Inhibition Activity of Plantaricin Q7 Produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and Its Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Culture and Determination of Titer
2.2. Growth Assay of L. monocytogenes
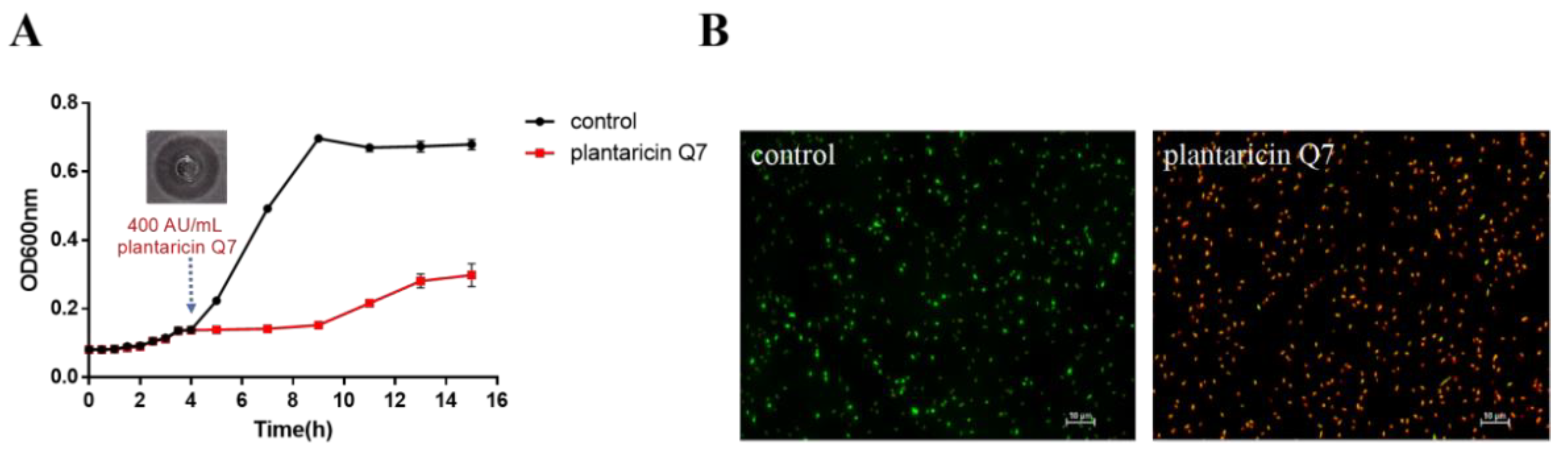
2.3. AO/EB Fluorescence Staining
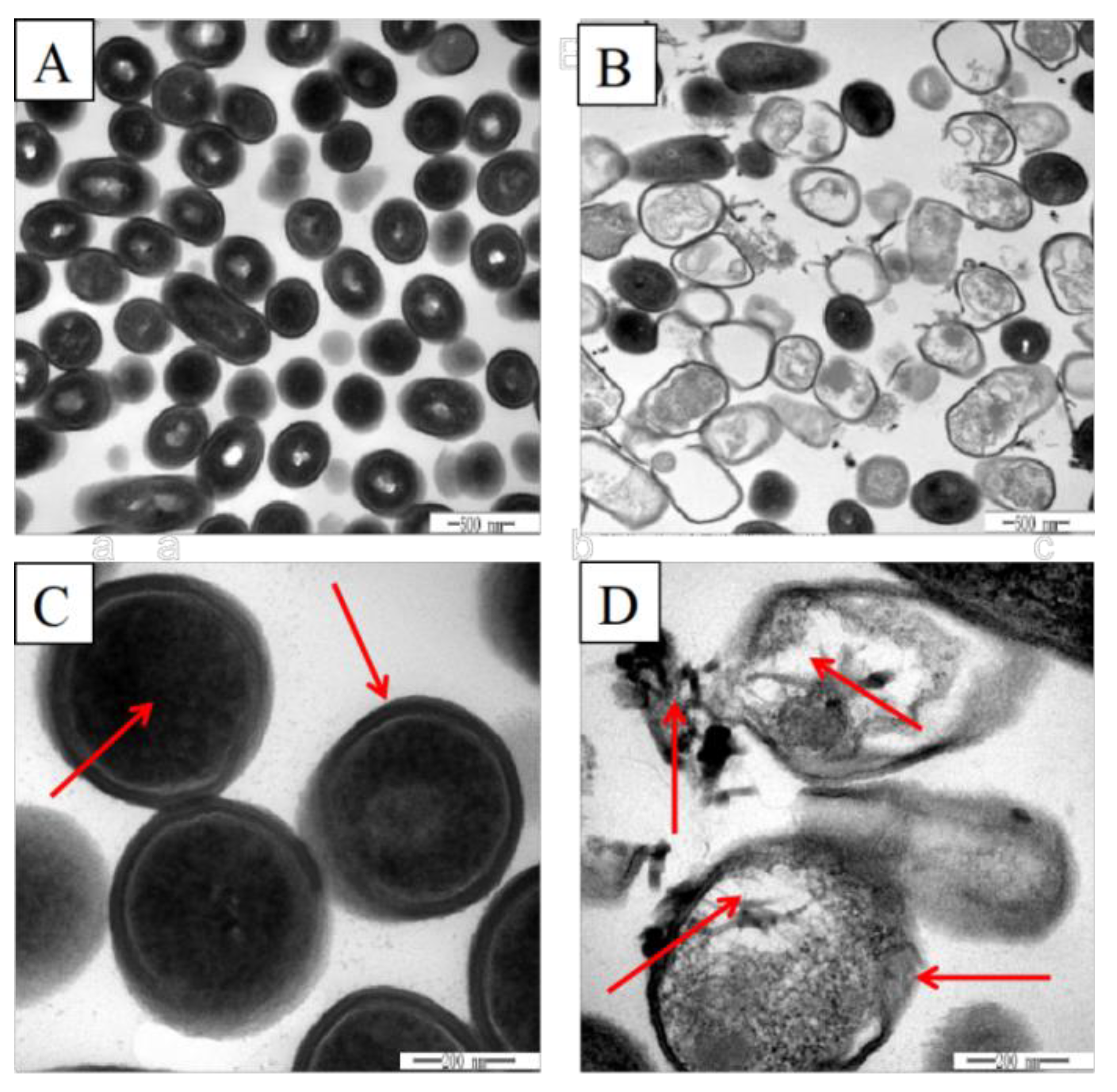
2.4. Transmission Electron Microscopy (TEM) Analyses
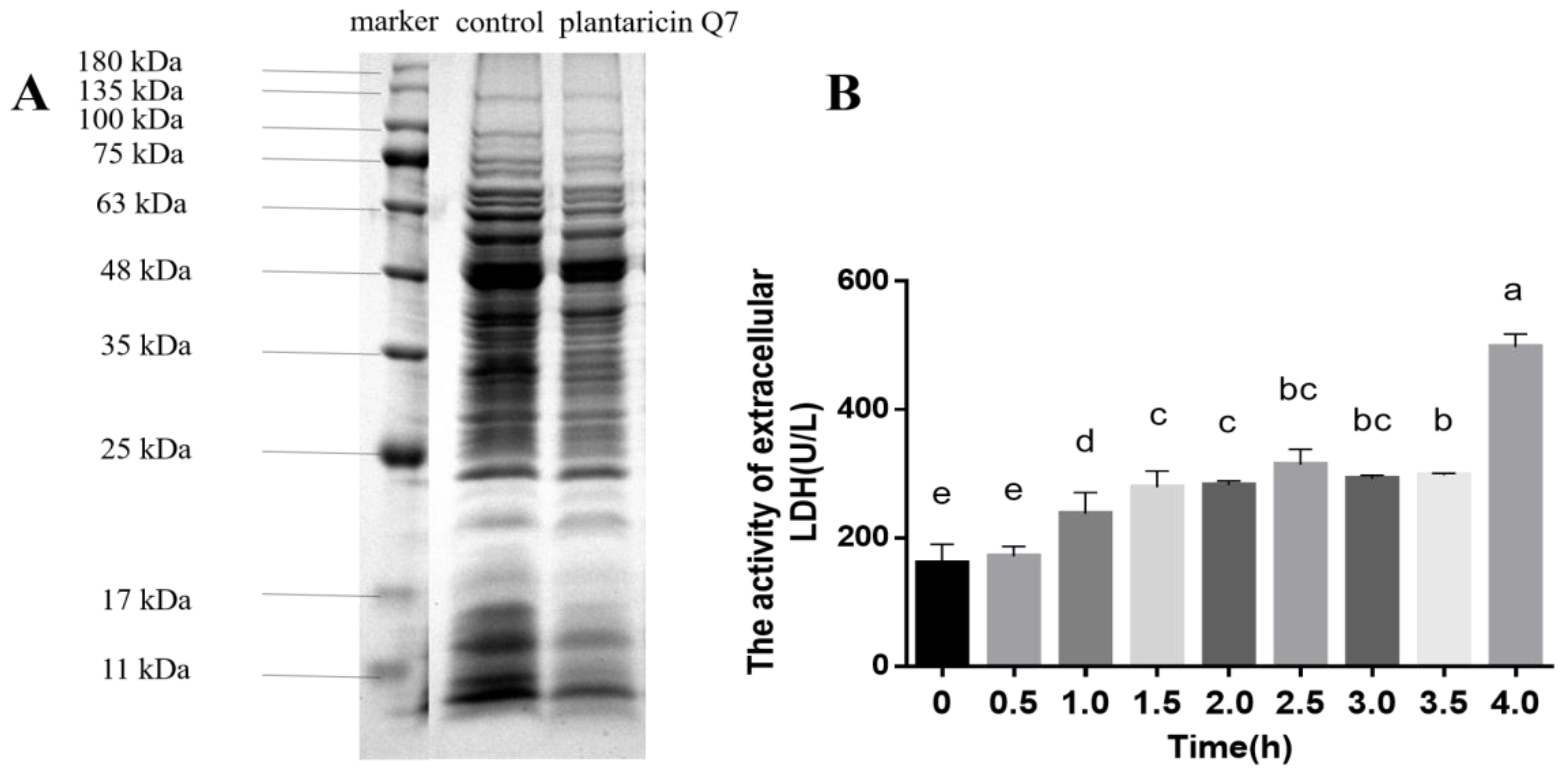
2.5. Determination of Intracellular Biomacromolecules
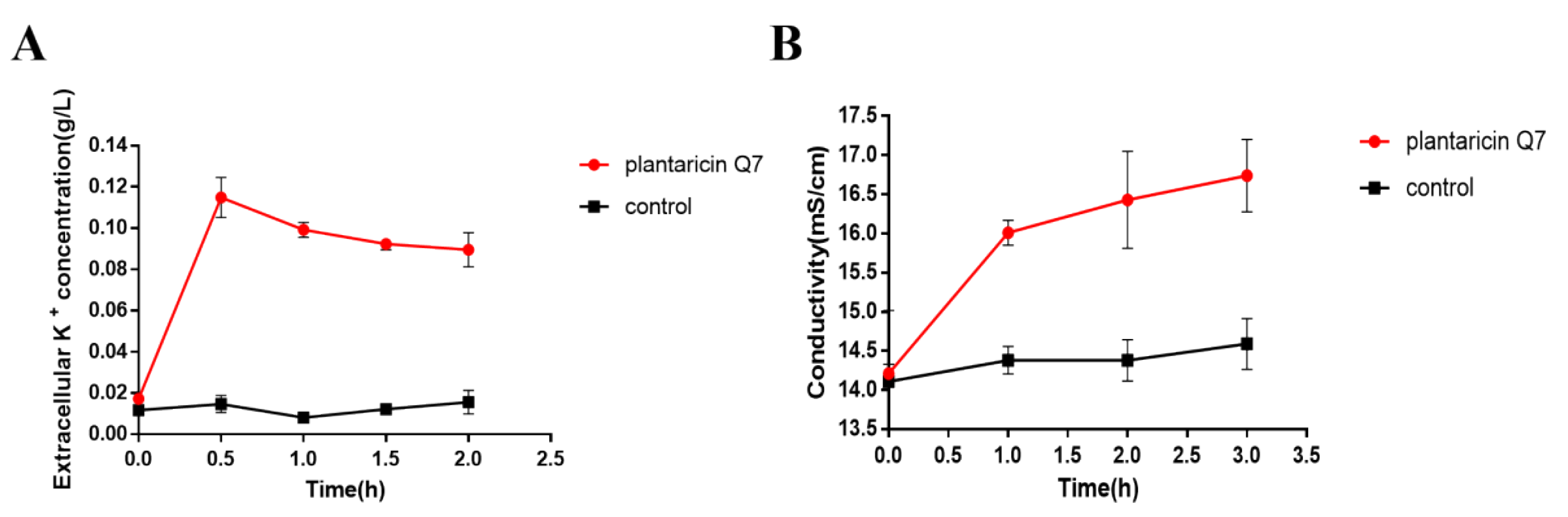
2.6. Cell Membrane Permeability Assay
2.7. Determination of the Motility of L. monocytogenes
2.8. Fibrinogen-Binding Assay
2.9. Effect of Plantaricin Q7 on L. monocytogenes Biofilm
2.10. Statistical Analyses
3. Results and Discussion
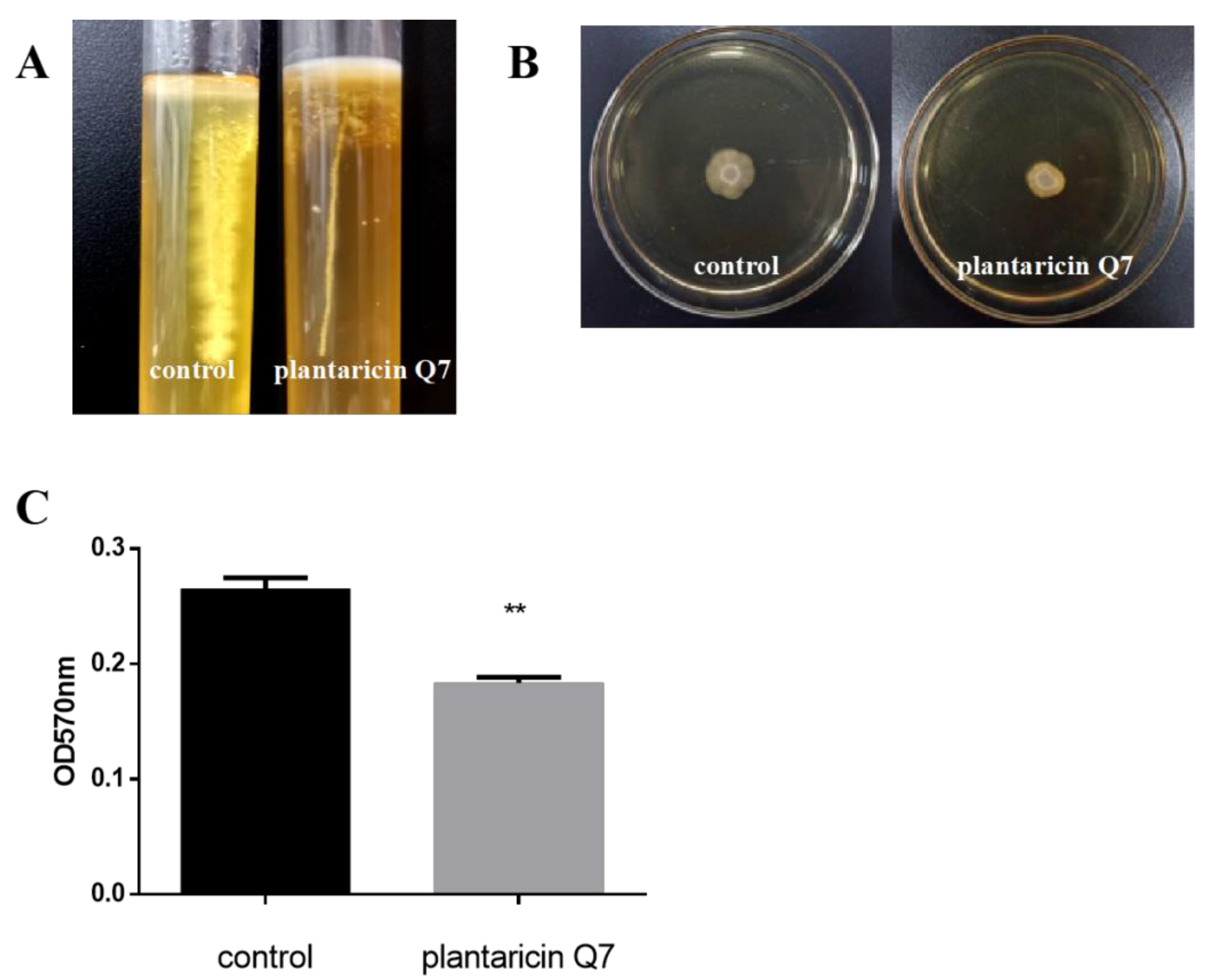
3.1. Antibacterial Activity of Plantaricin Q7 against L. monocytogenes
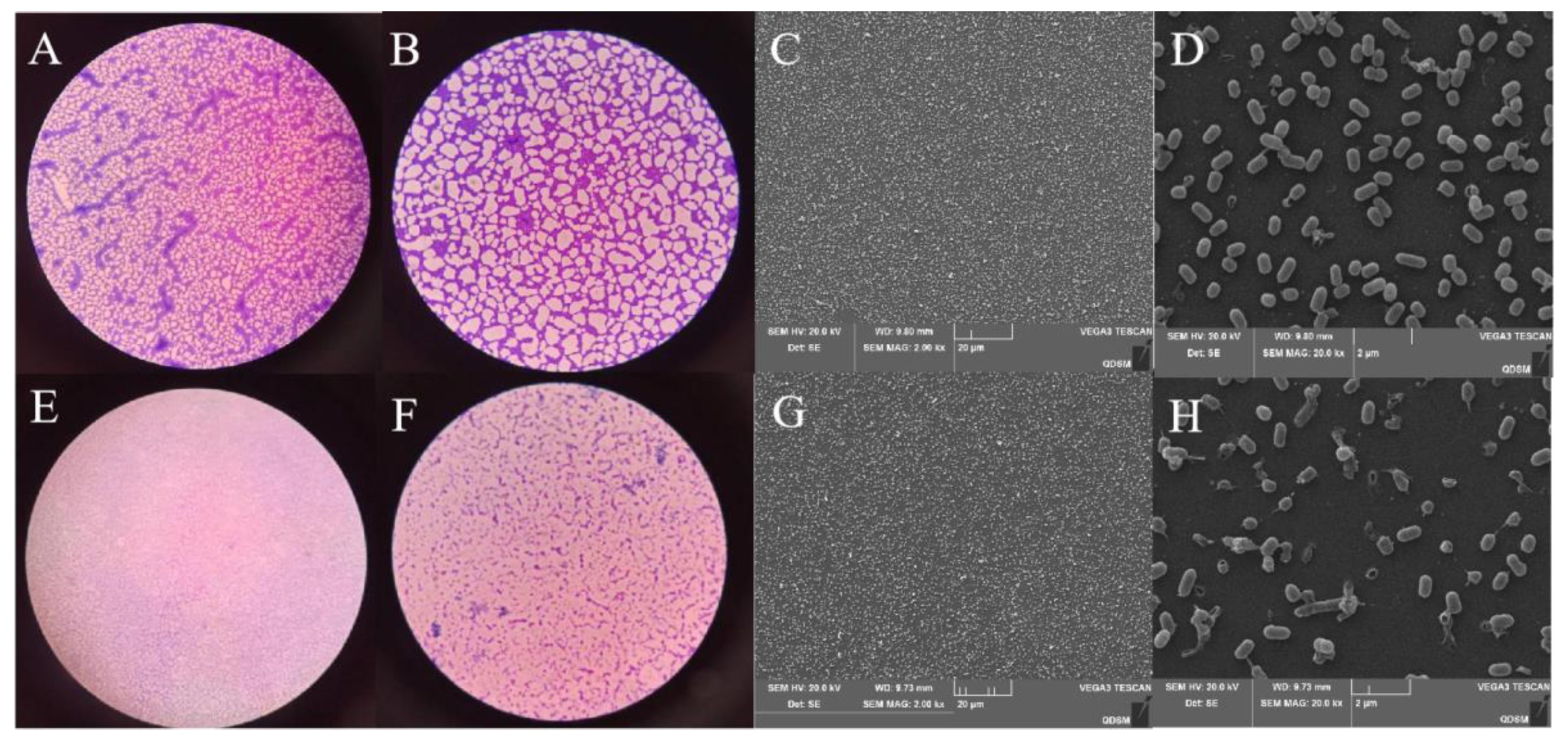
3.2. Effects of Plantaricin Q7 on Morphology and Internal Structure of L. monocytogenes
3.3. Effects of Plantaricin Q7 on Intracellular Substances Leakage of L. monocytogenes
3.4. Effects of Plantaricin Q7 on K+ Concentration and Electrical Conductivity of L. monocytogenes
3.5. Effects of Plantaricin Q7 on the Motility and Attachment of L. monocytogenes
3.6. Inhibitory Effect of Plantaricin Q7 on the Formation of L. monocytogenes Biofilm
