mL YPD medium was incubated at 30 °C and 150 rpm for 18 h in an orbital shaker. These fresh cells were used as inoculums in flasks. The total amount of inoculum was 10% (v/v) of SSF medium.
2.5.2. Simultaneous Saccharification and Fermentation (SSF)
The SSF experiments were executed in a 500 mL Erlenmeyer flask carrying a total capacity of 300 mL, containing 10% (w/v) of alkaline-extracted fibers (AEF) and 10 g of 10-fold concentrated YP medium (100 g/L yeast extract and 200 g/L peptone). The pH of the medium was adjusted to 5 ± 0.2 by 5 mM sodium citrate buffer and autoclaved at 121 °C for 30 min. Subsequently, the flasks were kept at room temperature to cool down, and then the mixture of Celluclast 15 FPU/g substrate, Novozyme 188, 15 IU/g substrate, and 10% (v/v) yeast inoculums was added through an aseptic technique.
The SSF fermentation step was carried out with three replications. The flasks were incubated in an orbital shaker at 150 rpm at 40 and 45 °C for 96 h. The samples (4 mL) were collected with three replicates from 0 to 96 h of fermentation, aseptically with sterile pipette tips, and analyzed for viable cells, glucose consumption, and ethanol production.
2.6. Analytical Methods
The chemical compositions of oil palm fronds (OPF) before pretreatment and after pretreatment were determined according to the standard protocols provided by the Technical Association of the Pulp and Paper Industry (TAPPI), that is, TAPPI T264 om-97 (1997) for the moisture content of OPF before pretreatment and after pretreatment, TAPPI T204 om-97 for extractive substances, TAPPI T223om-84 (TAPPI, 1983d) for the content of pentosan, TAPPI T211om-85 (TAPPI, 1983b) for ash, TAPPI T222om-98 (TAPPI, 1983a) for acid insoluble lignin, and TAPPI T203om-93 (TAPPI, 1983c) for the alpha-cellulose content.
2.7. Analysis of Cellobiose, Glucose, and Ethanol
The concentrations of ethanol, glucose, and cellobiose were determined by high performance liquid chromatography (HPLC A5333, KNAUER, Berlin, Germany) using an Aminex HPX-87H column at 50 °C. Sulfuric acid (0.005 M) was used as mobile phase with a flow rate of 0.6 mL/min [34].
2.8. Statistical Analysis
Statistically significant differences between ethanol concentrations (g/L), ethanol yield (g/g), ethanol productivity (g/L/h), and theoretical yield (%) were evaluated by Duncan’s new multiple range test through SPSS (23 version IBM Corp., USA) with a significance level of 95% (p < 0.05).
3. Results
3.1. Optimization of Alkaline Extraction by the Taguchi Method
