Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga and Growth Conditions
2.2. Growth Measurement
2.3. Total Lipid Content
2.4. Fatty Acid Profile
2.5. Estimated Biodiesel Characteristics
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sodium Nitrate
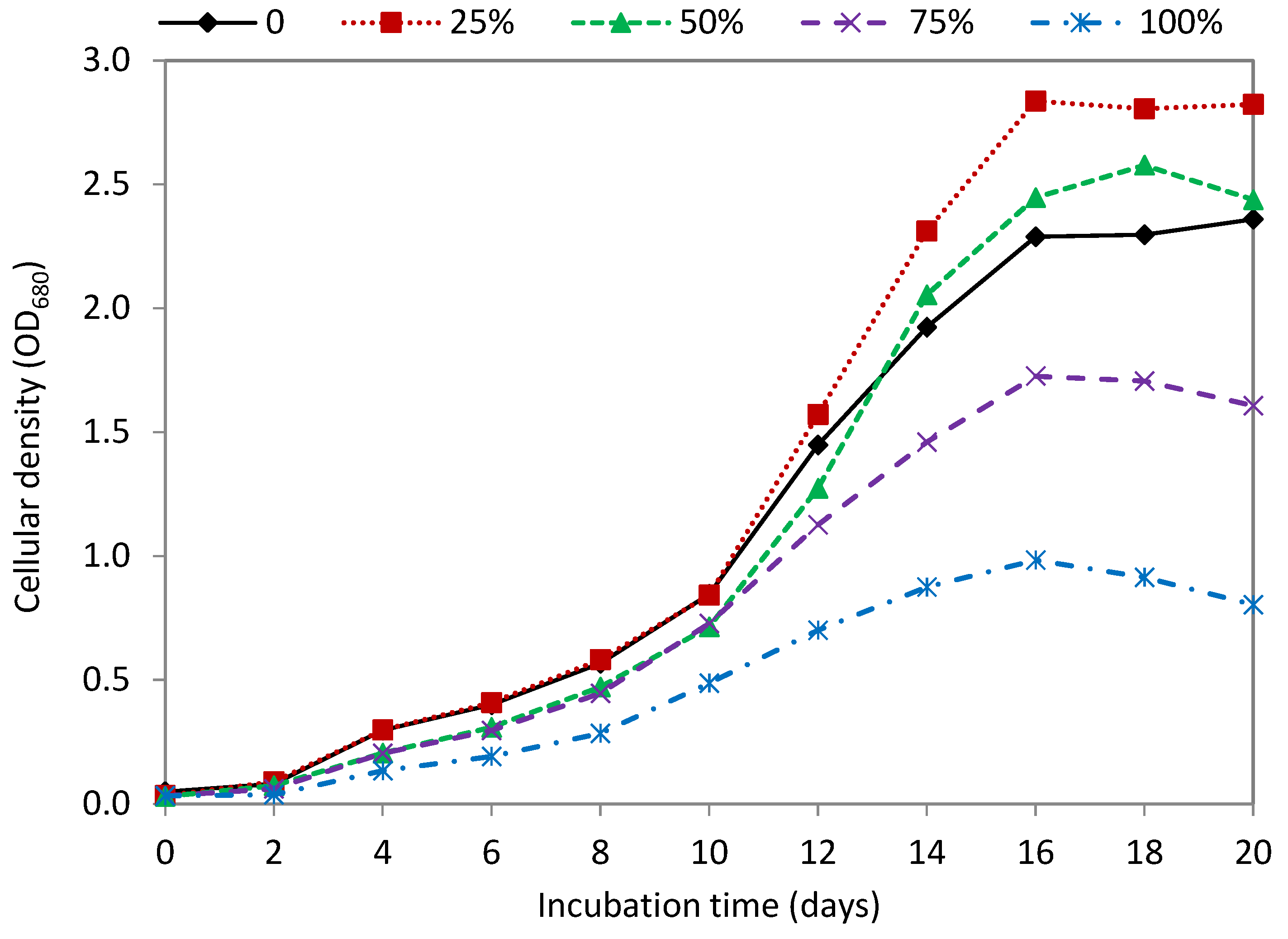
3.2. Effect of Seawater
3.3. Effect of Glycerol
3.4. Interaction Optimization
3.5. Lipid Productivity and Estimated Biodiesel Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Alotaibi, M.K.H.; Li, L.; Abomohra, A.E.-F. Enhanced waste glycerol recycling by yeast for efficient biodiesel production: Towards waste biorefinery. Biomass Bioenergy 2022, 159, 106410. [Google Scholar] [CrossRef]
- IEA. Are Aviation Biofuels Ready for Take Off? International Energy Agency: Paris, France, 2019; Available online: https://www.iea.org/commentaries/are-aviation-biofuels-ready-for-take-off (accessed on 27 September 2021).
