Contribution of Fermentation Technology to Building Blocks for Renewable Plastics
Abstract
:1. Introduction
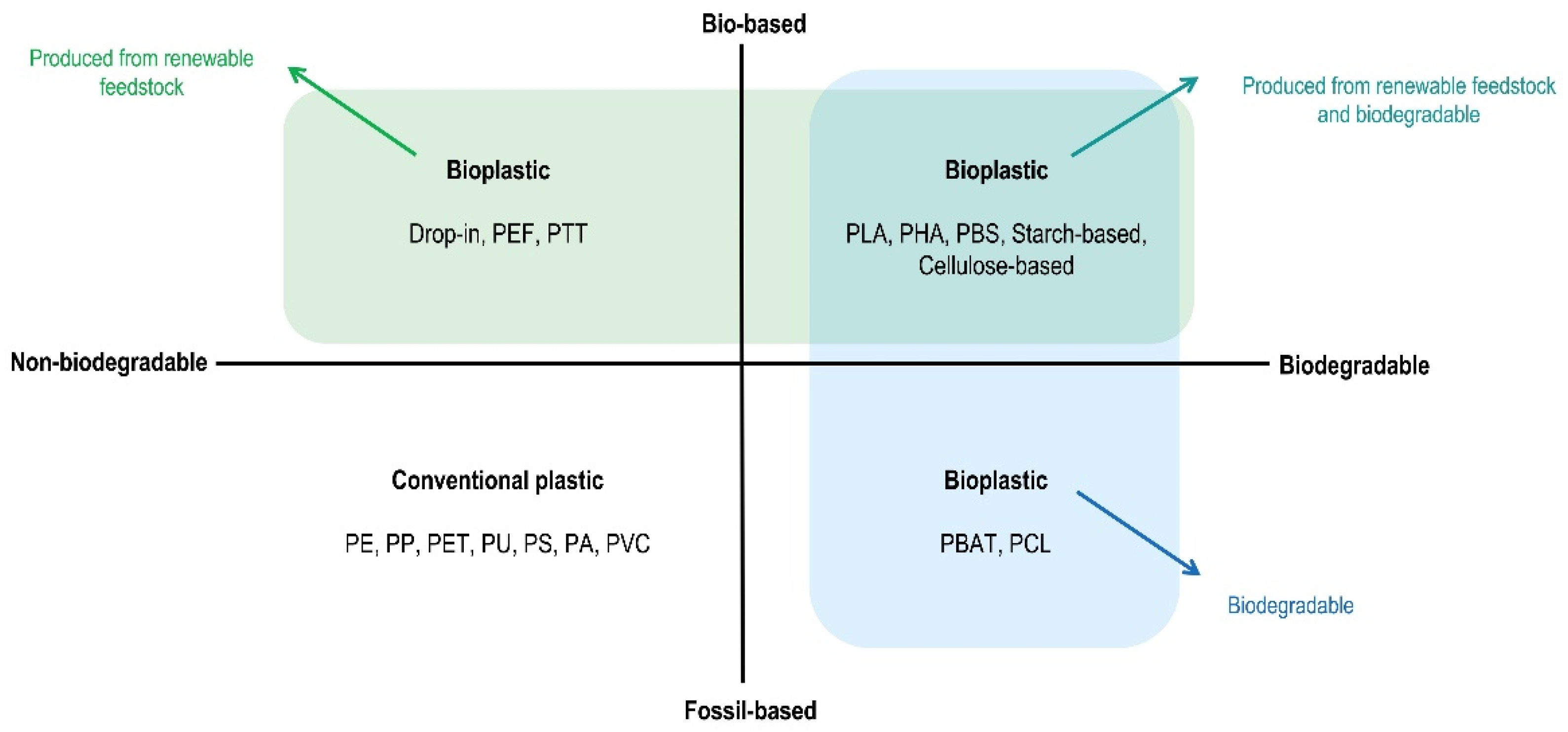
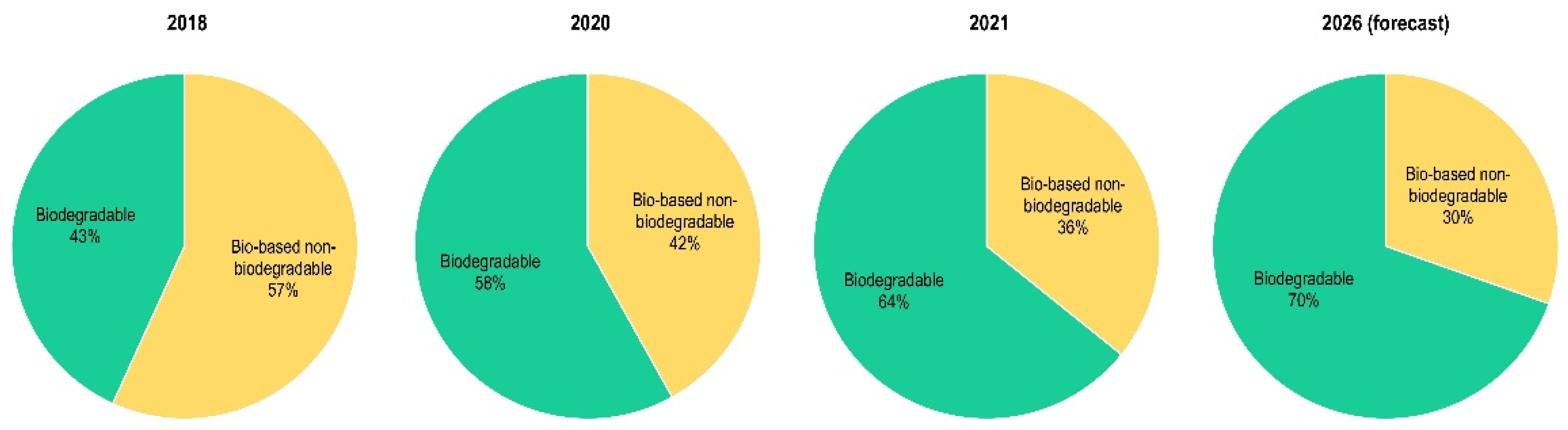
2. Renewable Plastics
2.1. Poly(ethylene furanoate) (PEF)
2.2. Poly(trimethylene terephthalate) (PTT)
2.3. Drop-In Plastic
2.3.1. Bio-Polyamides (Bio-PA)
2.3.2. Bio-Polyethylene (Bio-PE)
2.3.3. Bio-Polyethylene Terephthalate (Bio-PET)
2.3.4. Bio-Polypropylene (Bio-PP)
2.3.5. Bio-Polyurethane (Bio-PU)
2.4. Starch-Based Plastic
2.5. Cellulose-Based Plastic
2.6. Poly(lactic acid) (PLA)
2.7. Polyhydroxyalkanoates (PHA)
2.8. Polybutylene Adipate-co-Terephthalate (PBAT)
2.9. Polybutylene Succinate (PBS)
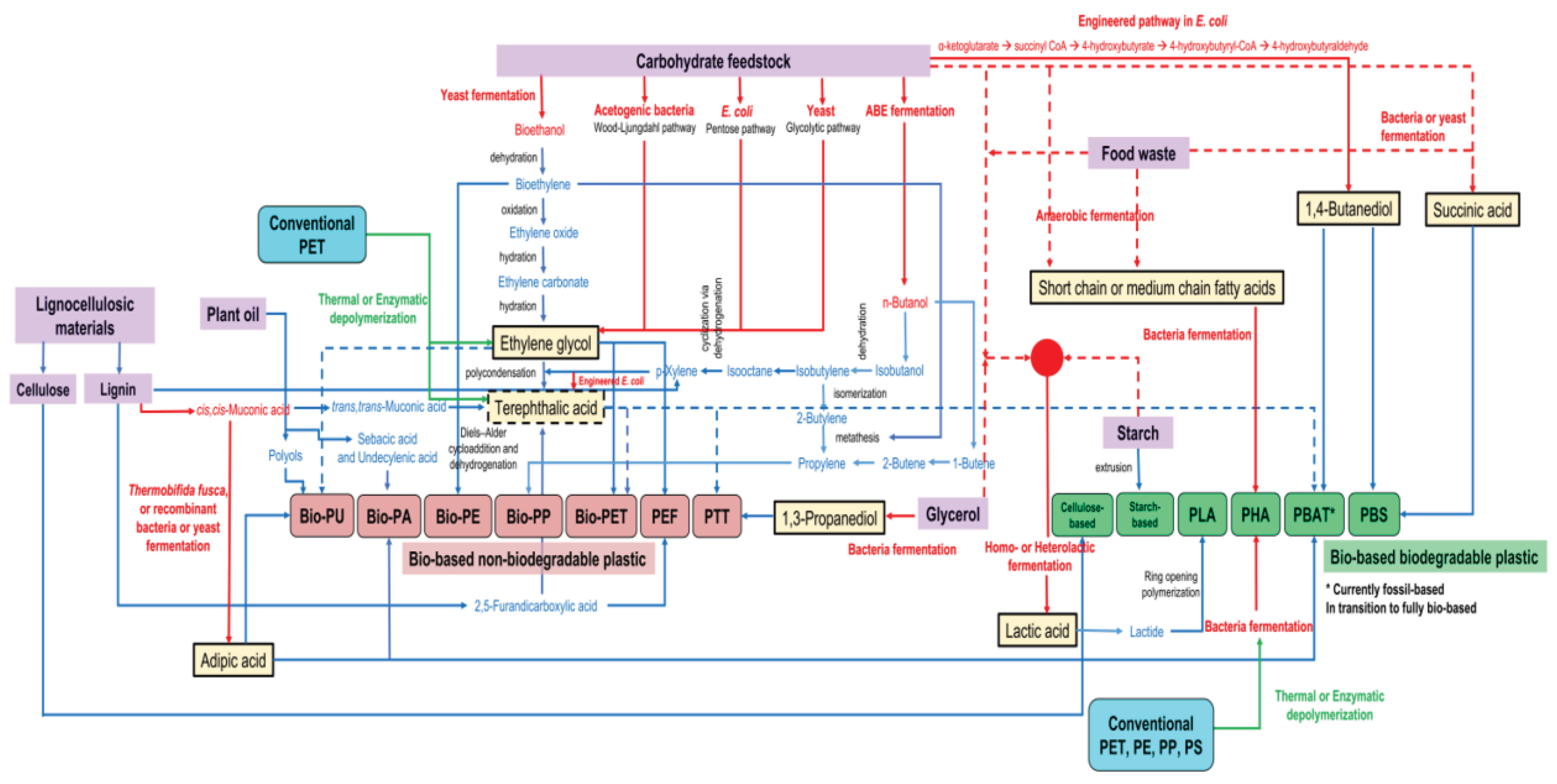
3. Fermentation Technology Providing Building Blocks for Renewable Plastics
3.1. 1,4-Butanediol (1,4-BDO)
3.2. 1,3-Propanediol (1,3-PDO)
3.3. Lactic Acid
3.4. Succinic Acid
3.5. Adipic Acid
3.6. New Emerging Bioplastic Monomers
3.6.1. Azelaic Acid
3.6.2. Lactones
3.6.3. 6-Hydroxyhexanoic Acid (6HA)
3.6.4. Itaconic Acid
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.G.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Jenkins, S.; Quer, A.M.I.; Fonseca, C.; Varrone, C. Microbial degradation of plastics: New plastic degraders, mixed cultures and engineering strategies. In Soil Microenvironment for Bioremediation and Polymer Production; Jamil, N., Kumar, P., Batool, R., Eds.; Wiley Online Book: Hoboken, NJ, USA, 2019; Chapter 12; pp. 215–238. ISBN 9781119592129. [Google Scholar]
- PlasticsEurope the Facts 2020. Available online: https://www.plasticseurope.org/application/files/3416/2270/7211/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 3 September 2021).
