Growth of Coniochaeta Species on Acetate in Biomass Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Rice Hull Dilute Acid Hydrolysate (RHH)
2.3. Metabolism of Sugars and Inhibitors in RHH Flask Cultures
2.4. Growth in Microbioreactors
2.5. Growth in Minibioreactors
2.6. Analytical Methods
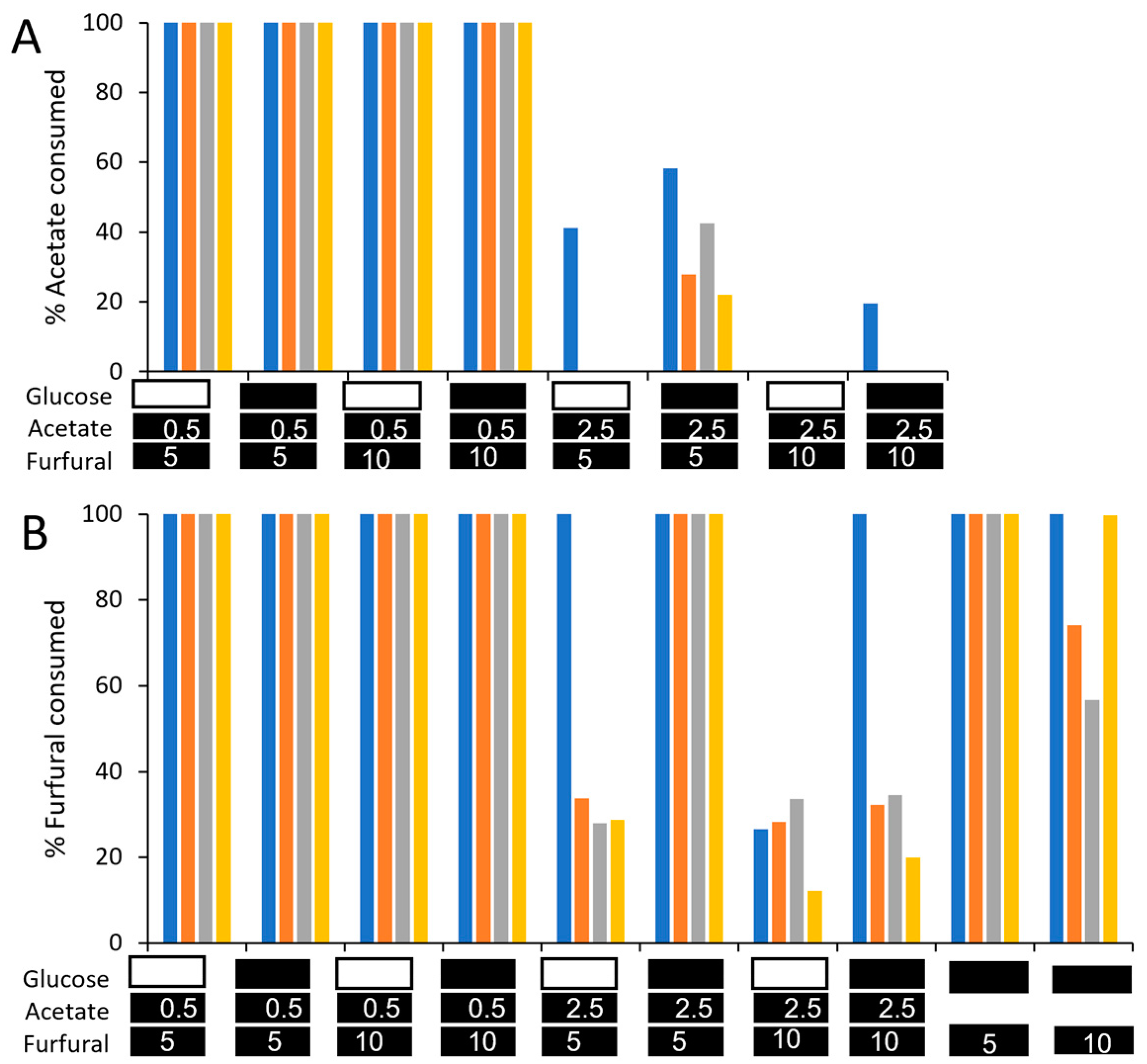
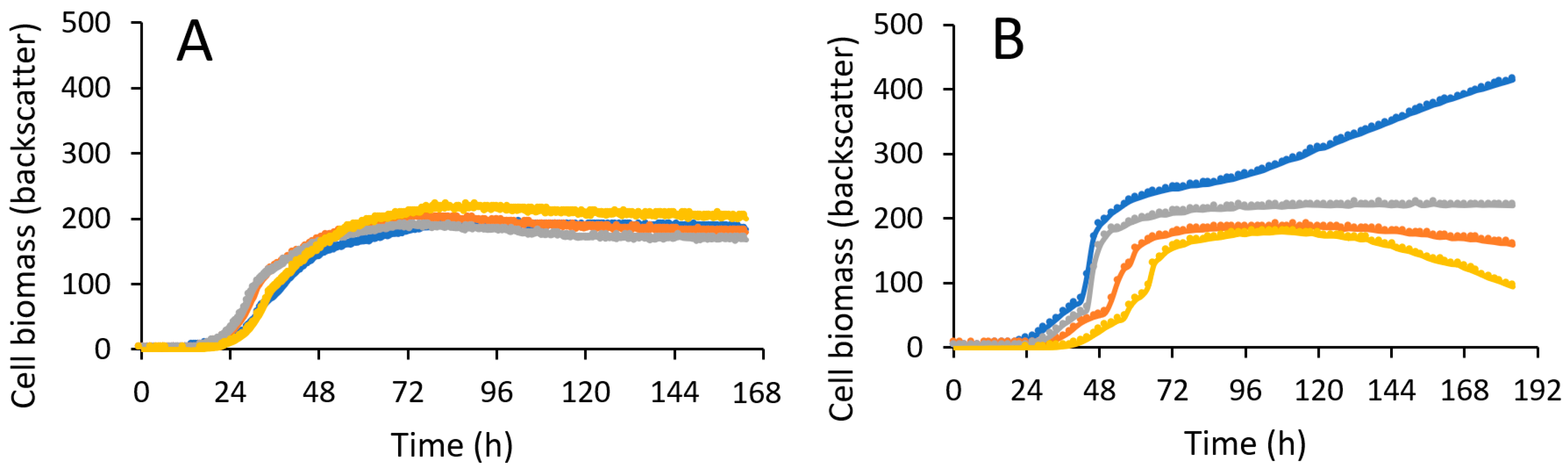
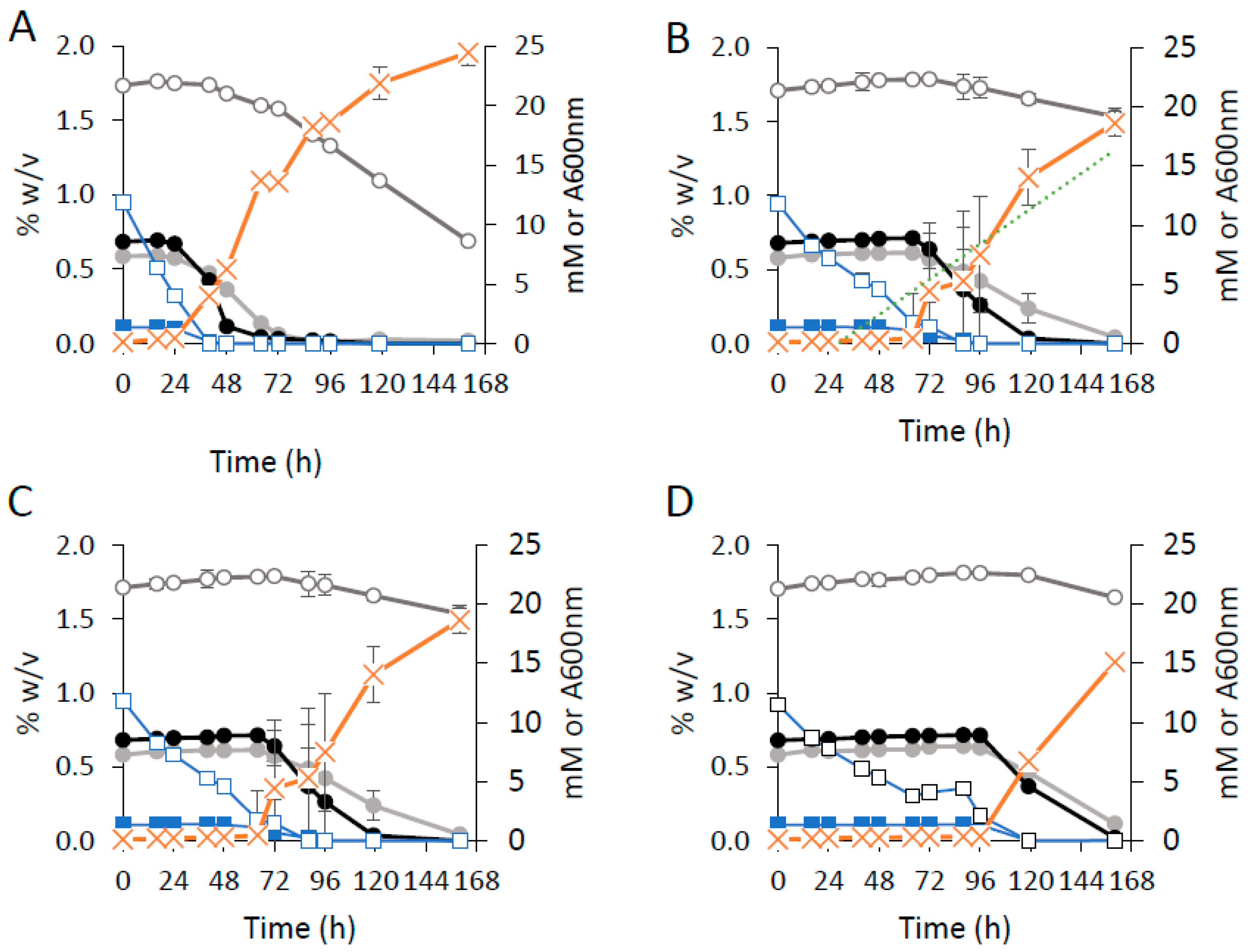
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.-O. The generation of fermentation inhibitors during dilute aid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mulako, I.; Tuffin, M.; Cowan, D. Understanding physiological responses to pre-treatment inhibitors in ethanologenic fermentations. Biotechnol. J. 2012, 7, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Runquist, D.; Nogué, V.S.I.; Lidén, G.; Gorwa-Grauslund, M.F. Stress-related challenges in pentose fermentation to ethanol by the yeast Saccharomyces cerevisiae. Biotechnol. J. 2011, 6, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M.T. Hemicelluloses. In Encylopedia of Food Science Food Technology and Nutrition, 2nd ed.; Macrae, R., Robinson, R.K., Sadler, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3060–3071. [Google Scholar] [CrossRef]
- Johnson, A.M.; Kim, H.; Ralph, J.; Mansfield, S.D. Natural acetylation impacts carbohydrate recovery during deconstruction of Populus trichocarpa wood. Biotechnol. Biofuels 2017, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegas, R.; Kabel, M.; Schols, H.A.; Alonso, J.L.; Parajó, J.C. Hydrothermal processing of rice husks: Effects of severity on product distribution. J. Chem. Technol. Biotechnol. 2008, 83, 965–972. [Google Scholar] [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; de Jong, H. Acetate metabolism and the inhibition of bacterial growth by acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef] [Green Version]