- Zhu, L.Y.; Zong, M.H.; Wu, H. Efficient lipid production with Trichosporonfermentans and its use for biodiesel preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, H.; Addy, M.; Anderson, E.; Liu, Y.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in wastewater with waste glycerol: Strategies for improving nutrients removal and enhancing lipid production. Bioresour. Technol. 2016, 207, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Abomohra; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Yildiz, I.; MacEachern, C. 1.21 Food and Energy. Compr. Energy Syst. 2018, 1–5, 850–874. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S.; Abomohra, A.E.-F. Integrated microalgal biorefinery—Routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; El-Sheekh, M.; Hanelt, D. Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew. Energy 2017, 101, 1266–1272. [Google Scholar] [CrossRef]
- Ashour, M.; Elshobary, M.E.; El-Shenody, R.; Kamil, A.W.; Abomohra, A.E.F. Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 2019, 120, 439–447. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Abomohra, A.E.-F.; Hanelt, D. Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J. Microbiol. Biotechnol. 2013, 29, 915–922. [Google Scholar] [CrossRef]
- Faisal, S.; Zaky, A.; Wang, Q.; Huang, J.; Abomohra, A. Integrated Marine Biogas: A Promising Approach towards Sustainability. Ferment 2022, 8, 520. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Jin, W.; Tu, R.; Han, S.-F.; Eid, M.; Eladel, H. Microalgal biomass production as a sustainable feedstock for biodiesel: Current status and perspectives. Renew. Sustain. Energy Rev. 2016, 64, 596–606. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; El-Naggar, A.H.; Alaswad, S.O.; Elsayed, M.; Li, M.; Li, W. Enhancement of biodiesel yield from a halophilic green microalga isolated under extreme hypersaline conditions through stepwise salinity adaptation strategy. Bioresour. Technol. 2020, 310, 123462. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.; Eladel, H.; El-Esawi, M.; Wang, S.; Wang, Q.; He, Z.; Feng, Y.; Shang, H.; Hanelt, D. Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: Innovative waste recycling for extraordinary lipid production. Bioresour. Technol. 2018, 249, 992–999. [Google Scholar] [CrossRef]
- Leoneti, A.B.; Aragão-Leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, T.G.; Holzer, G.; Lien, S.; Burris, N. Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzym. Microb. Technol. 1983, 5, 435–440. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A look back at the US Department of Energy’s aquatic species program: Biodiesel from algae. Natl. Renew. Energy Lab. 1998, 328, 1–294. [Google Scholar]
- Wani, T.A.; Ahmad, A.; Zargar, S.; Khalil, N.Y.; Darwish, I.A. Use of response surface methodology for development of new microwell-based spectrophotometric method for determination of atrovastatin calcium in tablets. Chem. Cent. J. 2012, 6, 134. [Google Scholar] [CrossRef]