3.7. Effect of Plantaricin Q7 on L. monocytogenes Biofilm Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Hartnett, E.; Chapman, B.; Donnelly, C.W.; Goodburn, K.E.; et al. Alternative approaches to the risk management of Listeria monocytogenes in low risk foods. Food Control 2020, 123, 107601. [Google Scholar] [CrossRef]
- Labite, H.; Lunani, I.; Van Der Steen, P.; Vairavamoorthy, K.; Drechsel, P.; Lens, P. Quantitative Microbial Risk Analysis to evaluate health effects of interventions in the urban water system of Accra, Ghana. J. Water Health 2010, 8, 417–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelaert, F.; Amore, G.; Van der Stede, Y.; Hugas, M. EU-wide monitoring of biological hazards along the food chain: Achievements, challenges and EFSA vision for the future. Curr. Opin. Food Sci. 2016, 12, 52–62. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lee, J.-J.; Chien, C.-C.; Tsai, W.-C.; Lu, C.-H.; Chang, W.-N.; Lien, C.-Y. High incidence of severe neurological manifestations and high mortality rate for adult Listeria monocytogenes meningitis in Taiwan. J. Clin. Neurosci. 2019, 71, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.; Wang, X.; Liu, B.; Dong, Q. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol. 2019, 312, 108358. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, A.; Stavru, F.; Cossart, P. Organelle targeting during bacterial infection: Insights from Listeria. Trends Cell Biol. 2015, 25, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Mammina, C.; Aleo, A.; Romani, C.; Pellissier, N.; Nicoletti, P.; Pecile, P.; Nastasi, A.; Pontello, M.M. Characterization of Listeria monocytogenes Isolates from Human Listeriosis Cases in Italy. J. Clin. Microbiol. 2009, 47, 2925–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forauer, E.; Wu, S.T.; Etter, A.J. Listeria monocytogenes in the retail deli environment: A review. Food Control 2020, 119, 107443. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Beleneva, I.A. Incidence and characteristics of Staphylococcus aureus and Listeria monocytogenes from the Japan and South China seas. Mar. Pollut. Bull. 2011, 62, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-Y.; Jeong, H.-W.; Kim, J.; Lee, J.-W.; Jang, J. Removal of biofilms using carbon dioxide aerosols. J. Aerosol Sci. 2010, 41, 1044–1051. [Google Scholar] [CrossRef]
- Shea, E.F.O.; Cotter, P.D.; Ross, R.P.; Hill, C. Strategies to improve the bacteriocin protection provided by lactic acid bacteria. Curr. Opin. Biotechnol. 2013, 24, 130–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Ross, R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Genet. 2012, 11, 95–105. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Chae, S.A.; Bang, W.Y.; Lee, M.; Ban, O.-H.; Kim, S.-J.; Jung, Y.H.; Yang, J. Anti-inflammatory potential of Lactiplantibacillus plantarum IDCC 3501 and its safety evaluation. Braz. J. Microbiol. 2021, 52, 2299–2306. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Coss, G.M.; Mandale, S.; Lovitt, R. Separation of lactobacilli bacteriocins from fermented broths using membranes. Process Biochem. 2013, 48, 1252–1261. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yi, H.; Han, X.; Chi, C. Identification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. LWT-Food Sci. Technol. 2016, 71, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Liu, Y.; Li, J.; Liu, T.; Gong, P.; Zhang, L.; Wang, Y.; Yi, H. Analyses of plantaricin Q7 synthesis by Lactobacillus plantarum Q7 based on comparative transcriptomics. Food Control 2021, 124, 107909. [Google Scholar] [CrossRef]
- An, Y.; Wang, Y.; Liang, X.; Yi, H.; Zuo, Z.; Xu, X.; Zhang, D.; Yu, C.; Han, X. Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control 2017, 81, 211–217. [Google Scholar] [CrossRef]
- Engelhardt, T.; Albano, H.; Kiskó, G.; Mohácsi-Farkas, C.; Teixeira, P. Antilisterial activity of bacteriocinogenic Pediococcus acidilactici HA6111-2 and Lactobacillus plantarum ESB 202 grown under pH and osmotic stress conditions. Food Microbiol. 2015, 48, 109–115. [Google Scholar] [CrossRef]