- European Environmental Agency. Plastics, the Circular Economy and Europe′s Environment—A Priority for Action; European Environmental Agency: Copenhagen, Denmark, 2021. [Google Scholar]
- Hamilton, L.A.; Feit, S.; Muffett, C.; Kelso, M.; Rubright, S.M.; Bernhardt, C.; Schaeffer, E.; Moon, D.; Morris, J.; Labbé-Bellas, R. Plastic & Climate the Hidden Costs of a Plastic Planet; Center for International Environmental Law (CIEL): Washington, DC, USA, 2019. [Google Scholar]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency Facts and Figures about Materials, Waste and Recycling. Available online: https://www.epa.gov/facts-and-s-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 20 December 2021).
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent Advances in Sustainable Plastic Upcycling and Biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Rennison, A.; Winther, J.R.; Varrone, C. Rational Protein Engineering to Increase the Activity and Stability of IsPETase Using the PROSS Algorithm. Polymers 2021, 13, 3884. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; Process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2021, 1–21. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Biorefin. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. Biosynthesis of polyesters and polyamide building blocks using microbial fermentation and biotransformation. Rev. Environ. Sci. Bio/Technol. 2016, 15, 639–663. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- European Bioplastics. Nova-Institute Bioplastics Market Data 2020. Available online: https://www.european-bioplastics.org/market/ (accessed on 8 September 2021).
- European Bioplastics. Nova-Institute Bioplastics Market Development Update 2021. Available online: https://www.european-bioplastics.org/global-bioplastics-production-will-more-than-triple-within-the-next-five-years/ (accessed on 12 December 2021).
- European Bioplastics Fact Sheet: What Are Bioplastics? Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 20 December 2021).
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- European Bioplastics; Nova-Institute Bioplastics Market Data 2018. Available online: https://www.european-bioplastics.org/wp-content/uploads/2016/02/Report_Bioplastics-Market-Data_2018.pdf (accessed on 20 December 2021).
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic production of 2,5-furandicarboxylic acid: Recent advances and future perspectives. Appl. Microbiol. Biotechnol. 2019, 104, 527–543. [Google Scholar] [CrossRef]
- Hwang, K.R.; Jeon, W.; Lee, S.Y.; Kim, M.S.; Park, Y.K. Sustainable bioplastics: Recent progress in the production of bio-building blocks for the bio-based next-generation polymer PEF. Chem. Eng. J. 2020, 390, 124636. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Laboratory (PNNL) and National Renewable Energy Laboratory (NREL): Richland, WA, USA, 2004. [CrossRef] [Green Version]
- Avantium Chemicals BV Sustainable and Carbon-Efficient Mono-Ethylene Glycol Generation in Demonstration PLANT. Available online: https://cordis.europa.eu/project/id/822956 (accessed on 12 December 2021).
- Scott, A. Avantium Starts Producing Biobased Ethylene Glycol. Available online: https://cen.acs.org/business/biobased-chemicals/Avantium-starts-producing-biobased-ethylene/97/i45 (accessed on 12 December 2021).
- Roux, M.; Varrone, C. Assessing the Economic Viability of the Plastic Biorefinery Concept and Its Contribution to a More Circular Plastic Sector. Polymers 2021, 13, 3883. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, S.; Haernvall, K.; Scaini, D.; Ghazaryan, G.; Zumstein, M.T.; Sander, M.; Pellis, A.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chem. 2017, 19, 5381–5384. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.R.; Coelho, J.F.J.; Silvestre, A.J.D.; Matos, M.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; et al. A New Generation of Furanic Copolyesters with Enhanced Degradability: Poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) Copolyesters. Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Cao, F.; Wen, B.; Zhu, X.; Wei, P. Chemosynthesis and characterization of fully biomass-based copolymers of ethylene glycol, 2,5-furandicarboxylic acid, and succinic acid. J. Appl. Polym. Sci. 2013, 130, 1415–1420. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Polymer Properties Database Polytrimethylene Terephthalate (PTT). Available online: https://polymerdatabase.com/Polymer%20Brands/PTT.html (accessed on 12 December 2021).
- Zhang, J. Study of Poly(Trimethylene Terephthalate) as an Engineering Thermoplastics Material. J. Appl. Polym. Sci. 2004, 91, 1657–1666. [Google Scholar] [CrossRef]
- Patel, M.; Angerer, G.; Crank, M.; Schleich, J.; Marscheider-Weidemann, F.; Wolf, O.; Hüsing, B. Techno-Economic Feasibility of Large-Scale Production of Bio-Based Polymers in Europe; Wolf, O., Ed.; Techncial Report EUR 22103 EN; European Communities: Sevilla, Spain, 2005; ISBN 9279012304. [Google Scholar]
- Sauer, M.; Marx, H.; Mattanovich, D. Microbial Production of 1,3-Propanediol. Recent Pat. Biotechnol. 2008, 2, 191–197. [Google Scholar] [CrossRef]
- Varrone, C.; Skiadas, I.V.; Gavala, H.N. Effect of hydraulic retention time on the modelling and optimization of joint 1,3 PDO and BuA production from 2G glycerol in a chemostat process. Chem. Eng. J. 2018, 347, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Yang, M.; Zhang, Y.; Zhu, L.; Fan, M.; Li, Q. Selective catalytic synthesis of bio-based terephthalic acid from lignocellulose biomass. Appl. Catal. A Gen. 2021, 630, 118440. [Google Scholar] [CrossRef]
- Luo, Z.W.; Lee, S.Y. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nat. Commun. 2017, 8, 15689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Nguyen, V.; Frost, J.W. Synthesis of terephthalic acid from methane. ACS Sustain. Chem. Eng. 2016, 4, 5998–6001. [Google Scholar] [CrossRef]
- Polymer Properties Database Biopolyamides. Available online: https://polymerdatabase.com/Polymer%20Brands/Biopolyamides.html (accessed on 12 October 2021).
- Bioplastics News Bio-Based Polyamides. Available online: https://bioplasticsnews.com/2016/11/21/bio-based-polyamides/ (accessed on 12 October 2021).