The cellulose and lignin content in the experimental data showed that among all the studied factors, the concentration of NaOH was the most noteworthy for the cellulose and lignin content (Table 3). This showed that the concentration of NaOH effectively declined lignin and augmented the cellulose content.

Table 3.
Analysis of variance (ANOVA) of factors affecting the cellulose and lignin content.
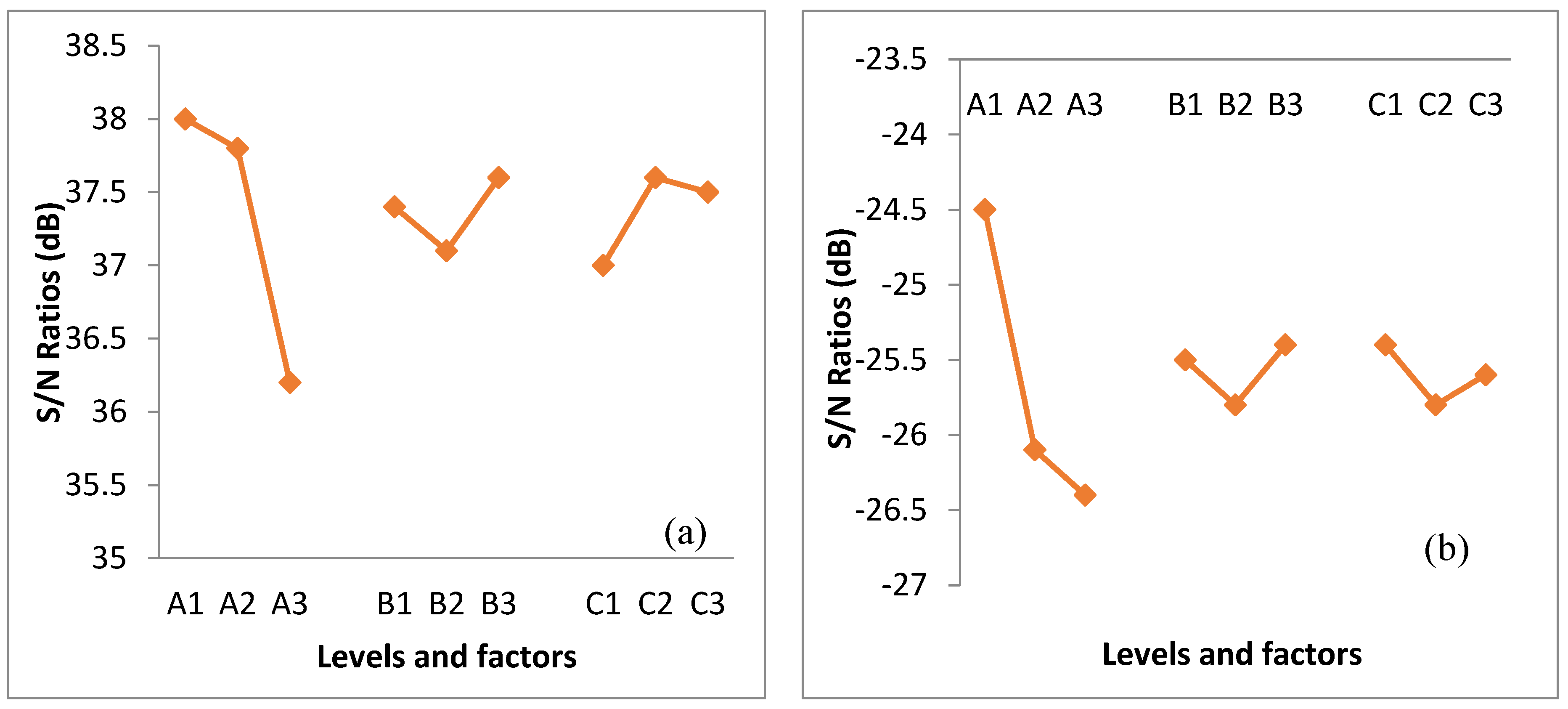
The data in Table 4 exhibits information to identify the optimized condition of NaOH extraction provided by prediction. The relation of S/N ratio plots is displayed in Figure 1. The main effect plot shows the change in factor level and effects of alkaline extraction. Out of three factors with three levels each, only one of the levels maximized the value of the mean S/N ratio. The results presented in Figure 1 suggest the optimum condition that gave the highest content of cellulose and the lowest content of lignin.

Table 4.
Optimized factor levels for the chemical composition of cellulose and lignin.
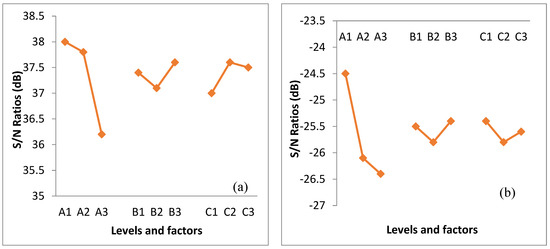
The equations presented in Table 5 were used to estimate the expected chemical content (Yexpected) of alkaline-extracted oil palm frond fibers under various conditions. The highest predicted cellulose content (83.38%) was obtained under the condition of 15% NaOH at 90 °C for 60 min. In addition, the lowest predicted lignin content (15.79%) was obtained under the condition of 15% NaOH at 70 °C for 90 min. Thus, both conditions were selected and used for further experiments.

Table 5.
Comparative analysis of predicted and experimental Yexpected values at the optimal 1qqqqqcondition for the chemical composition of cellulose (CON-1) and lignin (CON-2).
3.2. Confirmation under Optimal Conditions
For the confirmation of results, a single confirmation test with three replicates was conducted to analyze the chemical content using the above two recognized optimum conditions. The exhibition of the confirmatory test results for the optimized condition (Table 5) pointed to the presence of cellulose and lignin contents. The confirmation of CON-1 data showed the cellulose and lignin contents as 80.74% and 15.99%, respectively. The confirmation of CON-2 data showed the cellulose and lignin contents as 74.57% and 15.14%, respectively. These results indicate that CON-1 gave the higher cellulose content than CON-2, whereas the lignin contents of CON 1 and 2 were not different. Thus, CON-1 of 15% NaOH at 90 °C for 60 min was selected and used in the alkaline extraction step for the pretreatment of oil palm frond fibers. A detailed compositional analysis of oil palm frond after every step of the pretreatment is given in Table 6.

Table 6.
Chemical compositions of oil palm fronds after the optimized condition of the alkaline pretreatment.