- Wang, X.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; Wang, J. Combined Systems Approaches Reveal a Multistage Mode of Action of a Marine Antimicrobial Peptide against Pathogenic Escherichia coli and Its Protective Effect against Bacterial Peritonitis and Endotoxemia. Antimicrob. Agents Chemother. 2017, 61, e01056-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-W.; Xiang, Y.-Z.; Zhang, M.; Jiang, Y.-H.; Zhang, Y.; Liu, Y.-Y.; Lin, L.-B.; Zhang, Q.-L. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: Isolation, purification, identification, antibacterial and antibiofilm activity. LWT-Food Sci. Technol. 2020, 140, 110826. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, Y.; Wang, X. Ferulic Acid Inactivates Shigella flexneri through Cell Membrane Destructieon, Biofilm Retardation, and Altered Gene Expression. J. Agric. Food Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, G.; Zhan, H.; Liu, B.; Li, C.; Wang, L.; Wang, H.; Wang, J. Antimicrobial mechanism of 4-hydroxyphenylacetic acid on Listeria monocytogenes membrane and virulence. Biochem. Biophys. Res. Commun. 2021, 572, 145–150. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Young, G.M. Loss of Flagellum-Based Motility by Listeria monocytogenes Results in Formation of Hyperbiofilms. J. Bacteriol. 2008, 190, 6030–6034. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiao, M.; Zhang, X.; Li, J.; Meng, Q.; Qiao, J.; Li, Y.; Wang, X.; Zhang, G.; Zhang, K.; et al. Effects of Lmo2672 Deficiency on Environmental Adaptability, Biofilm Formation, and Motility of Listeria monocytogenes. Jundishapur J. Microbiol. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescensWCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.; Xu, L.; Wang, Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef]
- Yi, L.; Dang, J.; Zhang, L.; Wu, Y.; Liu, B.; Lü, X. Purification, characterization and bactericidal mechanism of a broad spectrum bacteriocin with antimicrobial activity against multidrug-resistant strains produced by Lactobacillus coryniformis XN8. Food Control 2016, 67, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Jin, F. Structural insights into the mechanism of a novel protein targeting pathway in Gram-negative bacteria. FEBS Open Bio 2020, 10, 561–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Guerrero, R.; Galán-Vásquez, E.; Pérez-Rueda, E. The protein architecture in Bacteria and Archaea identifies a set of promiscuous and ancient domains. PLoS ONE 2019, 14, e0226604. [Google Scholar] [CrossRef] [PubMed]
- Kolappan, S.; Shen, D.L.; Mosi, R.; Sun, J.; McEachern, E.; Vocadlo, D.; Craig, L. Structures of lactate dehydrogenase A (LDHA) in apo, ternary and inhibitor-bound forms. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Wakayama, M.; Takagi, K. Lactate Dehydrogenase Involved in Lactate Metabolism of Acetobacter Pasteurianus. Procedia Environ. Sci. 2015, 28, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Diao, W.-R.; Zhang, L.-L.; Feng, S.-S.; Xu, J.-G. Chemical Composition, Antibacterial Activity, and Mechanism of Action of the Essential Oil from Amomum kravanh. J. Food Prot. 2014, 77, 1740–1746. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamamoto, T.; Kawai, Y.; Inoue, N.; Yamazaki, K. Mode of action of piscicocin CS526 produced by Carnobacterium piscicola CS526. Appl. Microbiol. 2005, 98, 1146–1151. [Google Scholar] [CrossRef]
- Rurián-Henares, J.A.; Morales, F.J. Antimicrobial Activity of Melanoidins against Escherichia coli Is Mediated by a Membrane-Damage Mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [Green Version]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar Motility Is Critical for Listeria monocytogenes Biofilm Formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Armenta, F.; Bernal-Mercado, A.; Rodriguez, M.R.T.; Gonzalez-Aguilar, G.; Lopez-Zavala, A.; Martínez-Téllez, M.; Oñate, M.H.; Ayala-Zavala, J. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 2018, 90, 266–273. [Google Scholar] [CrossRef]
