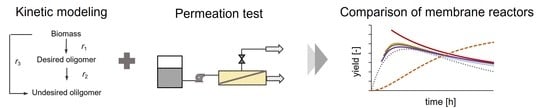
Kinetic Modeling of an Enzyme Membrane Reactor for the Selective Production of Oligosaccharides
Abstract
1. Introduction
2. Methodology
2.1. Materials
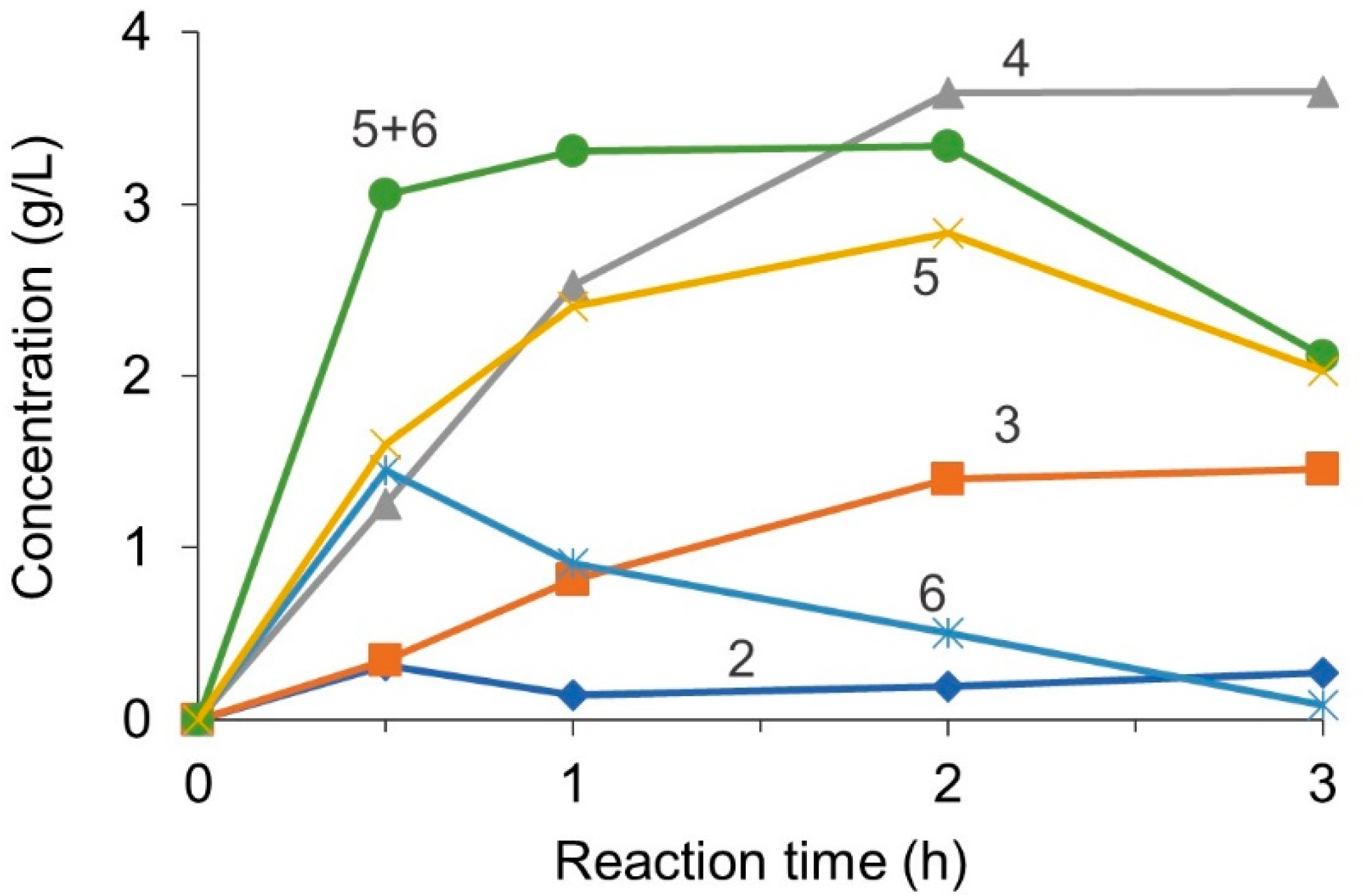
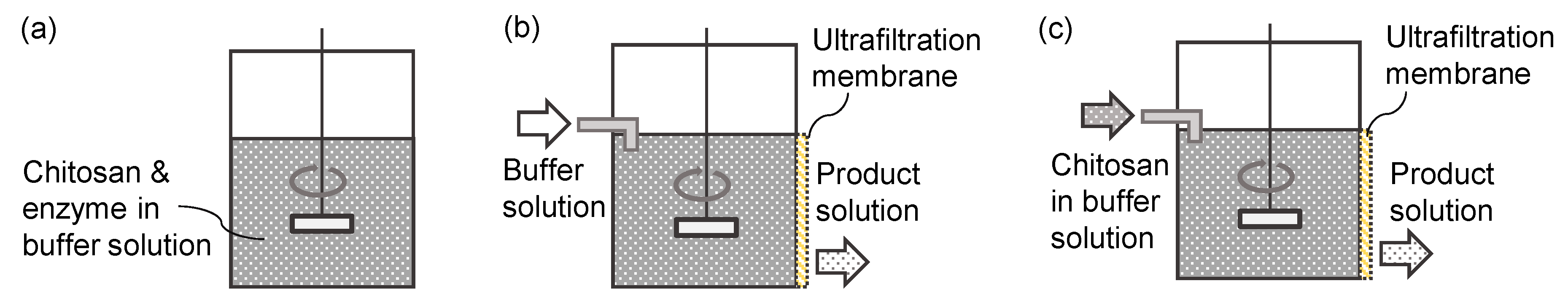
2.2. Batch Hydrolysis
2.3. Membrane Permeation
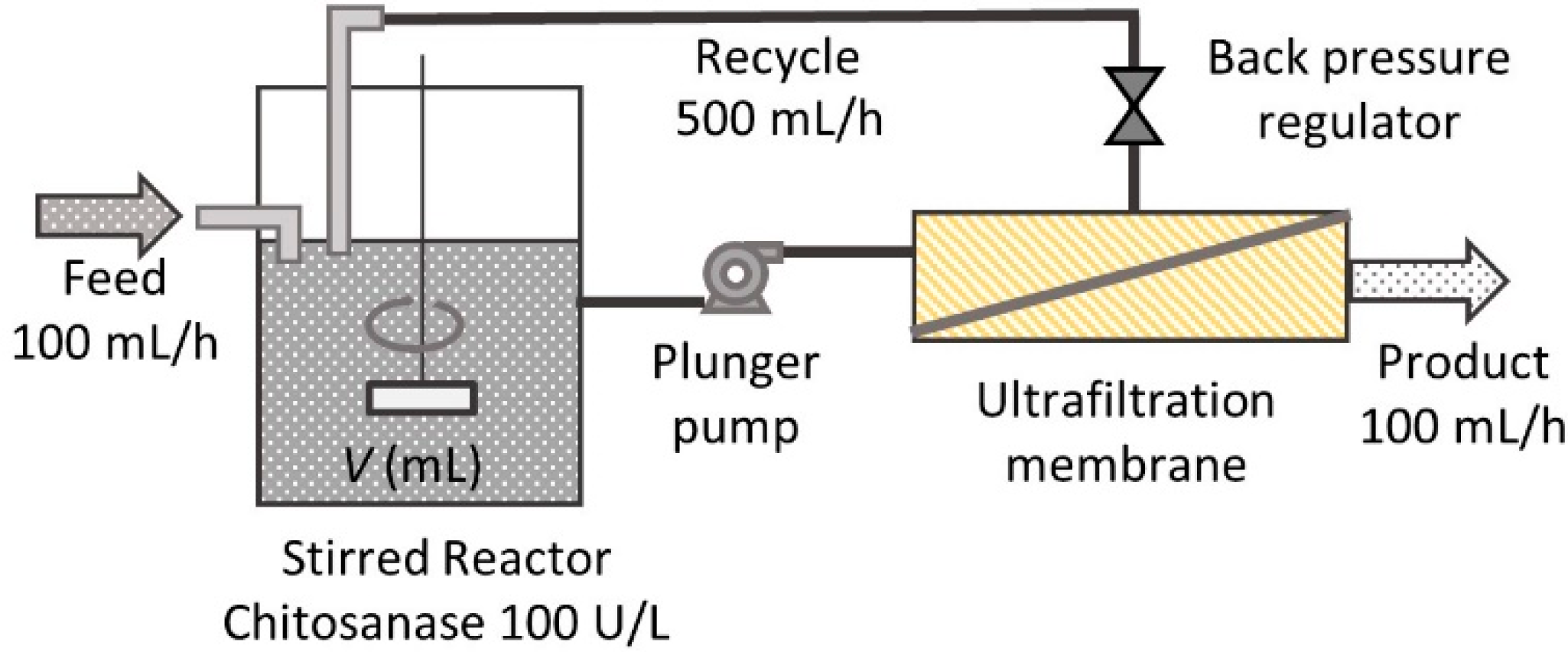
2.4. Continuous Hydrolysis with Membrane Reactor
3. Results and Discussion
3.1. Reaction Modeling and Permeation Evaluation
3.2. Reactor Modeling and Comparison
3.3. Experimental Validation
4. Conclusions
- (1)
- A tight membrane with a small MWCO value close to the target oligomer does not necessarily improve the reactor performance. Complete rejection of larger oligomers is difficult, even with the smallest MWCO. A coarse membrane with a larger MWCO is recommended to reduce the fouling risks and operating pressure;
- (2)
- Rejection of the membrane permeation concentrates the reacting medium at the residue side. Thus, a membrane reactor with continuous feeding of the reactant and recycling of the retentate solution can produce a higher yield in a shorter residence time than the batch reactor;
- (3)
- A membrane reactor with the semi-batch operation mode can obtain the target oligomer in a higher yield than with the continuous operation mode. However, the productivity is lower than the batch reactor because the permeation rate and concentration of the product are in a trade-off relationship;
- (4)
- Experimental results have an acceptable agreement with the model prediction. The membrane reactor model with the simplified reaction rate equations helps in designing the reactor blueprint. The discrepancy in the optimum residence time and yield will be reduced by considering the binding inhibition effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme Membrane Reactors for Production of Oligosaccharides: A Review on the Interdependence between Enzyme Reaction and Membrane Separation. Sep. Purif. Technol. 2020, 243, 116840. [Google Scholar] [CrossRef]