- Luli, G.W.; Strohl, W.R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 1990, 56, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.; Bellissimi, E.; de Hulster, E.; Wagner, A.; Pronk, J.T.; van Maris, A.J.A. Batch and continuous culture-based selection strategies for acetic acid tolerance in xylose-fermenting Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-G.; Liu, X.-Y.; He, X.-P.; Guo, X.-N.; Lu, Y.; Zhang, B. Improvement of acetic acid tolerance and fermentation performance of Saccharomyces cerevisiae by disruption of the FPS1 aquaglyceroporin gene. Biotechnol. Lett. 2011, 33, 277–284. [Google Scholar] [CrossRef]
- Helle, S.; Cameron, D.; Lam, J.; White, B.; Duff, S. Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzym. Microb. Technol. 2003, 33, 786–792. [Google Scholar] [CrossRef]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bellissimi, E.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J.A. Effect of acetic acid on the kinetics of xylose fermentation by an engineered, xylose-isomerase-based Saccharomyces cerevisiae strain. FEMS Yeast Res. 2009, 9, 358–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasunuma, T.; Sanda, T.; Yamada, R.; Yoshimura, K.; Ishii, J.; Kondo, A. Metabolic pathway engineering based on metabolomics confers acetic and formic acid tolerance to a recombinant xylose-fermenting strain of Saccharomyces cerevisiae. Microb. Cell Factories 2011, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inan, M.; Meagher, M.M. The effect of ethanol and acetate on protein expression in Pichia pastoris. J. Biosci. Bioeng. 2001, 92, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, B.M.; Hon, S.; Covalla, S.F.; Sonu, C.; Argyros, D.A.; Barret, T.F.; Wiswall, E.; Froehlich, A.C.; Zelle, R.M. Increasing anaerobic acetate consumption and ethanol yields in Saccharomyces cerevisiae with NADPH-specific alcohol dehydrogenase. Appl. Environ. Microbiol. 2015, 81, 8108–8117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, N.; Quarterman, J.; Kim, S.R.; Cate, J.H.D.; Jin, Y.-S. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat. Commun. 2013, 4, 2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapetridis, I.; van Dijk, M.; van Maris, A.J.A.; Pronk, J.T. Metabolic engineering strategies for optimizing acetate reduction, ethanol yield and osmotolerance in Saccharomyces cerevisiae. Biotechnol. Biofuels 2017, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Zhang, L.; Ladisch, M. Biological abatement of cellulase inhibitors. Bioresour. Technol. 2013, 146, 604–610. [Google Scholar] [CrossRef]