- Borges, A.; Maria, J.; Simoes, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Golla, R.M.; Lau, K.; Lushnikova, T.; Wang, G. Anti-Staphylococcal Biofilm Effects of Human Cathelicidin Peptides. ACS Med. Chem. Lett. 2015, 7, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Almendárez, B.E.; Cann, I.K.; Martin, S.E.; Guerrero-Legarreta, I.; Regalado, C. Effect of Lactococcus lactis UQ2 and its bacteriocin on Listeria monocytogenes biofilms. Food Control 2008, 19, 670–680. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Bu, Y.; Li, J.; Liu, Y.; Liu, A.; Gong, P.; Liu, T.; Zhang, L.; Wang, S.; Yi, H. Inhibition Activity of Plantaricin Q7 Produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and Its Biofilm. Fermentation 2022, 8, 75. https://doi.org/10.3390/fermentation8020075
Liu Y, Bu Y, Li J, Liu Y, Liu A, Gong P, Liu T, Zhang L, Wang S, Yi H. Inhibition Activity of Plantaricin Q7 Produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and Its Biofilm. Fermentation. 2022; 8(2):75. https://doi.org/10.3390/fermentation8020075
Chicago/Turabian StyleLiu, Yinxue, Yushan Bu, Jianxun Li, Yisuo Liu, Ao Liu, Pimin Gong, Tongjie Liu, Lanwei Zhang, Shumei Wang, and Huaxi Yi. 2022. "Inhibition Activity of Plantaricin Q7 Produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and Its Biofilm" Fermentation 8, no. 2: 75. https://doi.org/10.3390/fermentation8020075