- Abomohra; Zheng, X.; Wang, Q.; Huang, J.; Ebaid, R. Enhancement of biodiesel yield and characteristics through in-situ solvo-thermal co-transesterification of wet microalgae with spent coffee grounds. Bioresour. Technol. 2020, 323, 124640. [Google Scholar] [CrossRef] [PubMed]
- Policastro, G.; Luongo, V.; Frunzo, L.; Fabbricino, M. A comprehensive review of mathematical models of photo fermentation. Crit. Rev. Biotechnol. 2021, 41, 628–648. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 495–509. [Google Scholar] [CrossRef]
- Christie, W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology—Two; Adlof, R., Ed.; Oily Press: Dundee, UK, 1993; pp. 69–111. [Google Scholar]
- Abomohra, A.E.-F.; Jin, W.; El-Sheekh, M. Enhancement of lipid extraction for improved biodiesel recovery from the biodiesel promising microalga Scenedesmus obliquus. Energy Convers. Manag. 2016, 108, 23–29. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Song, M.; Pei, H.; Hu, W.; Ma, G. Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour. Technol. 2013, 141, 245–251. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agric. Nat. Resour. 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Piorreck, M.; Baasch, K.H.; Pohl, P. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 1984, 23, 207–216. [Google Scholar] [CrossRef]
- Turpin, D.H. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J. Phycol. 1991, 27, 14–20. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Jiménez, C.; Figueroa, F.L.; Niell, F.X. Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 1998, 10, 461. [Google Scholar] [CrossRef]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.F.; Wagner, M.; El-Sheekh, M.; Hanelt, D. Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: Screening studies towards biodiesel production. J. Appl. Phycol. 2013, 25, 931–936. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef]
- Janssen, J.H.; Kastenhofer, J.; de Hoop, J.A.; Lamers, P.P.; Wijffels, R.H.; Barbosa, M.J. Effect of nitrogen addition on lipid productivity of nitrogen starved Nannochloropsis gaditana. Algal Res. 2018, 33, 125–132. [Google Scholar] [CrossRef]
- Santucci, P.; Johansen, M.D.; Point, V.; Poncin, I.; Viljoen, A.; Cavalier, J.F.; Kremer, L.; Canaan, S. Nitrogen deprivation induces triacylglycerol accumulation, drug tolerance and hypervirulence in mycobacteria. Sci. Rep. 2019, 9, 8667. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, R.; Chisti, Y.; Banerjee, U.C. Botryococcus braunii: A renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotechnol. 2002, 22, 245–279. [Google Scholar] [CrossRef]
- Dayananda, C.; Sarada, R.; Bhattacharya, S.; Ravishankar, G.A. Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii. Process Biochem. 2005, 40, 3125–3131. [Google Scholar] [CrossRef]