- Karnaouri, A.; Matsakas, L.; Bühler, S.; Muraleedharan, M.N.; Christakopoulos, P.; Rova, U. Tailoring Celluclast® Cocktail’s Performance towards the Production of Prebiotic Cello-Oligosaccharides from Waste Forest Biomass. Catalysts 2019, 9, 897. [Google Scholar] [CrossRef]
- Das, R.; Sen, D.; Sarkar, A.; Bhattacharyya, S.; Bhattacharjee, C. A Comparative Study on the Production of Galacto-Oligosaccharide from Whey Permeate in Recycle Membrane Reactor and in Enzymatic Batch Reactor. Ind. Eng. Chem. Res. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Botelho, V.A.; Mateus, M.; Petrus, J.C.C.; de Pinho, M.N. Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides. Membranes 2022, 12, 171. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Izuta, H.; Nabetani, H.; Nakajima, M.; Sato, S.; Mukataka, S.; Ichikawa, S. Selective and Stable Production of Physiologically Active Chitosan Oligosaccharides Using an Enzymatic Membrane Bioreactor. Process Biochem. 2009, 44, 283–287. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Pinelo, M.; Wan, Y. Directing Filtration to Narrow Molecular Weight Distribution of Oligodextran in an Enzymatic Membrane Reactor. J. Membr. Sci. 2018, 555, 268–279. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Kovacs, Z.; Quitmann, H.; Ebrahimi, M.; Czermak, P. Enzymatic Production of Fructooligosaccharides from Inexpensive and Abundant Substrates Using a Membrane Reactor System. Sep. Sci. Technol. 2016, 51, 1537–1545. [Google Scholar] [CrossRef]
- Burghardt; Coletta; van der Bolt; Ebrahimi; Gerlach; Czermak Development and Characterization of an Enzyme Membrane Reactor for Fructo-Oligosaccharide Production. Membranes 2019, 9, 148. [CrossRef]
- Baldassarre, S.; Babbar, N.; Van Roy, S.; Dejonghe, W.; Maesen, M.; Sforza, S.; Elst, K. Continuous Production of Pectic Oligosaccharides from Onion Skins with an Enzyme Membrane Reactor. Food Chem. 2018, 267, 101–110. [Google Scholar] [CrossRef]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the Manufacture, Purification and Applications of Xylo-Oligosaccharides as Food Additives and Nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Swiatkiewicz, M.; Arczewska-Wlosek, A.; Jozefiak, D. Chitosan and Its Oligosaccharide Derivatives (Chito-Oligosaccharides) as