3.3. Enzyme Hydrolysis
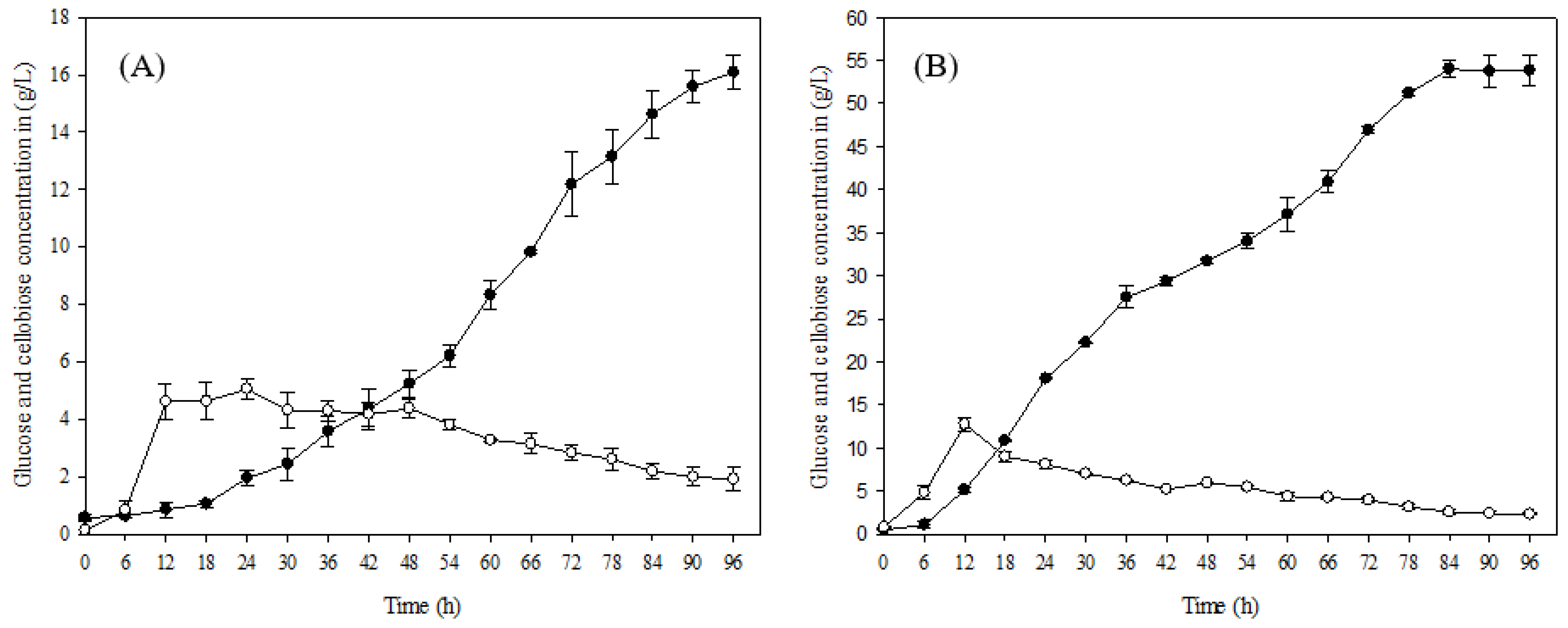
The enzyme hydrolysis was carried out separately with 10% raw material and alkaline pretreated oil palm frond (OPF) fibers. Enzyme hydrolysis with raw material gave a glucose concentration of 18.08 g/L after 96 h of hydrolysis, while the glucose concentration significantly increased when OPF fibers were treated with alkaline pretreatment. The alkaline-treated OPF fibers gave a substantial glucose concentration of 54.30 g/L after 96 h of enzyme hydrolysis (Figure 2A,B).
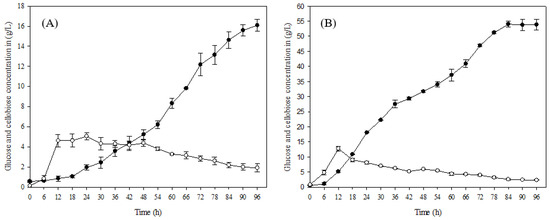
Figure 2.
Enzyme hydrolysis of oil palm frond (A) before pretreatment (raw material) and (B) after pretreatment: (●) glucose concentration and (○) cellobiose concentration in g/L.
3.4. Simultaneous Saccharification and Fermentation of Alkaline Pretreated Oil Palm Frond Fibers
Ethanol production through SSF was performed with alkaline pretreated oil palm frond (OPF) fibers using Celluclast 1.5 L, Novozyme 188, and S. cerevisiae SC 90. The relations of ethanol, glucose cellobiose, and viable cells at 40 and 45 °C are shown in Figure 3 and Figure 4, respectively.