- Nichols, N.N.; Hector, R.E.; Saha, B.C.; Frazer, S.E.; Kennedy, G.J. Biological abatement of inhibitors in rice hull hydrolysate and fermentation to ethanol using conventional engineered microbes. Biomass Bioenergy 2014, 67, 79–88. [Google Scholar] [CrossRef]
- Nichols, N.N.; Dien, B.S.; Guisado, G.M.; López, M.J. Bioabatement to remove inhibitors from biomass-derived sugar hydrolysates. Appl. Biochem. Biotechnol. 2005, 121–124, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Sharma, L.H.; Mowery, R.A.; Chambliss, C.K.; van Walsum, G.P.; Dien, B.S.; Iten, L.B. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzym. Microb. Technol. 2008, 42, 624–630. [Google Scholar] [CrossRef]
- Donoso, R.A.; González-Toro, F.G.; Pérez-Pantoja, D. Widespread distribution of hmf genes in Proteobacteria reveals key enzymes for 5-hydroxymethylfurfural conversion. Comput. Struct. Biotechnol. J. 2021, 19, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Dien, B.S.; Cotta, M.A. Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour. Technol. 2010, 101, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Mertens, J.A.; Dien, B.S.; Hector, R.E.; Frazer, S.E. Recycle of fermentation process water through mitigation of inhibitors in dilute-acid corn stover hydrolysate. Bioresour. Technol. Rep. 2020, 9, 100349. [Google Scholar] [CrossRef]
- Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. (Eds.) Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington DC, USA, 1994. [Google Scholar]
- López, M.J.; Nichols, N.N.; Dien, B.S.; Moreno, J.; Bothast, R.J. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl. Microbiol. Bitoechnol. 2004, 64, 125–131. [Google Scholar] [CrossRef]
- Weber, E.; Görke, C.; Begerow, D. The Lecythophora-Coniochaeta complex II. Molecular studies based on sequences of the large subunit of ribosomal DNA. Nova Hedwig. 2002, 74, 187–200. [Google Scholar] [CrossRef]