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Salvador, M.; Abdulmutalib, U.; Gonzalez, J.; Kim, J.; Smith, A.A.; Faulon, J.L.; Wei, R.; Zimmermann, W.; Jimenez, J.I. Microbial Genes for a Circular and Sustainable Bio-PET Economy. Genes 2019, 10, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- The Coca-Cola Company Coca-Cola Collaborates with Tech Partners to Create Bottle Prototype Made from 100% Plant-Based Sources. Available online: https://www.coca-colacompany.com/news/100-percent-plant-based-plastic-bottle (accessed on 19 December 2021).
- Barecka, M.H.; Skiborowski, M.; Górak, A. Process Intensification in Practice: Ethylene Glycol Case Study. In Practical Aspects of Chemical Engineering; Ochowiak, M., Doligalski, M., Woziwodzki, S., Mitkowski, P.T., Eds.; Springer: Cham, Switzerland, 2018; pp. 17–34. ISBN 978-3-319-73978-6. [Google Scholar]
- Cabulong, R.B.; Valdehuesa, K.N.G.; Ramos, K.R.M.; Nisola, G.M.; Lee, W.K.; Lee, C.R.; Chung, W.J. Enhanced yield of ethylene glycol production from d-xylose by pathway optimization in Escherichia coli. Enzym. Microb. Technol. 2017, 97, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, M.; Feng, X.; Liu, M.; Zhao, G. Biosynthesis of ethylene glycol from d-xylose in recombinant Escherichia coli. Bioengineered 2018, 9, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.; Zhang, H.; De Mey, M.; Lim, C.G.; Li, Z.J.; Stephanopoulos, G. Engineering a novel biosynthetic pathway in Escherichia coli for production of renewable ethylene glycol. Biotechnol. Bioeng. 2016, 113, 376–383. [Google Scholar] [CrossRef]
- Islam, M.A.; Hadadi, N.; Ataman, M.; Hatzimanikatis, V.; Stephanopoulos, G. Exploring biochemical pathways for mono-ethylene glycol (MEG) synthesis from synthesis gas. Metab. Eng. 2017, 41, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Uranukul, B.; Woolston, B.M.; Fink, G.R.; Stephanopoulos, G. Biosynthesis of monoethylene glycol in Saccharomyces cerevisiae utilizing native glycolytic enzymes. Metab. Eng. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Gevo An Overview of Gevo’s Biobased Isobutananol Production Process. Available online: https://gevo.com/wp-content/uploads/2019/11/Gevo-WP_Isobutanol.1.pdf (accessed on 15 October 2021).
- Carraher, J.M.; Pfennig, T.; Rao, R.G.; Shanks, B.H.; Tessonnier, J.P. cis,cis-Muconic acid isomerization and catalytic conversion to biobased cyclic-C6-1,4-diacid monomers. Green Chem. 2017, 19, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Shiramizu, M.; Toste, F.D. On the Diels-Alder Approach to Solely Biomass-Derived Polyethylene Terephthalate (PET): Conversion of 2,5-Dimethylfuran and Acrolein into p-Xylene. Chem. Eur. J. 2011, 17, 12452. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Kimura, S.; Kasuya, K.I. Synthesis and Verification of Biobased Terephthalic Acid from Furfural. Sci. Rep. 2015, 5, 8249. [Google Scholar] [CrossRef]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.; Ferreira, A.; Materials, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Utomo, R.N.C.; Li, W.J.; Tiso, T.; Eberlein, C.; Doeker, M.; Heipieper, H.J.; Jupke, A.; Wierckx, N.; Blank, L.M. Defined Microbial Mixed Culture for Utilization of Polyurethane Monomers. ACS Sustain. Chem. Eng. 2020, 8, 17466–17474. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of vegetable oil based polyurethane derived from low viscous bio aliphatic isocyanate: Adhesion strength to wood-wood substrate bonding. Macromol. Res. 2017, 25, 772–778. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Singh, R.P.; Pandey, J.K.; Rutot, D.; Degée, P.; Dubois, P. Biodegradation of poly(ε-caprolactone)/starch blends and composites in composting and culture environments: The effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res. 2003, 338, 1759–1769. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Mathiot, C.; Ponge, P.; Gallard, B.; Sassi, J.F.; Delrue, F.; Le Moigne, N. Microalgae starch-based bioplastics: Screening of ten strains and plasticization of unfractionated microalgae by extrusion. Carbohydr. Polym. 2019, 208, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.N.; Canejo, J.P.; Echeverria, C.; Godinho, M.H. Functional materials from liquid crystalline cellulose derivatives: Synthetic routes, characterization and applications. In Liquid Crystalline Polymers; Springer: Cham, Switzerland, 2015; pp. 339–368. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M. Cellulose Plastics. In Brydson’s Plastics Materials: Eighth Edition; Gilbert, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 617–630. ISBN 9780323358248. [Google Scholar]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Zhang, W.; Jing, Z.; Shan, Y.; Ge, X.; Mu, X.; Jiang, Y.; Li, H.; Wu, P. Paper reinforced with regenerated cellulose: A sustainable and fascinating material with good mechanical performance, barrier properties and shape retention in water. J. Mater. Chem. A 2016, 4, 17483–17490. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L.; et al. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Wiley Online Libr. 2019, 30, 1906307. [Google Scholar] [CrossRef]
- Su, Z.; Huang, S.; Wang, Y.; Ling, H.; Yang, X.; Jin, Y.; Wang, X.; Zhang, W. Robust, high-barrier, and fully recyclable cellulose-based plastic replacement enabled by a dynamic imine polymer. J. Mater. Chem. A 2020, 8, 14082–14090. [Google Scholar] [CrossRef]
- Emergen Research Cellulose-Based Plastics Market. Available online: https://www.emergenresearch.com/industry-report/cellulose-based-plastics-market (accessed on 15 December 2021).