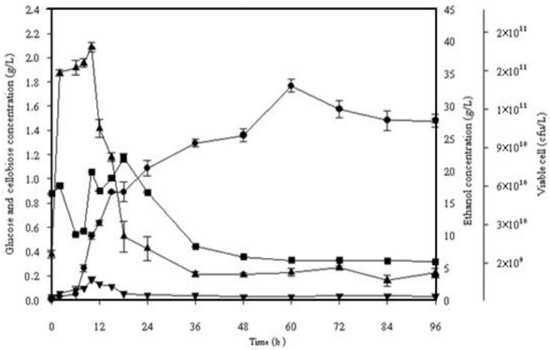
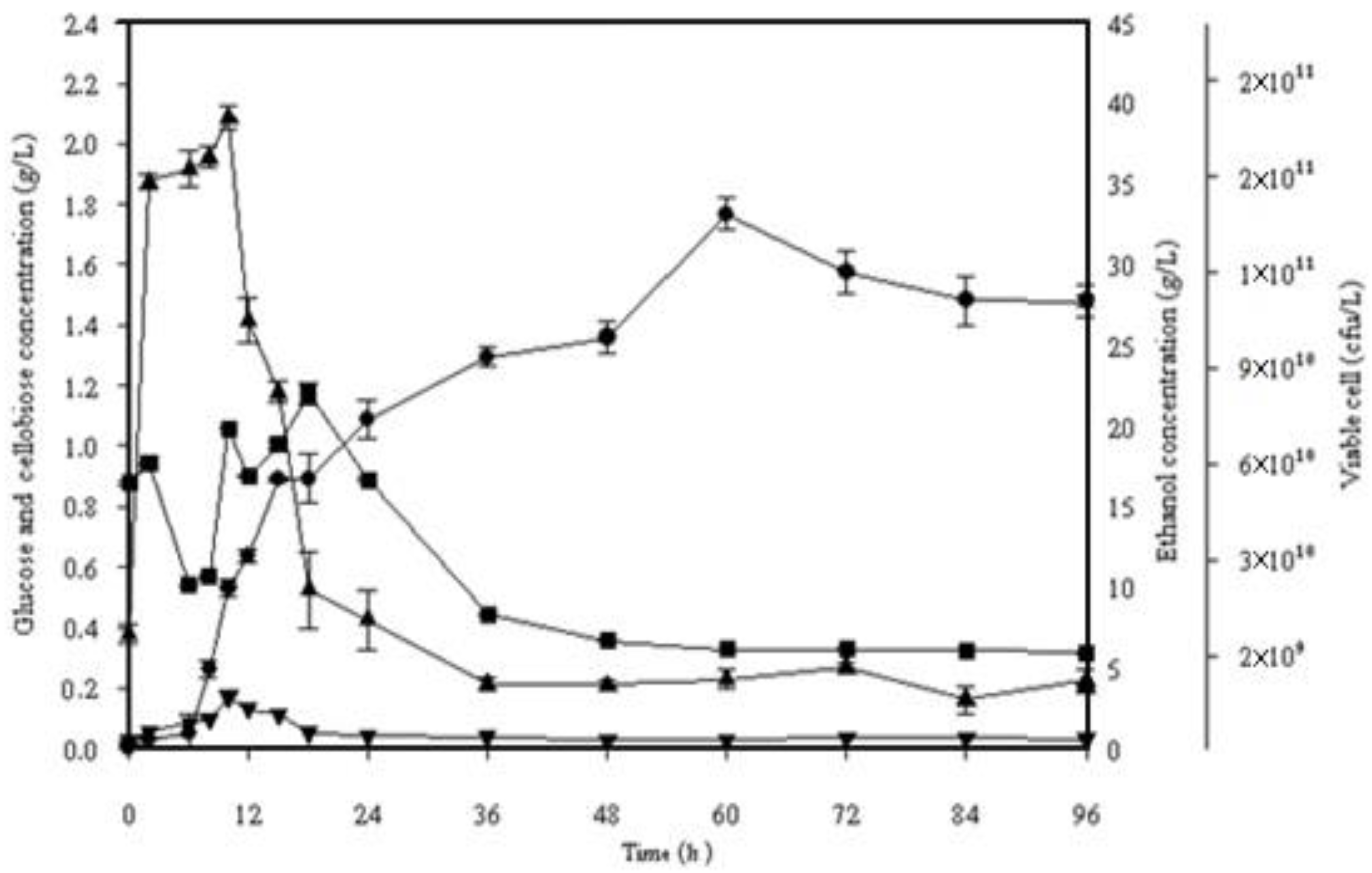
Figure 3.
Simultaneous saccharification and fermentation of 10% (w/v) alkaline pretreated frond fibers incubated with S. cerevisiae SC 90 at 40 °C for 96 h. (■) Viable cells, (▼) cellobiose, (▲) glucose, (●) ethanol.
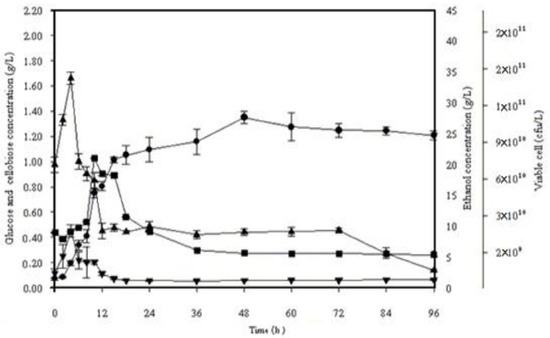
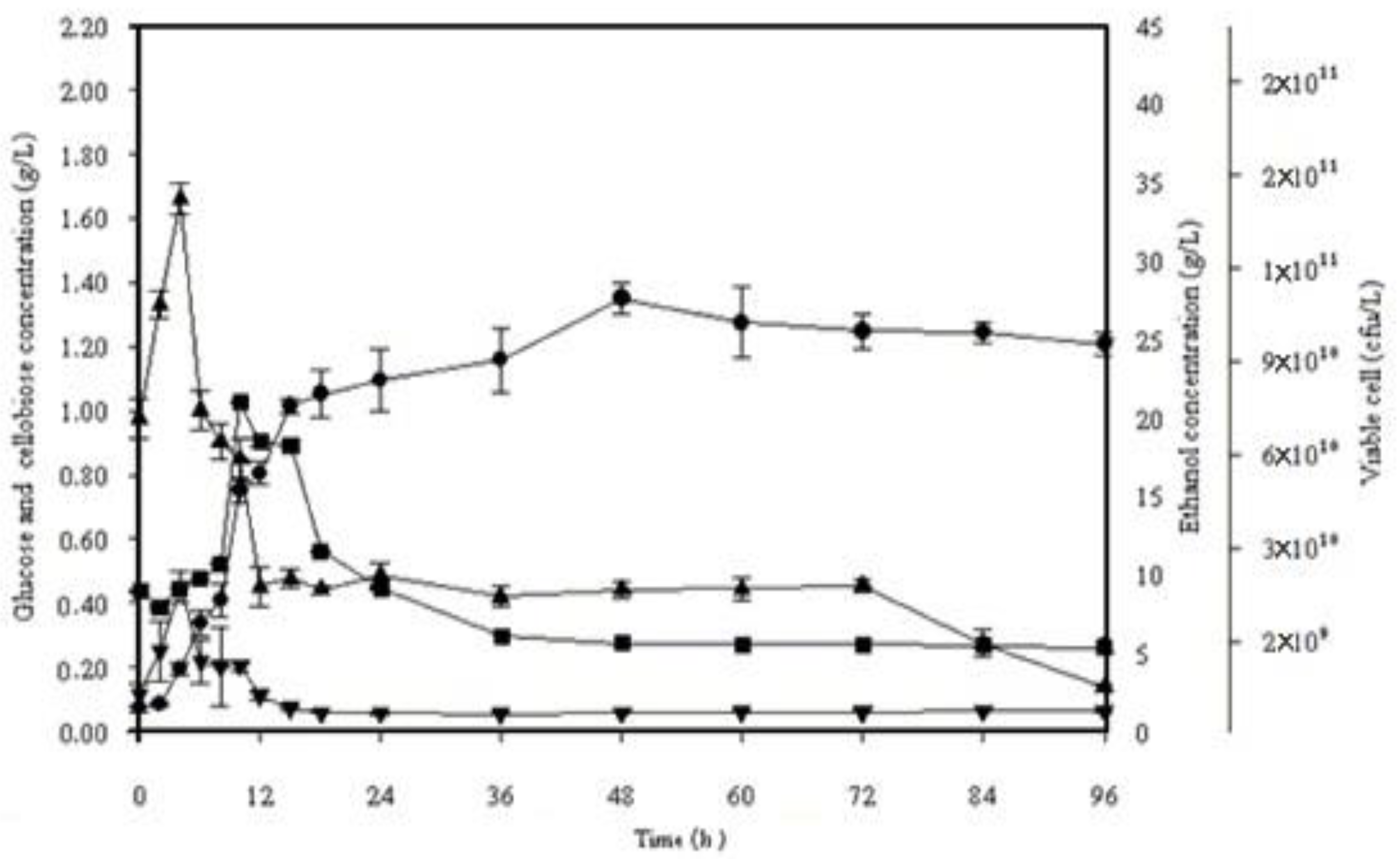
Figure 4.
Simultaneous saccharification and fermentation of 10% (w/v) alkaline pretreated frond fibers incubated with S. cerevisiae SC 90 at 45 °C for 96 h. (■) Viable cells, (▼) cellobiose, (▲) glucose, (●) ethanol.
To compare culturing temperatures of 40 and 45 °C, the simultaneous saccharification and fermentation (SSF) was performed with 10% dry w/v of alkaline pretreated OPF fibers. In Figure 3, it was observed that in the first 6 h of fermentation, the thermotolerant Saccharomyces cerevisiae SC90 yeast amended to the new atmosphere. The results depict the maximum ethanol concentration and theoretical ethanol yield at 40 °C, that is, 33.15 g/L and 72.54%, respectively.
Figure 4 displays the profile of ethanol production using alkaline pretreated OPF fibers at 45 °C. It was exposed through the data that in the initial 6 h of fermentation, the glucose concentration amplified exponentially but started lessening afterward till 96 h. During SSF, the ethanol production started slowly and gradually after 6 h. The peak value of ethanol concentration, that is, 27.62 g/L, was observed after 48 h, which was expressively lower than 40 °C. However, the obtained values of ethanol productivity at 40 and 45 °C (0.55 and 0.54 g/L/h, respectively) were not significantly different. A comparative analysis of bioethanol production using lignocellulosic biomass is given in Table 7.

Table 7.
Comparative analysis of the current study with previous relevant studies for the production of bioethanol.