Feed Supplements in Poultry and Swine Nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nedaei, S.; Noori, A.; Valipour, A.; Khanipour, A.A.; Hoseinifar, S.H. Effects of Dietary Galactooligosaccharide Enriched Commercial Prebiotic on Growth Performance, Innate Immune Response, Stress Resistance, Intestinal Microbiota and Digestive Enzyme Activity in Narrow Clawed Crayfish (Astacus Leptodactylus Eschscholtz, 1823). Aquaculture 2019, 499, 80–89. [Google Scholar] [CrossRef]
- Qin, Z.; Luo, S.; Li, Y.; Chen, Q.; Qiu, Y.; Zhao, L.; Jiang, L.; Zhou, J. Biochemical Properties of a Novel Chitosanase from Bacillus Amyloliquefaciens and Its Use in Membrane Reactor. LWT 2018, 97, 9–16. [Google Scholar] [CrossRef]
- Asano, S.; Yatabe, S.; Maki, T.; Mae, K. Numerical and Experimental Quantification of the Performance of Microreactors for Scaling-up Fast Chemical Reactions. Org. Process Res. Dev. 2019, 23, 807–817. [Google Scholar] [CrossRef]
- Asano, S.; Maki, T.; Nakayama, R.; Utsunomiya, R.; Muranaka, Y.; Kuboyama, T.; Mae, K. Precise Analysis and Control of Polymerization Kinetics Using a Micro Flow Reactor. Chem. Eng. Process. Process Intensif. 2017, 119, 73–80. [Google Scholar] [CrossRef]
- Asano, S.; Maki, T.; Inoue, S.; Sogo, S.; Furuta, M.; Watanabe, S.; Muranaka, Y.; Kudo, S.; Hayashi, J.; Mae, K. Incorporative Mixing in Microreactors: Influence on Reactions and Importance of Inlet Designation. Chem. Eng. J. 2023, 451, 138942. [Google Scholar] [CrossRef]
- Fan, R.; Burghardt, J.P.; Dresler, J.; Czermak, P. Process Design for the Production of Prebiotic Oligosaccharides in an Enzyme Membrane Bioreactor: Interaction between Enzymatic Reaction and Membrane Filtration. Chem. Ing. Tech. 2021, 93, 306–310. [Google Scholar] [CrossRef]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Al-Zuhair, S. Dynamic Model of Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Membrane Bioreactor. Biochem. Eng. J. 2021, 174, 108107. [Google Scholar] [CrossRef]