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Ebnesajjad, S., Ed.; Elsevier Inc.: Chadds Ford, PA, USA, 2012; ISBN 9781455730032. [Google Scholar]
- Shah, S.; Matkawala, F.; Garg, S.; Nighojkar, S.; Nighojkar, A.; Kumar, A. Emerging Trend of Bio-plastics and Its Impact on Society. Biotechnol. J. Int. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Prados, E.; Maicas, S. Bacterial Production of Hydroxyalkanoates (PHA). Univers. J. Microbiol. Res. 2016, 4, 23–30. [Google Scholar] [CrossRef]
- Bartels, M.; Gutschmann, B.; Widmer, T.; Grimm, T.; Neubauer, P.; Riedel, S.L. Recovery of the PHA Copolymer P(HB-co-HHx) with Non-halogenated Solvents: Influences on Molecular Weight and HHx-Content. Front. Bioeng. Biotechnol. 2020, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Rhu, D.H.; Lee, W.H.; Kim, J.Y.; Choi, E. Polyhydroxyalkanoate (PHA) production from waste. Water Sci. Technol. 2003, 48, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, R. Production of Polyhydroxyalkanoates (PHA) by Haloferax mediterranei from Food Waste Derived Nutrients for Biodegradable Plastic Applications. J. Microbiol. Biotechnol. 2021, 31, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Panda, A.N.; Ray, L. An investigation for recovery of polyhydroxyalkanoates (PHA) from Bacillus sp. BPPI-14 and Bacillus sp. BPPI-19 isolated from plastic waste landfill. Int. J. Biol. Macromol. 2019, 134, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zain, N.F.; Paramasivam, M.; Tan, J.S.; Lim, V.; Lee, C.K. Response surface methodology optimization of polyhydroxyalkanoate production by Burkholderia cepacia BPT1213 using waste glycerol from palm oil-based biodiesel production. Biotechnol. Prog. 2021, 37, e3077. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Tabatabaei, M.; Ghazali, F.M.; Abd-Aziz, S.; Shirai, Y.; Hassan, M.A. Polyhydroxyalkanoate production from anaerobically treated palm oil mill effluent by new bacterial strain Comamonas sp. EB172. World J. Microbiol. Biotechnol. 2010, 26, 767–774. [Google Scholar] [CrossRef]
- Arumugam, A.; Senthamizhan, S.G.; Ponnusami, V.; Sudalai, S. Production and optimization of polyhydroxyalkanoates from non-edible Calophyllum inophyllum oil using Cupriavidus necator. Int. J. Biol. Macromol. 2018, 112, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Prajapati, V.; Patel, K.; Trivedi, U. Optimization and characterization of PHA from isolate Pannonibacter phragmitetus ERC8 using glycerol waste. Int. J. Biol. Macromol. 2016, 86, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ling, C.; Hajnal, I.; Wu, Q.; Chen, G.Q. Construction of Halomonas bluephagenesis capable of high cell density growth for efficient PHA production. Appl. Microbiol. Biotechnol. 2018, 102, 4499–4510. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M.; Thakur, I.S. Analysis and optimization of process parameters for production of polyhydroxyalkanoates along with wastewater treatment by Serratia sp. ISTVKR1. Bioresour. Technol. 2017, 242, 55–59. [Google Scholar] [CrossRef]
- Kanavaki, I.; Drakonaki, A.; Geladas, E.D.; Spyros, A.; Xie, H.; Tsiotis, G. Polyhydroxyalkanoate (PHA) Production in Pseudomonas sp. phDV1 Strain Grown on Phenol as Carbon Sources. Microorganisms 2021, 9, 1636. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yoon, J.J.; Kim, H.J.; Hong, J.W.; Gi Hong, Y.; Song, H.S.; Moon, Y.M.; Jeon, J.M.; Kim, Y.G.; Yang, Y.H. Engineering of artificial microbial consortia of Ralstonia eutropha and Bacillus subtilis for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production from sugarcane sugar without precursor feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef]
- Pereira, J.; Queirós, D.; Lemos, P.C.; Rossetti, S.; Serafim, L.S. Enrichment of a mixed microbial culture of PHA-storing microorganisms by using fermented hardwood spent sulfite liquor. New Biotechnol. 2020, 56, 79–86. [Google Scholar] [CrossRef]
- Akdoğan, M.; Çelik, E. Enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer by recombinant Bacillus megaterium in fed-batch bioreactors. Bioprocess Biosyst. Eng. 2021, 44, 403–416. [Google Scholar] [CrossRef]
- Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Choi, T.R.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Kim, E.J.; Kim, B.G.; Yang, Y.H. Construction of Efficient Platform Escherichia coli Strains for Polyhydroxyalkanoate Production by Engineering Branched Pathway. Polymers 2019, 11, 509. [Google Scholar] [CrossRef] [Green Version]
- Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P.M. Microalgae as Contributors to Produce Biopolymers. Mar. Drugs 2021, 19, 466. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Das, S.K.; Sathish, A.; Stanley, J. Production of Biofuel and Bioplastic from Chlorella Pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; de Jesus Assis, D.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Ganesh Saratale, R.; Cho, S.K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Kenny, S.T.; Runic, J.N.; Kaminsky, W.; Woods, T.; Babu, R.P.; Keely, C.M.; Blau, W.; O’Connor, K.E. Up-Cycling of PET (Polyethylene Terephthalate) to the Biodegradable Plastic PHA (Polyhydroxyalkanoate). Environ. Sci. Technol. 2008, 42, 7696–7701. [Google Scholar] [CrossRef]
- Kenny, S.T.; Runic, J.N.; Kaminsky, W.; Woods, T.; Babu, R.P.; O’Connor, K.E. Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 2012, 95, 623–633. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Narancic, T.; Salvador, M.; Hughes, G.M.; Beagan, N.; Abdulmutalib, U.; Kenny, S.T.; Wu, H.; Saccomanno, M.; Um, J.; O’Connor, K.E.; et al. Genome analysis of the metabolically versatile Pseudomonas umsongensis GO16: The genetic basis for PET monomer upcycling into polyhydroxyalkanoates. Microb. Biotechnol. 2021, 14, 2463–2480. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Black-Solís, J.D.; Ortega-Gudiño, P.; Sabino-Gutiérrez, M.A.; Benítez-Jiménez, J.J.; Barajas-Cervantes, A.; Bautista-Baños, S.; Hurtado-Colmenares, L.B. Preparation and Characterization of Bio-Based PLA/PBAT and Cinnamon Essential Oil Polymer Fibers and Life-Cycle Assessment from Hydrolytic Degradation. Polymers 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, R.; Rajeswari, N.; Tamilselvi, A. Antimicrobial, mechanical, barrier, and thermal properties of bio-based poly (butylene adipate-co-terephthalate) (PBAT)/Ag2O nanocomposite films for packaging application. Polym. Adv. Technol. 2018, 29, 61–68. [Google Scholar] [CrossRef]
- Meng, D.; Xie, J.; Waterhouse, G.I.N.; Zhang, K.; Zhao, Q.; Wang, S.; Qiu, S.; Chen, K.; Li, J.; Ma, C.; et al. Biodegradable Poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: Preparation, enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 48485. [Google Scholar] [CrossRef]
- Chen, S.; Lin, S.; Hu, Y.; Ma, M.; Shi, Y.; Liu, J.; Zhu, F.; Wang, X. A lignin-based flame retardant for improving fire behavior and biodegradation performance of polybutylene succinate. Polym. Adv. Technol. 2018, 29, 3142–3150. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-based monomers and polymers using microbes for a sustainable bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef] [PubMed]
- Mitsubishi Chemical Coporation BIOPBSTM. Available online: https://www.mcpp-global.com/en/mcpp-asia/products/brand/biopbsTM/ (accessed on 12 January 2022).