4. Discussion
In this study, oil palm frond fibers were subjected to steam explosion, hot water extraction, and optimized alkaline pretreatment, followed by simultaneous saccharification and fermentation (SSF), to produce ethanol. The alkaline pretreatment was optimized using the Taguchi method with a three-factor design constituted by a number of NaOH concentrations, time, and temperature. The Taguchi method employs full fractional design called orthogonal arrays (minimum set of experiments, representing various factor combinations) and ANOVA as a tool for analysis. The orthogonal arrays are optimized in relation to the signal-to-noise S/N ratio of the responses instead of the responses itself. This feature marks the difference between the conventional statistical technique and the Taguchi method. Additionally, in comparison with the central composite design (CCD) and response surface methodology (RSM), the Taguchi method is suitable and economical owing to the minimum number of experiments, less time requirement, and computational experience for finding out the optimized condition [39]. The optimized alkaline pretreatment (15% NaOH/90 °C/60 min) showed that the concentration of NaOH effectively diminished lignin and improved cellulose content. Similar observations were reported by Tareen et al. [40], in whose study, the lignin content decreased from 21.64% to 6.13% in oil palm trunk by using NaOH pretreatment. Furthermore, in a study by Nlewem and Thrash [41], switch grass pretreated with NaOH showed a momentous shrinkage in lignin content and enhanced pore formation with approachability of the surface area to the enzymes. In comparison with acid pretreatment, alkaline pretreatment was proven to be more favorable by Rizal et al. [12] because acid pretreatment could significantly reduce lignin. In addition, lignin plays a vital function in plant cell wall texture, as it is heterogeneous in nature and composed of phenolic and biopolymers, owing to which it shows resistance to degradation [41,42].
The chemical composition of OPF fibers was analyzed after every step of pretreatment. The optimized alkaline pretreatment significantly removed the lignin content to 15.99%, which in turn improved the overall cellulose content up to 80.74%. The content of alpha-cellulose and lignin was found to be relatively improved than the results of a study by Barlianti et al. [43], who obtained 54.97% cellulose and 19.23% lignin while using alkaline pretreated oil palm frond (OPF) fibers. However, Mahmood et al. [44] used alkaline extraction for OPF in their study and achieved only 31% of lignin removal with 21.57% percentage yield of alkaline pretreated fibers.
The presence of a higher percentage of lignin in lignocellulosic biomass remarkably influences the process of enzyme hydrolysis by producing toxicity and nonspecific enzyme adsorption within the structure of lignocellulose [45]. Prior to the fermentation process for ethanol production, the enzyme hydrolysis was carried out using 10% OPF fibers. The results of enzyme hydrolysis were parallel to the impact observed using alkaline treatment, which significantly removed lignin and increased cellulose content. Therefore, the leftover cellulose was more prone to be hydrolyzed by enzymes into glucose [5]. Hence, more glucose availability for conversion to ethanol indicates the efficacy of alkaline pretreatment. Likewise, the findings of this study expressed a post–pretreated accelerated amount of glucose from 18.08 g/L in raw material to 54.30 g/L after alkaline pretreatment after 96 h of enzyme hydrolysis. The significant escalation in glucose concentration is due to the potential effect of alkaline treatment by severe delignification, which augmented biomass porosity and diminished cellulose crystallinity, which led to an increase in the surface area of fibers for enzyme accessibility [46] and digestibility due to reducing nonspecific bonding to enzyme [47].
Simultaneous saccharification and fermentation of alkaline pretreated oil palm frond fibers was performed using Celluclast 1.5 L, Novozyme 188, and S. cerevisiae SC 90 at 40 and 45 °C. It was observed that during SSF at 40 °C, in commencing 6 h of fermentation, the thermotolerant Saccharomyces cerevisiae SC90 yeast amended to the new atmosphere. The glucose concentration steadily increased, which could be due to poor mixing of the enzymes Celluclast 1.5 L, Novozyme 188, and OPF fibers. Similar observations were noted by Cara et al. [48] and Tareen et al. [40], stating that an enzyme requires time to adjust to the new environment and mix properly with a substrate for the production of glucose. After the initial 6 h, the growth of the cells was observed due to the release of glucose and the production of ethanol on a continuous basis. The results depicted the maximum ethanol concentration and theoretical ethanol yield at 40 °C, that is, 33.15 g/L and 72.54%, respectively.
In contrast, the data of SSF at 45 °C for ethanol production using alkaline pretreated OPF fibers indicated the exponential growth of glucose in the first 6 h of SSF, which later decreased steadily up to 96 h; nonetheless, it was vice versa for ethanol production (lower in the initial 6 h and later rose till 96 h) owing to high temperature (higher than 43 °C), which may considerably affect yeast cells [48]. The maximum ethanol concentration at 45 °C was 27.62 g/L (48 h). It exhibited that a temperature of 40 °C was the optimum condition, which gave the highest ethanol concentration after 60 h using SSF. These results were found on a higher hand when compared with the findings of Boateng and Lee [49], who achieved the maximum ethanol concentration and yield of 21.96 g/L and 84.65%, respectively. On the other hand, Boonsawang et al. [35] managed to produce only 12.1 g/L of ethanol concentration using alkaline pretreated OPF fibers in SSF fermentation at 35 °C.
5. Conclusions
The study exhibited the optimum condition of alkaline extraction of oil palm fronds obtained by the Taguchi method. The optimized condition of alkaline extraction with 15% NaOH, 90 °C of temperature, and 60 min of extraction time significantly reduced the biomass recalcitrance. The results revealed that the highest cellulose and lowest lignin were 80.74% and 15.99% on a dry weight basis, respectively. For ethanol production by the SSF process, an optimized temperature of 40 °C significantly gave the uppermost ethanol concentration along with a theoretical ethanol yield of 33.15 g/L and 72.54%. Thus, oil palm frond is an alternative material for ethanol production.