- Lu, C.; Vonshak, A. Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol. Plant. 2002, 114, 405–413. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-Scavenging Systems to Salt Stress During Seedling and Reproductive Stages in Rice. Ann. Bot. 2007, 99, 1161. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Dikshit, P.K.; Kumar, P.; Kim, B.S. Co-fermentation of agricultural and industrial waste by Naganishia albida for microbial lipid production in fed-batch fermentation. J. Chem. Technol. Biotechnol. 2020, 95, 813–821. [Google Scholar] [CrossRef]
- Szymanowska-PowaŁowska, D. The effect of high concentrations of glycerol on the growth, metabolism and adaptation capacity of Clostridium butyricum DSP1. Electron. J. Biotechnol. 2015, 18, 128–133. [Google Scholar] [CrossRef]
- Kot, A.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous Production of Lipids and Carotenoids by the Red Yeast Rhodotorula from Waste Glycerol Fraction and Potato Wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Singh, A.; Sinha, S.; Choudhary, A.K.; Panchal, H.; Elkelawy, M.; Sadasivuni, K.K. Optimization of performance and emission characteristics of CI engine fueled with Jatropha biodiesel produced using a heterogeneous catalyst (CaO). Fuel 2020, 280, 118611. [Google Scholar] [CrossRef]
- Kemper, P.; Müller, D.; Thümmler, A. Combining response surface methodology with numerical methods for optimization of Markovian models. IEEE Trans. Dependable Secur. Comput. 2006, 3, 259–269. [Google Scholar] [CrossRef]
- Eladel, H.; Abomohra, A.E.-F.; Battah, M.; Mohmmed, S.; Radwan, A.; Abdelrahim, H. Evaluation of Chlorella sorokiniana isolated from local municipal wastewater for dual application in nutrient removal and biodiesel production. Bioprocess Biosyst. Eng. 2019, 42, 425–433. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, N.; Farooqi, H.; Abdin, M.Z.; Mock, T.; Kumar, S. Phycoremediation of municipal wastewater by microalgae to produce biofuel. Int. J. Phytoremediation 2017, 19, 805–812. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M.; Seifi, M.H. Cultivation of newly isolated microalgae Coelastrum sp. in wastewater for simultaneous CO2 fixation, lipid production and wastewater treatment. Bioprocess Biosyst. Eng. 2018, 41, 519–530. [Google Scholar] [CrossRef]
- Xin, L.; Hong-ying, H.; Jia, Y. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Rinna, F.; Buono, S.; Cabanelas, I.T.D.; Nascimento, I.A.; Sansone, G.; Barone, C.M.A. Wastewater treatment by microalgae can generate high quality biodiesel feedstock. J. Water Process Eng. 2017, 18, 144–149. [Google Scholar] [CrossRef]
- Han, S.; Jin, W.; Chen, Y.; Tu, R.; Abomohra, A.E.-F. Enhancement of Lipid Production of Chlorella Pyrenoidosa Cultivated in Municipal Wastewater by Magnetic Treatment. Appl. Biochem. Biotechnol. 2016, 180, 1043–1055. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Beardall, J. Effect of salinity on fatty acid composition of a green microalga from an antarctic hypersaline lake. Phytochemistry 1997, 45, 655–658. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kalacheva, G.S.; Volova, T.G. Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J. Appl. Phycol. 2011, 23, 47–52. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L. Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J. Appl. Phycol. 1994, 6, 347–356. [Google Scholar] [CrossRef]