- PTT MCC Biochem Co., Ltd. What’s BioPBS. Available online: https://www.pttmcc.com/what-is-biopbs (accessed on 13 January 2022).
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-based poly (butylene succinate): Recent progress, challenges and future opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Mordor Intelligence 1,4 Butanediol Market—Growth, Trends, COVID-19 Impact, and Forecast (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/1-4-butanediol-market (accessed on 28 September 2021).
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef]
- Barton, N.R.; Burgard, A.P.; Burk, M.J.; Crater, J.S.; Osterhout, R.E.; Pharkya, P.; Steer, B.A.; Sun, J.; Trawick, J.D.; Van Dien, S.J.; et al. An integrated biotechnology platform for developing sustainable chemical processes. J. Ind. Microbiol. Biotechnol. 2015, 42, 349–360. [Google Scholar] [CrossRef]
- Burgard, A.; Burk, M.J.; Osterhout, R.; Van Dien, S.; Yim, H. Development of a commercial scale process for production of 1,4-butanediol from sugar. Curr. Opin. Biotechnol. 2016, 42, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Sung, L.Y.; Li, H.; Huang, C.H.; Hu, Y.C. Combining CRISPR and CRISPRi Systems for Metabolic Engineering of E. coli and 1,4-BDO Biosynthesis. ACS Synth. Biol. 2017, 6, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Genomatica GENO BDOTM Process: Commercial Scale; Proven; Available to License. Available online: https://www.genomatica.com/products/#bdo (accessed on 28 September 2021).
- DuPont Tate & Lyle BioProducts Genomatica and DuPont Tate & Lyle Bio Products Successfully Produce 1,4-Butanediol (BDO) on Commercial Scale. Available online: https://duponttateandlyle.com/genomatica-and-dupont-tate-lyle-bio-products-successfully-produce-14-butanediol-bdo-on-commercial-scale/ (accessed on 28 September 2021).
- Novamont Opening of The World’s First Industrial Scale Plant for the Production of Butanediol via Fermentation of Renewable Raw Materials. Available online: https://www.novamont.com/eng/read-press-release/mater-biotech/ (accessed on 28 September 2021).
- Mordor Intelligence 1,3-Propanediol (PDO) Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/1-3-propanediol-pdo-market (accessed on 28 September 2021).
- PRNewswire 1,3-Propanediol (PDO) Market to be Driven by Rising Infiltration of Polyurethane & High Demand for Polyesters Till 2022. Available online: https://www.prnewswire.com/news-releases/1-3-propanediol-pdo-market-to-be-driven-by-rising-infiltration-of-polyurethane--high-demand-for-polyesters-till-2022--million-insights-300984122.html (accessed on 3 October 2021).
- Biebl, H.; Menzel, K.; Zeng, A.P.; Deckwer, W.D. Microbial production of 1,3-propanediol. Appl. Microbiol. Biotechnol. 1999, 52, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tabah, B.; Varvak, A.; Pulidindi, I.N.; Foran, E.; Banin, E.; Gedanken, A. Production of 1,3-propanediol from glycerol via fermentation by Saccharomyces cerevisiae. Green Chem. 2016, 18, 4657–4666. [Google Scholar] [CrossRef]
- Durgapal, M.; Kumar, V.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, S. Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour. Technol. 2014, 159, 223–231. [Google Scholar] [CrossRef]
- Ju, J.H.; Wang, D.; Heo, S.Y.; Kim, M.S.; Seo, J.W.; Kim, Y.M.; Kim, D.H.; Kang, S.A.; Kim, C.H.; Oh, B.R. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53. Microb. Cell Fact. 2020, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Kongjan, P.; Jariyaboon, R.; Reungsang, A.; Sittijunda, S. Co-fermentation of 1,3-propanediol and 2,3-butanediol from crude glycerol derived from the biodiesel production process by newly isolated Enterobacter sp.: Optimization factors affecting. Bioresour. Technol. Rep. 2021, 13, 100616. [Google Scholar] [CrossRef]
- Przystałowska, H.; Zeyland, J.; Szymanowska-Powałowska, D.; Szalata, M.; Słomski, R.; Lipiński, D. 1,3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiol. Res. 2015, 171, 1–7. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, J.J.; Shen, J.T.; Zheng, Y.F.; Sun, Y.Q.; Xiu, Z.L. Sequential fed-batch fermentation of 1,3-propanediol from glycerol by Clostridium butyricum DL07. Appl. Microbiol. Biotechnol. 2020, 104, 9179–9191. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Gao, H.; Wang, H.; Wan, Z.; Jiang, Y.; Xin, F.; Zhang, W.; Jiang, M. Current advances in microbial production of 1,3-propanediol. Biofuels Bioprod. Biorefin. 2021, 15, 1566–1583. [Google Scholar] [CrossRef]
- Varrone, C.; Floriotis, G.; Heggeset, T.M.B.; Le, S.B.; Markussen, S.; Skiadas, I.V.; Gavala, H.N. Continuous fermentation and kinetic experiments for the conversion of crude glycerol derived from second-generation biodiesel into 1,3 propanediol and butyric acid. Biochem. Eng. J. 2017, 128, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Emptage, M.; Haynie, S.L.; Laffend, L.A.; Pucci, J.P.; Whited, G. Process for the Biological Production of 1,3-Propanediol with High Titer. U.S. Patent No. 6514733, 4 February 2003. [Google Scholar]
- Nova-Institute USA. World’s First Propanediol Production from Corn Sugar Opened. Available online: https://renewable-carbon.eu/news/u-s-a-worlds-first-propanediol-production-from-corn-sugar-opened/ (accessed on 5 October 2021).