Author Contributions
Investigation and writing—original draft, P.K.; conceptualization, W.V.; resources, S.S.; project administration, N.L.; editing and reviewing the manuscript, A.K.T.; validation of data, Z.U.; supervision, resources, and conceptualization, P.P.; supervision, formal analysis, and data curation, I.N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the Graduate School, Kasetsart University, Thailand, and the Thai Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Conversion of Lignocellulosic Biomass into Platform Chemicals for Biobased Polyurethane Application. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–213. [Google Scholar]
- Malinee, R.; Stratoulias, D.; Nuthammachot, N. Detection of oil palm disease in plantations in krabi province, Thailand with high spatial resolution satellite imagery. Agriculture 2021, 11, 251. [Google Scholar] [CrossRef]
- Theerapong, J. The Path to Success is the Production of Palm Oil. Oil Palm Research and Development Center, Faculty of Natural Resources, Prince of Songkla University. 2016. Available online: http://www.natres.psu.ac.th/researchcenter/Palm-Research/menu/pic-book/2559-palmbook.pdf (accessed on 13 July 2022).
- Nutongkaew, P.; Waewsak, J.; Riansut, W.; Kongruang, C.; Gagnon, Y. The potential of palm oil production as a pathway to energy security in Thailand. Sustain. Energy Technol. Assess. 2019, 1, 189–203. [Google Scholar] [CrossRef]
- Murphy, D.J. The future of oil palm as a major global crop: Opportunities and challenges. J. Oil Palm Res. 2014, 1, 1–24. [Google Scholar]
- Schoneveld, G.C.; Ekowati, D.; Andrianto, A.; Haar, S.V.D. Modeling peat and forestland conversion by oil palm smallholders in Indonesian Borneo. Environ. Res. Lett. 2019, 14, 014006. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 5th ed.; Wiley-Blackwell Science: Oxford, UK, 2015; pp. 1–680. [Google Scholar]
- Moraidi, A.; Sung, C.T.; Joo, C.K.; Hanif, A.H.; Ishak, C.F. Evaluation of four soil conservation practices in a nonterraced oil palm plantation. Agron. J. 2012, 104, 1727–1740. [Google Scholar] [CrossRef]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sust. Energ. Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Conversion of steam exploded hydrolyzate of oil palm trunk to furfural by using sulfuric acid, solid acid, and base catalysts in one pot. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–12. [Google Scholar] [CrossRef]
- Rizal, N.; Ibrahim, M.F.; Zakaria, M.R.; Abd-Aziz, S.; Yee, P.L.; Hassan, M.A. Pretreatment of oil palm biomass for fermentable sugars production. Molecules 2018, 23, 1381. [Google Scholar] [CrossRef]
- Azmi, I.S.; Azizan, A.; Salleh, M.D. Pretreatment of oil palm frond (OPF) with ionic liquid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012071. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, S.K.; Kim, J.H.; Park, S.Y.; Kim, J.C.; Jeong, H.; Kim, H.Y.; Choi, I.G. Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Investigation of alkaline hydrogen peroxide pretreatment to enhance enzymatic hydrolysis and phenolic compounds of oil palm trunk. 3 Biotech 2020, 10, 179. [Google Scholar] [CrossRef]
- Devi, A.; Singh, A.; Bajar, S.; Pant, D.; Din, Z.U. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Kuboon, S.; Kraithong, W.; Damaurai, J.; Faungnawakij, K. Hydro-fractionation for biomass upgrading. In Renewable Resources and Biorefineries; Jacob-Lopes, E., Zepka, Q., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, O.Y.; Persson, G.; Olssonc, E.; Rova, U.; Olsson, E.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2018, 273, 521–528. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotech. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Koopraserting, P.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Effect of temperature and time of steam explosion on chemical compositions of oil palm frond. In Proceedings of the 50th Kasetsart University Annual Conference (Subject Agro—Industry) Kasetsart University, Bangkok, Thailand; 2012; pp. 362–369. [Google Scholar]
- Wilaithup, A.; Sultan, I.N.; Tareen, A.K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Bioethanol production from oil palm trunk fibers using activated immobilized Saccharomyces cerevisiae SC90 under simultaneous saccharification and fermentation. Bioenerg. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, J.; Wu, J.; Zhang, L.; Lyu, X.; Xiao, W.; Gong, Y.; Xu, Y.; Liu, Z. Vacuum-assisted black liquor-recycling enhances the sugar yield of sugarcane bagasse and decreases water and alkali consumption. Bioresour. Technol. 2020, 309, 123349. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Mier-Alba, E.; da Silva, S.S.; Chandel, A.K. Commercial Washing Detergents-Assisted Alkaline Pretreatment for Lignocellulosic Sugars Production: A First Report. Sugar Tech. 2021, 23, 1425–1431. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Bisht, A.; Pagolu, R.; Lai, C.; Lestari, R.; Kumar, A.; Das, D.; Kalia, V.C.; Lee, J.K. Mild Alkaline Pretreatment of Rice Straw as a Feedstock in Microbial Fuel Cells for Generation of Bioelectricity. Indian J. Microbiol. 2022, 62, 447–455. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Salini, C.N.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef]