- Sinha, S.; Chand, S.; Tripathi, P. Production, Purification and Characterization of a New Chitosanase Enzyme and Improvement of Chitosan Pentamer and Hexamer Yield in an Enzyme Membrane Reactor. Biocatal. Biotransform. 2014, 32, 208–213. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B.H.; Zhang, D. Examining the Interplay between Streptococcus agalactiae, the Biopolymer Chitin and Its Derivative. MicrobiologyOpen 2019, 8, e00733. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The Effect of Preparation Processes on the Physicochemical Characteristics and Antibacterial Activity of Chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Noguchi, Y.; Nakajima, M.; Sato, S.; Mukataka, S.; Ichikawa, S. Production of Chitosan Oligosaccharides Using Chitosanase Immobilized on Amylose-Coated Magnetic Nanoparticles. Process Biochem. 2008, 43, 62–69. [Google Scholar] [CrossRef]
- Fukamizo, T.; Honda, Y.; Goto, S.; Boucher, I.; Brzezinski, R. Reaction Mechanism of Chitosanase from Streptomyces Sp. N174. Biochem. J. 1995, 311, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fukamizo, T.; Yoshikawa, T.; Katsumi, T.; Amano, S.; Miki, K.; Ando, A.; Brzezinski, R. Substrate-Binding Mode of Bacterial Chitosanases. J. Appl. Glycosci. 2005, 52, 7. [Google Scholar] [CrossRef][Green Version]
- Boucher, I.; Fukamizo, T.; Honda, Y.; Willick, G.E.; Neugebauer, W.A.; Brzezinski, R. Site-Directed Mutagenesis of Evolutionary Conserved Carboxylic Amino Acids in the Chitosanase from Streptomyces Sp. N174 Reveals Two Residues Essential for Catalysis. J. Biol. Chem. 1995, 270, 6. [Google Scholar] [CrossRef]
- Neggaz, Y.; Vargas, M.L.; Dris, A.O.; Riera, F.; Alvarez, R. A Combination of Serial Resistances and Concentration Polarization Models along the Membrane in Ultrafiltration of Pectin and Albumin Solutions. Sep. Purif. Technol. 2007, 54, 18–27. [Google Scholar] [CrossRef]
- García, A.; Álvarez, S.; Riera, F.; Álvarez, R.; Coca, J. Sunflower Oil Miscella Degumming with Polyethersulfone Membranes. J. Food Eng. 2006, 74, 516–522. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Wong, F.-S. A New Method for the Prediction of Pore Size Distribution and MWCO of Ultrafiltration Membranes. J. Membr. Sci. 2006, 279, 558–569. [Google Scholar] [CrossRef]