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Lactic Acid Market Share, Industry Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market (accessed on 13 October 2021).
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Florou-Paneri, P.; Christaki, E.; Bonos, E. Lactic Acid Bacteria as Source of Functional Ingredients. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; IntechOpen: London, UK, 2013; pp. 589–614. ISBN 978-953-51-5349-8. [Google Scholar]
- Awasthi, D.; Wang, L.; Rhee, M.S.; Wang, Q.; Chauliac, D.; Ingram, L.O.; Shanmugam, K.T. Metabolic engineering of Bacillus subtilis for production of D-lactic acid. Biotechnol. Bioeng. 2018, 115, 453–463. [Google Scholar] [CrossRef]
- Danner, H.; Neureiter, M.; Madzingaidzo, L.; Gartner, M.; Braun, R. Bacillus stearothermophilus for thermophilic production of L-lactic acid. Appl. Biochem. Biotechnol. 1998, 70–72, 895–903. [Google Scholar] [CrossRef]
- Gao, T.; Wong, Y.; Ng, C.; Ho, K. L-lactic acid production by Bacillus subtilis MUR1. Bioresour. Technol. 2012, 121, 105–110. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. L-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Patel, M.; Ou, M.; Ingram, L.O.; Shanmugam, K.T. Fermentation of sugar cane bagasse hemicellulose hydrolysate to l(+)-lactic acid by a thermotolerant acidophilic Bacillus sp. Biotechnol. Lett. 2004, 26, 865–868. [Google Scholar] [CrossRef]
- Sakai, K.; Yamanami, T. Thermotolerant Bacillus licheniformis TY7 produces optically active l-lactic acid from kitchen refuse under open condition. J. Biosci. Bioeng. 2006, 102, 132–134. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Assavasirijinda, N.; Yu, B.; Wang, L.; Ma, Y. Non-sterilized fermentation of high optically pure d-lactic acid by a genetically modified thermophilic Bacillus coagulans strain. Microb. Cell Fact. 2017, 16, 213. [Google Scholar] [CrossRef]
- Qiu, Z.; Gao, Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer d-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.T.; Salem, S.S.; Fouda, A.; El-Gamal, M.S.; Abdel-Rahman, M.A. Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1. Fermentation 2021, 7, 111. [Google Scholar] [CrossRef]
- Ferone, M.; Raganati, F.; Olivieri, G.; Salatino, P.; Marzocchella, A. Continuous Succinic Acid Fermentation by Actinobacillus Succinogenes: Assessment of Growth and Succinic Acid Production Kinetics. Appl. Biochem. Biotechnol. 2019, 187, 782–799. [Google Scholar] [CrossRef]
- Yan, D.; Wang, C.; Zhou, J.; Liu, Y.; Yang, M.; Xing, J. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour. Technol. 2014, 156, 232–239. [Google Scholar] [CrossRef]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, D.; Li, Z.; Ye, Q. Simultaneous saccharification and fermentation of cassava to succinic acid by Escherichia coli NZN111. Bioresour. Technol. 2014, 163, 100–105. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Bio-Conversion and Separation Technology. WP 8.1.Determination of Market Potentialfor Selected Platform Chemicals—Itaconic Acid, Succinic Acid, 2,5-Furandicarboxylic Acid; WEASTRA s.r.o.: Bratislava, Slovakia, 2013. [Google Scholar]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ma, L.; Mao, Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016, 105, 16–26. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, Y. Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. Wiley Online Libr. 2015, 119, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, K.; Partow, S.; Correia, K.; Khusnutdinova, A.N.; Yakunin, A.F.; Mahadevan, R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metab. Eng. Commun. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Blankschien, M.D.; Vick, J.E.; Chou, A.; Kim, S.; Gonzalez, R. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metab. Eng. 2015, 28, 202–212. [Google Scholar] [CrossRef]
- Todea, A.; Deganutti, C.; Spennato, M.; Asaro, F.; Zingone, G.; Milizia, T.; Gardossi, L.; Pellis, A. Azelaic Acid: A Bio-Based Building Block for Biodegradable Polymers. Polymers 2021, 13, 4091. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S.; Karagiannidis, N. Synthesis, Crystallization, and Enzymatic Degradation of the Biodegradable Polyester Poly(ethylene azelate). Macromol. Chem. Phys. 2010, 211, 2585–2595. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jun, M.W.; Seong, Y.J.; Park, H.; Ahn, J.; Park, Y.C. Direct Biotransformation of Nonanoic Acid and Its Esters to Azelaic Acid by Whole Cell Biocatalyst of Candida tropicalis. ACS Sustain. Chem. Eng. 2019, 7, 17958–17966. [Google Scholar] [CrossRef]
- Otte, K.B.; Kirtz, M.; Nestl, B.M.; Hauer, B. Synthesis of 9-Oxononanoic Acid, a Precursor for Biopolymers. ChemSusChem 2013, 6, 2149–2156. [Google Scholar] [CrossRef]
- Otte, K.B.; Kittelberger, J.; Kirtz, M.; Nestl, B.M.; Hauer, B. Whole-Cell One-Pot Biosynthesis of Azelaic Acid. ChemCatChem 2014, 6, 1003–1009. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Seo, J.H.; Kang, W.R.; Kim, M.J.; Lee, J.H.; Oh, D.K.; Park, J.B. Simultaneous enzyme/Whole-Cell biotransformation of plant oils into C9 carboxylic acids. ACS Catal. 2016, 6, 7547–7553. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial Synthesis of Medium-Chain α,ω-Dicarboxylic Acids and ω-Aminocarboxylic Acids from Renewable Long-Chain Fatty Acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Seo, E.J.; Yeon, Y.J.; Seo, J.H.; Lee, J.H.; Boñgol, J.P.; Oh, Y.; Park, J.M.; Lim, S.M.; Lee, C.G.; Park, J.B. Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 2018, 251, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gantrade Corporation Caprolactone Monomer: A Gateway Building Block for Advanced Performance Intermediates. Available online: https://www.gantrade.com/blog/caprolactone-monomer-a-gateway-building-block-for-advanced-performance-intermediates (accessed on 16 December 2021).