- Kadar, Z.; Szengyel, Z.; Reczey, K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crops Prod. 2004, 20, 103–110. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, X.; Li, H.; Du, X.; Liang, S.; Zhao, X. Screening and Mutation of Saccharomyces cerevisiae UV-20 with a High Yield of Second Generation Bioethanol and High Tolerance of Temperature, Glucose and Ethanol. Indian J. Microbiol. 2018, 58, 440–447. [Google Scholar] [CrossRef]
- Deepanraj, B.; Bivasubramanian, V.; Jayaraj, S. Multi-response optimization of process parameters in biogas production from food waste using taguchi-grey relational analysis. Energy Convers. Manag. 2017, 141, 429. [Google Scholar] [CrossRef]
- Taguchi, G.; Konishi, S. Taguchi Methods Orthogonal Arrays and Linear Graphs: Tools for Quality Engineering; American Supplier Institute: Dearborn, MI, USA, 1987. [Google Scholar]
- Sultan, I.N.; Khienpanya, N.; Tareen, A.K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatida, P. Kinetic study of ethanol production from different sizes of two-step pretreated oil palm trunk by fed-batch simultaneous saccharification and fermentation. Agric. Nat. Resour. 2022, 56, 287–297. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory Technical Report, NREL/TP510-42629; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Tareen, A.K.; Punsuvon, V.; Sultan, I.N.; Khan, M.W.; Parakulsuksatid, P. Cellulase addition and pre-hydrolysis effect of high solid fed-batch simultaneous saccharification and ethanol fermentation from a combined pretreated oil palm trunk. ACS Omega 2021, 6, 26119–26129. [Google Scholar] [CrossRef]
- Boateng, O.C.; Lee, K.T. Same-vessel enzymatic saccharification and fermentation of organosolv/H2O2 pretreated oil palm (Elaeis guineensis Jacq.) fronds for bioethanol production: Optimization of process parameters. Energy Convers. Manag. 2014, 78, 421–430. [Google Scholar] [CrossRef]
- Boonsawang, P.; Subkaree, Y.; Srinorakutara, T. Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy 2012, 40, 127–132. [Google Scholar] [CrossRef]
- Hong, L.S.; Ibrahim, D.; Omar, C.I. Oil palm frond for the production of bioethanol. Int. J. Biochem. Biotechnol. 2012, 1, 7–11. [Google Scholar] [CrossRef]
- Danbamrongtrakool, N.; Sultan, I.N.; Laemsak, N.; Tareen, A.K.; Sirisansaneeyakul, S.; Parakulsuksatid, P. Utilization of urea as a nitrogen source for ethanol production from oil palm trunk using simultaneous saccharification and fermentation. Agric. Nat. Resour. 2022, 56, 175–186. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Raman, A.A.; Daud, W.M.A.W.A. Comparison of Central Composite Design and Taguchi Method for Optimizing Fenton Process. Sci. World J. 2014, 2014, 869120. [Google Scholar] [CrossRef]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-step pretreatment of oil palm trunk for ethanol production by thermotolerent Saccharomyces cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef]
- Nlewem, K.C.; Thrash, M.E. Comparison of different pretreatment methods based on residual lignin effect on the enzymatic hydrolysis of switchgrass. Bioresour. Technol. 2010, 101, 5426–5430. [Google Scholar] [CrossRef]
- Schoenherr, S.; Ebrahimi, M.; Czermak, P. Lignin degradation processes and the purification of valuable products. In Trends and Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Barlianti, V.; Dahnum, D.; Hendarsyah, H.; Abimanyu, H. Effect of alkaline pretreatment on properties of lignocellulosic oil palm waste. Procedia Chem. 2015, 16, 195–201. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Akil, H.M. Ionic liquid pretreatment at high solids loading: A clean approach for fabrication of renewable resource based particulate composites. Polym. Compos. 2018, 39, 1994–2003. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.; Tang, Y.; Xia, H.; Zhang, X.; Yue, M.; Qiu, X.; Xu, K.; Wang, Z. Transcriptomic insights into citrus segment membrane’s cell wall components relating to fruit sensory texture. BMC Genom. 2018, 19, 280. [Google Scholar] [CrossRef]
- Pezoa, R.; Cortinez, V.; Hyvarinen, S. Use of ionic liquids in the pretreatment of forest and agricultural residues for the production of bioethanol. Cell. Chem. Technol. 2010, 44, 165–172. [Google Scholar]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 2–9. [Google Scholar] [CrossRef]
- Cara, C.; Moya, M.; Ballesteros, I.; Negro, M.J.; Gonzalez, A.; Ruiza, E. Influence of solid loading on enzymatic hydrolysis of steam exploded or liquid hot water pretreated olive tree biomass. Process Biochem. 2007, 42, 1003–1009. [Google Scholar] [CrossRef]
- Reed, G.; Peppler, H.J. Yeast Technology; The AVI Publication Company Inc.: Westport, CT, USA, 1973; Volume 19, p. 380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).