- Hu, H.; Gao, K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006, 28, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; Kim, J.Y.H.; Woo, H.M.; Jin, E.S.; Min, B.K.; Sim, S.J. Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res. 2016, 19, 215–224. [Google Scholar] [CrossRef]
- Almutairi, A.W.; Al-Hasawi, Z.M.; Abomohra, A.E.F. Valorization of lipidic food waste for enhanced biodiesel recovery through two-step conversion: A novel microalgae-integrated approach. Bioresour. Technol. 2021, 342, 125966. [Google Scholar] [CrossRef] [PubMed]
- Altun, Ş. Effect of the degree of unsaturation of biodiesel fuels on the exhaust emissions of a diesel power generator. Fuel 2014, 117, 450–457. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN 14214 Automotive Fuels D Fatty Acid Methylesters (FAME) for Diesel Engines. Requirements and Test Methods; European Committee for Standardization (CEN): Brussels, Belgium, 2008; ISBN 9780580707810. [Google Scholar]
- Francisco, É.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
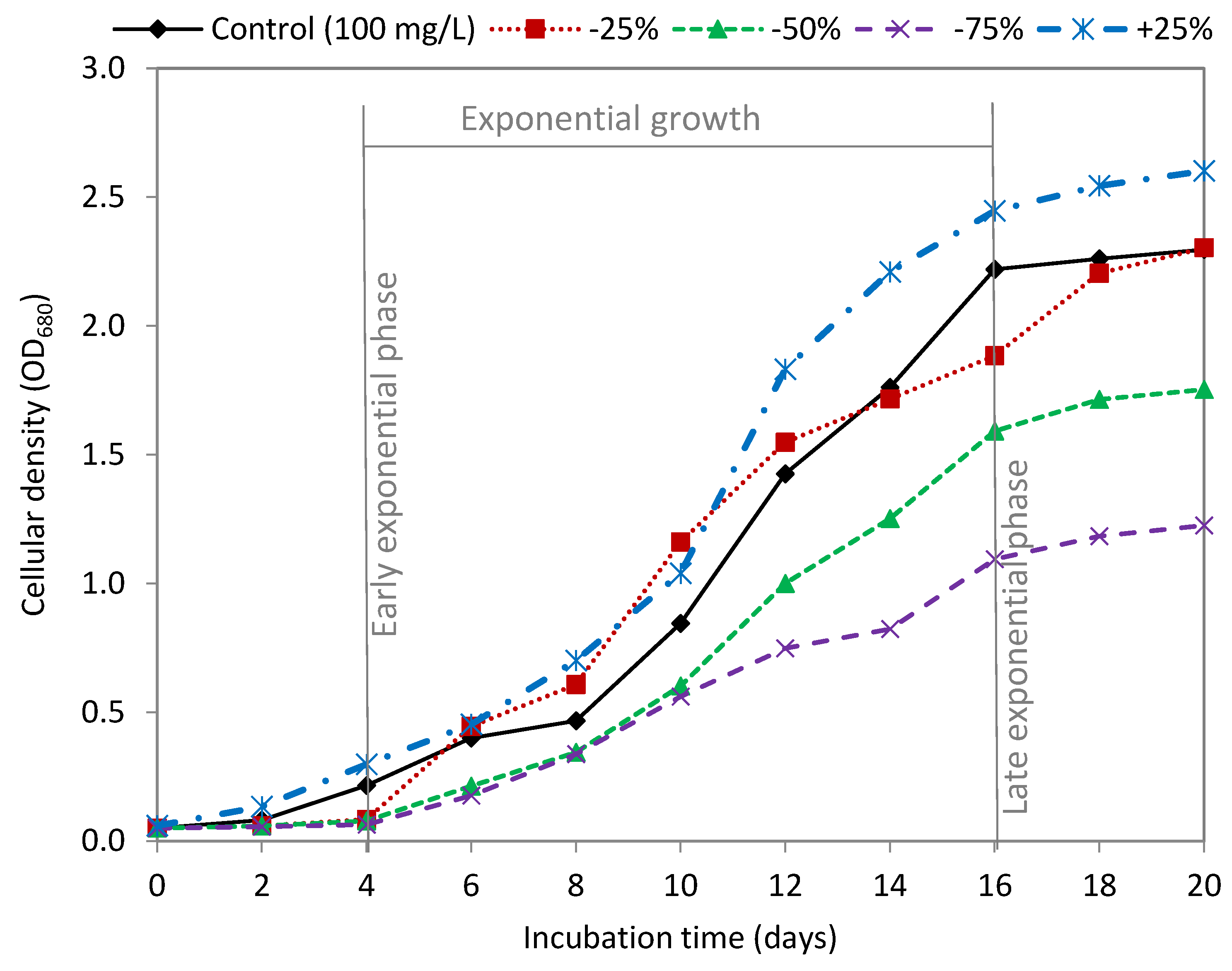




| Sodium Nitrate | Dry Weight (g/L) | Biomass Productivity (g/L Day) | Lipid Content (mg/g DW) | Lipid Productivity (mg/L Day) |
|---|---|---|---|---|
| Control (100 mg/L) | 2.49 ± 0.079 a | 0.187 ± 0.007 a | 160.06 ± 4.27 a | 27.27 ± 0.728 a |
| −25% | 2.15 ± 0.071 b | 0.171 ± 0.006 b | 175.72 ± 6.39 b | 25.36 ± 0.978 ab |
| −50% | 1.78 ± 0.011 c | 0.141 ± 0.001 c | 204.81 ± 8.99 c | 24.57 ± 0.013 b |
| −75% | 1.23 ± 0.013 d | 0.096 ± 0.001 d | 258.23 ± 3.61 d | 22.26 ± 0.319 c |
| +25% | 2.85 ± 0.054 e | 0.210 ± 0.004 e | 158.23 ± 7.55 a | 35.76 ± 1.965 d |
| Seawater Ratio (%, v/v) | Dry Weight (g/L) | Biomass Productivity (g/L Day) | Lipid Content (mg/g DW) | Lipid Productivity (mg/L Day) |
|---|---|---|---|---|
| Control (0) | 2.56 ± 0.011 a | 0.186 ± 0.005 a | 157.81 ± 8.78 a | 27.46 ± 0.893 a |
| 25% | 3.18 ± 0.039 b | 0.237 ± 0.003 b | 164.90 ± 5.06 a | 37.60 ± 0.459 b |
| 50% | 2.74 ± 0.121 c | 0.209 ± 0.009 c | 132.27 ± 5.04 b | 27.05 ± 0.504 a |
| 75% | 1.93 ± 0.020 d | 0.142 ± 0.003 d | 123.17 ± 7.09 b | 16.80 ± 0.720 c |
| 100% | 1.10 ± 0.043 e | 0.079 ± 0.003 e | 111.24 ± 3.23 c | 8.38 ± 0.664 d |
| Glycerol (g L−1) | Dry Weight (g/L) | Biomass Productivity (g/L Day) | Lipid Content (mg/g DW) | Lipid Productivity (mg/L Day) |
|---|---|---|---|---|
| Control (0) | 2.42 ± 0.053 a | 0.180 ± 0.003 ab | 163.48 ± 5.02 a | 27.17 ± 0.388 a |
| 2 | 2.74 ± 0.060 b | 0.195 ± 0.007 c | 181.69 ± 8.09 b | 33.29 ± 0.759 b |
| 4 | 2.64 ± 0.225 ab | 0.190 ± 0.013 ac | 203.63 ± 5.33 c | 42.61 ± 0.898 c |