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Schmidt, S.; Scherkus, C.; Muschiol, J.; Menyes, U.; Winkler, T.; Hummel, W.; Grçger, H.; Liese, A.; Herz, H.-G.; Bornscheuer, U.T.; et al. An Enzyme Cascade Synthesis of ε-Caprolactone and its Oligomers. Angew. Chem. Int. Ed. 2015, 54, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Schäfer, L.; Karande, R.; Bühler, B. Maximizing Biocatalytic Cyclohexane Hydroxylation by Modulating Cytochrome P450 Monooxygenase Expression in P. taiwanensis VLB120. Front. Bioeng. Biotechnol. 2020, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Salamanca, D.; Bühler, K.; Engesser, K.H.; Schmid, A.; Karande, R. Whole-cell biocatalysis using the Acidovorax sp. CHX100 Δ6HX for the production of ω-hydroxycarboxylic acids from cycloalkanes. New Biotechnol. 2021, 60, 200–206. [Google Scholar] [CrossRef]
- Bretschneider, L.; Heuschkel, I.; Wegner, M.; Lindmeyer, M.; Bühler, K.; Karande, R.; Bühler, B. Conversion of Cyclohexane to 6-Hydroxyhexanoic Acid Using Recombinant Pseudomonas taiwanensis in a Stirred-Tank Bioreactor. Front. Catal. 2021, 1, 683248. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Chiloeches, A.; Funes, A.; Cuervo-Rodríguez, R.; López-Fabal, F.; Fernández-García, M.; Echeverría, C.; Muñoz-Bonilla, A. Biobased polymers derived from itaconic acid bearing clickable groups with potent antibacterial activity and negligible hemolytic activity. Polym. Chem. 2021, 12, 3190–3200. [Google Scholar] [CrossRef]
- Verified Market Research Itaconic Acid Market Size and Forecast. Available online: https://www.verifiedmarketresearch.com/product/itaconic-acid-market/ (accessed on 18 December 2021).
- Cunha da Cruz, J.; Machado de Castro, A.; Camporese Sérvulo, E.F. World market and biotechnological production of itaconic acid. 3 Biotech 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Gopaliya, D.; Kumar, V.; Khare, S.K. Recent advances in itaconic acid production from microbial cell factories. Biocatal. Agric. Biotechnol. 2021, 36, 102130. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Recent Advances in Biotechnological Itaconic Acid Production, and Application for a Sustainable Approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.R.; Chen, X.Z.; Niu, D.D.; Tian, K.M.; Prior, B.A.; Shen, W.; Shi, G.Y.; Singh, S.; Wang, Z.X. Evaluation of genetic manipulation strategies on d-lactate production by Escherichia coli. Curr. Microbiol. 2011, 62, 981–989. [Google Scholar] [CrossRef]
- Jantama, K.; Zhang, X.; Moore, J.C.; Shanmugam, K.T.; Svoronos, S.A.; Ingram, L.O. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 2008, 101, 881–893. [Google Scholar] [CrossRef]
- Chudasama, A. Exploiting Technologies in the Emerging Bioeconomy. Sugar Sugar Deriv. Chang. Consum. Prefer. 2020, 25–38. [Google Scholar] [CrossRef]
- Hagen, A.; Poust, S.; De Rond, T.; Fortman, J.L.; Katz, L.; Petzold, C.J.; Keasling, J.D. Engineering a Polyketide Synthase for in Vitro Production of Adipic Acid. ACS Synth. Biol. 2016, 5, 21–27. [Google Scholar] [CrossRef]
- Li, W.J.; Jayakody, L.N.; Franden, M.A.; Wehrmann, M.; Daun, T.; Hauer, B.; Blank, L.M.; Beckham, G.T.; Klebensberger, J.; Wierckx, N. Laboratory evolution reveals the metabolic and regulatory basis of ethylene glycol metabolism by Pseudomonas putida KT2440. Environ. Microbiol. 2019, 21, 3669–3682. [Google Scholar] [CrossRef]
- Li, W.J.; Narancic, T.; Kenny, S.T.; Niehoff, P.J.; O’Connor, K.; Blank, L.M.; Wierckx, N. Unraveling 1,4-Butanediol Metabolism in Pseudomonas putida KT2440. Front. Microbiol. 2020, 11, 382. [Google Scholar] [CrossRef] [Green Version]
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; De Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Biobased Building Blocks and Polymers—Global Capacities, Production and Trends, 2018–2023. Ind. Biotechnol. 2019, 15, 237–241. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomwongsopon, P.; Varrone, C. Contribution of Fermentation Technology to Building Blocks for Renewable Plastics. Fermentation 2022, 8, 47. https://doi.org/10.3390/fermentation8020047
Lomwongsopon P, Varrone C. Contribution of Fermentation Technology to Building Blocks for Renewable Plastics. Fermentation. 2022; 8(2):47. https://doi.org/10.3390/fermentation8020047
Chicago/Turabian StyleLomwongsopon, Passanun, and Cristiano Varrone. 2022. "Contribution of Fermentation Technology to Building Blocks for Renewable Plastics" Fermentation 8, no. 2: 47. https://doi.org/10.3390/fermentation8020047