- Samorski, M.; Müller-Newen, G.; Büchs, J. Quasi-continuous combined scattered light and fluorescence measurements: A novel measurement technique for shaken microtiter plates. Biotechnol. Bioeng. 2005, 92, 61–68. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef]
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism—Cell Homeostasis and Stress Response; Bubulya, P., Ed.; InTech: London, UK, 2012; Available online: https://www.intechopen.com/chapters/26771 (accessed on 7 December 2022). [CrossRef]
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol. Biofuels 2009, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Verma, A.; Singhania, R.R.; Varjani, S.; Dong, C.D.; Patel, A.K. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour. Technol. 2021, 332, 125042. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Quarterman, J.C.; Frazer, S.E. Use of green fluorescent protein to monitor fungal growth in biomass hydrolysate. Biol. Methods Protoc. 2018, 3, bpx012. [Google Scholar] [CrossRef] [PubMed]

| Strain | Microorganism | Source or Reference |
|---|---|---|
| 1 | Coniochaeta ligniaria NRRL 30616 a | [28] |
| 2 | Lecythophora hoffmannii NRRL 31961 a | DSM2693 b |
| 3 | Lecythophora mutabilis NRRL 31962 a | DSM10716 b |
| 4 | Lecythophora lignicola NRRL 31963 a | DSM63551 b |
| 5 | Coniochaeta ligniaria NRRL 32068 a | 95.605 c |
| 6 | Coniochaeta ligniaria NRRL 32069 a | 98.1105 c |
| 7 | Coniochaeta ligniaria NRRL 32070 a | 98.1126 c |
| 8 | Coniochaeta ligniaria NRRL 32071 a | F3331 c |
| 9 | Coniochaeta ligniaria NRRL 32072 a | F3343 c |
| 10 | Phialophora decumbens NRRL 32073 a | CBS153.42 d |
| 11 | Phialophora fasciculatus NRRL 32074 a | CBS205.38 d |
| 14 | Lecythophora lignicola NRRL 32077 a | CBS267.33 d |
| 16 | Lecythophora mutabilis NRRL 32080 a | CBS157.44 d |
| 17 | Lecythophora mutabilis NRRL 32081 a | CBS303.62 d |
| 18 | Coniochaeta ligniaria NRRL 32082 a | CBS620.69 d |
| 19 | Coniochaeta ligniaria NRRL 32083 a | CBS178.75 d |
| 20 | Coniochaeta malacotricha NRRL 32084 a | CBS323.72 d |
| 21 | Coniochaeta subcorticalis NRRL 32085 a | CBS551.75 d |