| 6 | 2.28 ± 0.129 c | 0.167 ± 0.012 b | 204.15 ± 3.50 c | 37.13 ± 0.624 d |
| 8 | 1.98 ± 0.174 d | 0.146 ± 0.014 d | 188.32 ± 3.27 b | 29.29 ± 0.861 e |
| Parameters | Control | Optimized |
|---|---|---|
| Dry weight (g/L) | 2.48 ± 0.06 | 3.27 ± 0.06 * |
| Lipid content (mg/g dw) | 161.62 ± 3.66 | 194.41 ± 9.01 * |
| Lipid productivity (mg/L day) | 27.40 ± 1.55 | 46.98 ± 3.38 * |
| Total FAMEs (mg/g dw) | 147.2 ± 3.25 | 181.0 ± 8.53 * |
| C14:0 | 1.12 | 1.47 |
| C16:0 | 20.18 | 25.84 |
| C16:1n7 | 10.96 | 9.45 |
| C16:1n9 | 3.88 | 2.36 |
| C16:3n4 | 4.1 | 6.14 |
| C18:0 | 16.47 | 18.18 |
| C18:1n9 | 15.96 | 17.05 |
| C18:1n7 | 3.45 | 5.96 |
| C18:2n6 | 9.85 | 6.04 |
| C18:3n3 | 6.47 | 5.42 |
| C18:4n3 | 2.07 | 1.13 |
| C20:0 | 5.49 | 0.96 |
| SFAs | 43.26 ± 1.05 | 46.45 ± 1.06 * |
| MUFAs | 34.25 ± 0.95 | 34.82 ± 0.86 ns |
| PUFAs | 22.49 ± 0.85 | 18.73 ± 0.74 * |
| Wastewater | Microalgae | Pmass (g/L Day) | Plipid (mg/L Day) | RE (%) | Ref. |
|---|---|---|---|---|---|
| Municipal wastewater | Chlorella sorokiniana | 0.073 | 16.20 | 74.2 NO3 83.3 NH4 | [50] |
| Urban wastewater | Chlorella vulgaris | 0.190 | 14.31 | 87.9 NO3 | [51] |
| Cattle manure leachate | Coelastrum sp. | 0.171 | 11.08 | 72.3 NO3 | [52] |
| Municipal wastewater | Scenedesmus sp. LX1 | 0.007 | 8.00 | 98.5 NO3 | [53] |
| Domestic effluents | Botryococcus braunii | 0.065 | 15.80 | 62.0 NO3 | [54] |
| Carpet mill | Chlorella saccharophila | 0.023 | 4.20 | - | [56] |
| Municipal wastewater | Chlorella pyrenoidosa | 0.229 | 48.90 | 59.4 NH4 | [55] |
| Optimized synthetic wastewater | Chlorella sp. | 0.277 | 46.98 | 98.0 TN | This study |
| Parameters | Control | Optimized | US (ASTM D6751-08) | Europe (EN 14214) |
|---|---|---|---|---|
| ADU | 0.94 | 0.86 | - | - |
| Kinematic viscosity (mm2 s−1) | 4.61 | 4.66 | 1.9–6.0 | 3.5–5.0 |
| Specific gravity | 0.88 | 0.88 | 0.85–0.9 | - |
| Cloud point | 7.45 | 8.49 | - | - |
| Cetane number | 56.61 | 57.13 | Min. 47 | 51–120 |
| Iodine value (g I2/100 g oil) | 82.58 | 76.75 | - | Max. 120 |
| HHV (MJ kg−1) | 40.19 | 40.05 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abomohra, A.; Li, M.; Faisal, S.; Li, L.; Elsayed, M. Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production. Fermentation 2022, 8, 682. https://doi.org/10.3390/fermentation8120682
Abomohra A, Li M, Faisal S, Li L, Elsayed M. Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production. Fermentation. 2022; 8(12):682. https://doi.org/10.3390/fermentation8120682
Chicago/Turabian StyleAbomohra, Abdelfatah, Mei Li, Shah Faisal, Li Li, and Mahdy Elsayed. 2022. "Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production" Fermentation 8, no. 12: 682. https://doi.org/10.3390/fermentation8120682
APA StyleAbomohra, A., Li, M., Faisal, S., Li, L., & Elsayed, M. (2022). Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production. Fermentation, 8(12), 682. https://doi.org/10.3390/fermentation8120682