- Długołęcki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the Resistances of Membrane, Diffusion Boundary Layer and Double Layer in Ion Exchange Membrane Transport. J. Membr. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Arahman, N.; Jakfar, J.; Dzulhijjah, W.A.; Halimah, N.; Silmina, S.; Aulia, M.P.; Fahrina, A.; Bilad, M.R. Hydrophilic Antimicrobial Polyethersulfone Membrane for Removal of Turbidity of Well-Water. Water 2022, 14, 3769. [Google Scholar] [CrossRef]
- Saleem, H.; Goh, P.S.; Saud, A.; Khan, M.A.W.; Munira, N.; Ismail, A.F.; Zaidi, S.J. Graphene Quantum Dot-Added Thin-Film Composite Membrane with Advanced Nanofibrous Support for Forward Osmosis. Nanomaterials 2022, 12, 4154. [Google Scholar] [CrossRef]
- Tian, H.; Wu, X.; Zhang, K. Tailoring Morphology and Properties of Tight Utrafiltration Membranes by Two-Dimensional Molybdenum Disulfide for Performance Improvement. Membranes 2022, 12, 1071. [Google Scholar] [CrossRef] [PubMed]






| Value (h−1) | Standard Error | Correlation Coefficient | ||||
|---|---|---|---|---|---|---|
| (h−1) | (%) | |||||
| 9.64 | 0.502 | 5.2 | 1 | - | - | |
| 2.91 | 0.273 | 9.4 | 0.49 | 1 | - | |
| 4.78 | 0.436 | 9.1 | −0.318 | −0.797 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, S.; Muranaka, Y.; Maki, T.; Ikeda, K.; Mae, K. Kinetic Modeling of an Enzyme Membrane Reactor for the Selective Production of Oligosaccharides. Fermentation 2022, 8, 701. https://doi.org/10.3390/fermentation8120701
Asano S, Muranaka Y, Maki T, Ikeda K, Mae K. Kinetic Modeling of an Enzyme Membrane Reactor for the Selective Production of Oligosaccharides. Fermentation. 2022; 8(12):701. https://doi.org/10.3390/fermentation8120701
Chicago/Turabian StyleAsano, Shusaku, Yosuke Muranaka, Taisuke Maki, Koki Ikeda, and Kazuhiro Mae. 2022. "Kinetic Modeling of an Enzyme Membrane Reactor for the Selective Production of Oligosaccharides" Fermentation 8, no. 12: 701. https://doi.org/10.3390/fermentation8120701
APA StyleAsano, S., Muranaka, Y., Maki, T., Ikeda, K., & Mae, K. (2022). Kinetic Modeling of an Enzyme Membrane Reactor for the Selective Production of Oligosaccharides. Fermentation, 8(12), 701. https://doi.org/10.3390/fermentation8120701