| 22 | Coniochaeta velutina NRRL 32086 a | CBS176.59 d |
| 23 | Coniochaeta velutina NRRL 32087 a | CBS948.72 d |
| 24 | Coniochaeta velutina NRRL 32088 a | CBS981.68 d |
| Strain | Acetate (% w/v) | |||||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.5 | 5.0 | 7.5 | 10.0 | |
| 4 | 174.3 | 186.9 | 196.9 | 0 | 0 | 0 |
| 14 | 155.0 | 194.4 | 200.3 | 0 | 0 | 0 |
| 17 | 126.3 | 117.5 | 109.6 | 0 | 0 | 0 |
| 19 | 162.0 | 147.7 | 111.1 | 0 | 0 | 0 |
| 20 | 160.3 | 189.9 | 137.3 | 0 | 0 | 0 |
| 21 | 126.4 | 126.1 | 146.0 | 0 | 0 | 0 |
| 22 | 181.6 | 192.7 | 193.2 | 0 | 0 | 0 |
| 23 | 172.5 | 223.5 | 259.1 | 0 | 0 | 0 |
| 24 | 193.6 | 285.1 | 281.7 | 0 | 0 | 0 |
| 1 | 128.1 | 149.6 | 187.7 | 156.3 | 14.9 | 14.2 |
| 3 | 131.3 | 139.9 | 170.0 | 126.1 | 21.9 | 22.3 |
| 7 | 132.4 | 175.3 | 171.5 | 122.7 | 25.6 | 10.6 |
| 10 | 123.1 | 136.3 | 118.5 | 127.7 | 5.8 | 18.2 |
| 11 | 187.3 | 159.5 | 121.9 | 87.2 | 17.2 | 20.0 |
| 16 | 90.2 | 98.9 | 143.6 | 142.6 | 30.0 | 29.3 |
| 18 | 116.1 | 145.8 | 213.4 | 107.5 | 1.5 | 14.6 |
| 5 | 154.2 | 192.6 | 183.6 | 179.1 | 96.6 | 50.0 |
| 6 | 127.3 | 148.8 | 158.2 | 137.3 | 20.2 | 49.4 |
| 8 | 191.5 | 173.7 | 143.9 | 189.5 | 67.7 | 16.1 |
| 9 | 153.3 | 124.1 | 118.4 | 132.4 | 100.6 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichols, N.N.; Mertens, J.A.; Frazer, S.E.; Hector, R.E. Growth of Coniochaeta Species on Acetate in Biomass Sugars. Fermentation 2022, 8, 721. https://doi.org/10.3390/fermentation8120721
Nichols NN, Mertens JA, Frazer SE, Hector RE. Growth of Coniochaeta Species on Acetate in Biomass Sugars. Fermentation. 2022; 8(12):721. https://doi.org/10.3390/fermentation8120721
Chicago/Turabian StyleNichols, Nancy N., Jeffrey A. Mertens, Sarah E. Frazer, and Ronald E. Hector. 2022. "Growth of Coniochaeta Species on Acetate in Biomass Sugars" Fermentation 8, no. 12: 721. https://doi.org/10.3390/fermentation8120721
APA StyleNichols, N. N., Mertens, J. A., Frazer, S. E., & Hector, R. E. (2022). Growth of Coniochaeta Species on Acetate in Biomass Sugars. Fermentation, 8(12), 721. https://doi.org/10.3390/fermentation8120721








