pH Auto-Sustain-Based Fermentation Supports Efficient Gamma-Aminobutyric Acid Production by Lactobacillus brevis CD0817
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strain, Starting Media, and Preparation of Inoculum
2.3. Optimization Experiments
2.4. Fermentation Trial
2.5. Analytic Procedures
2.6. Calculations
2.7. Statistical Analysis
3. Results and Discussion
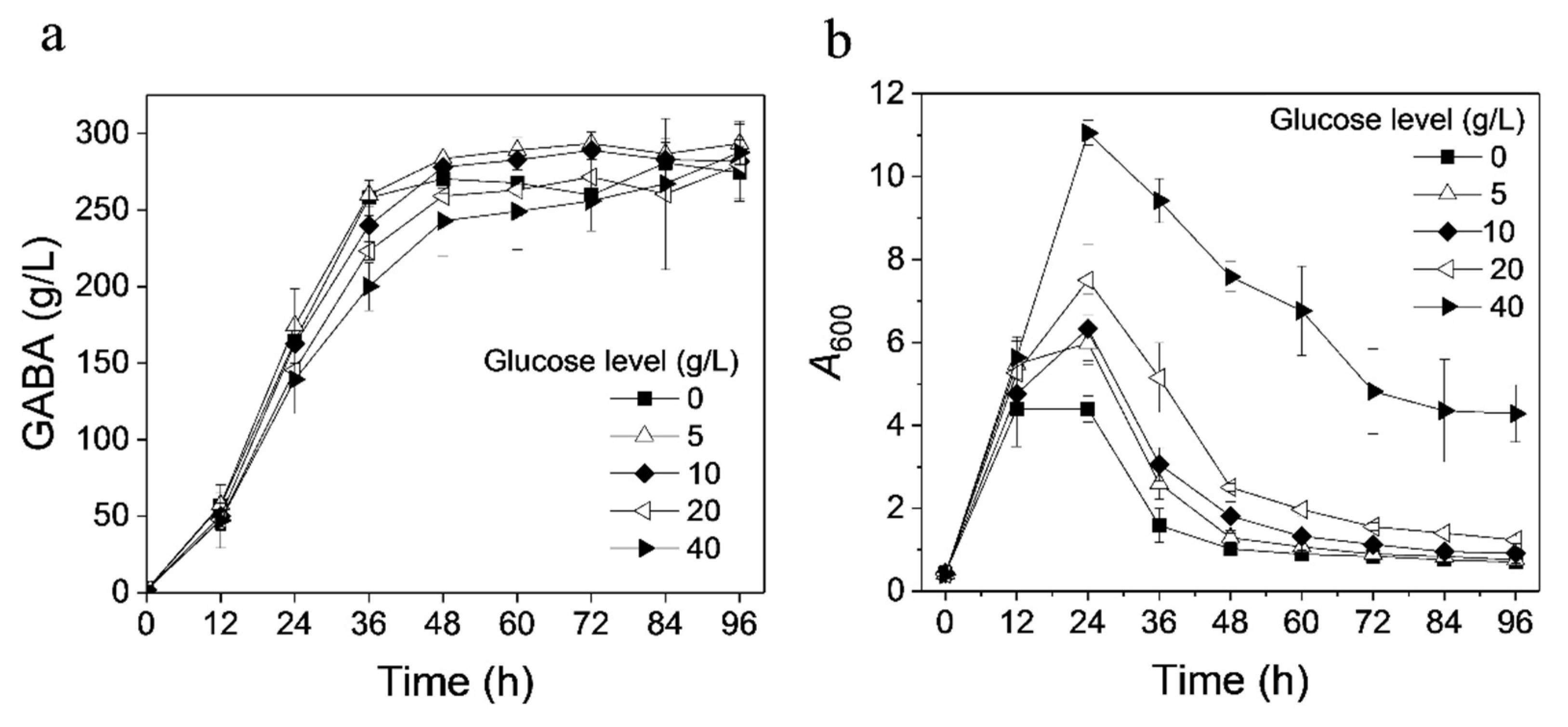
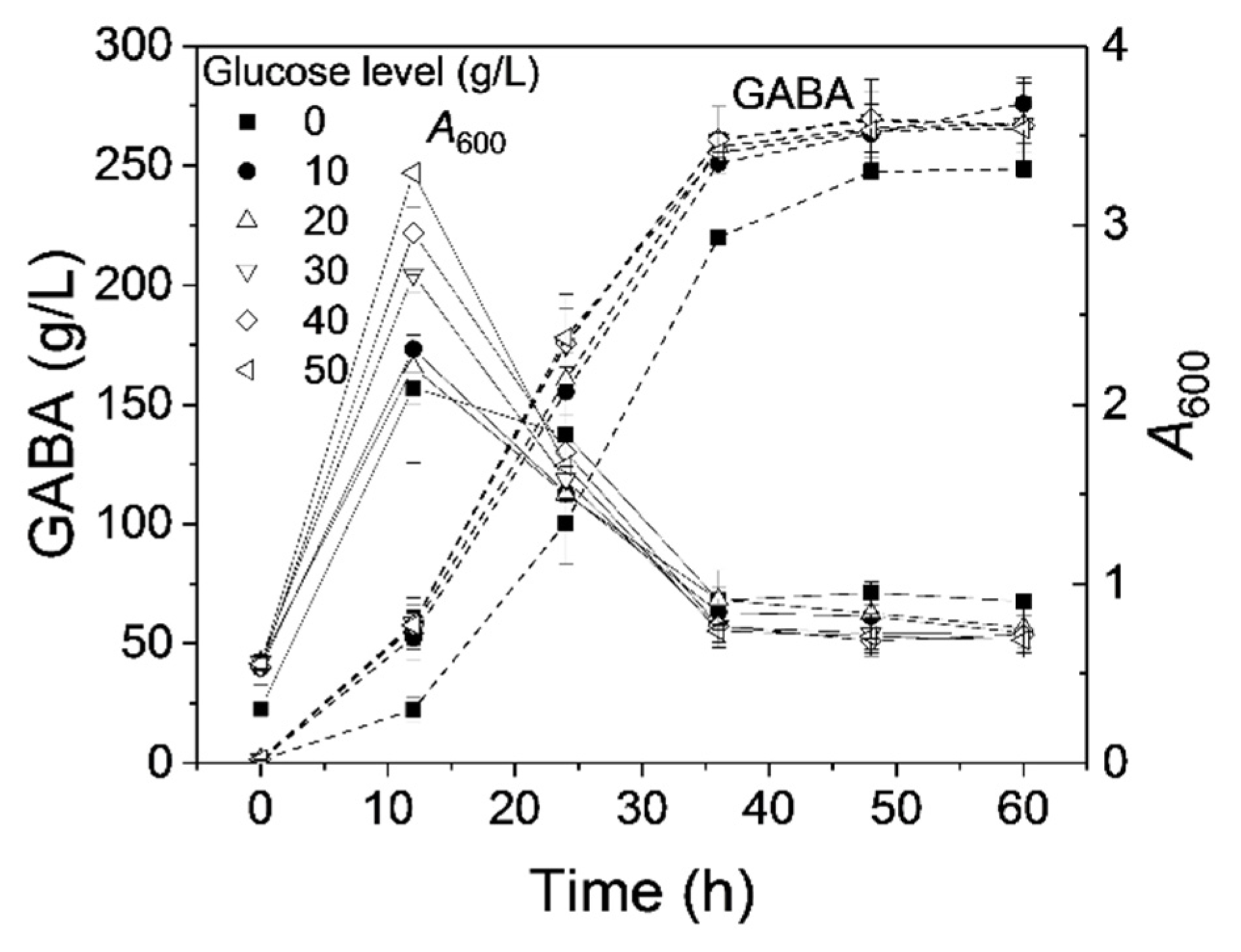
3.1. Effect of Glucose on GABA Production
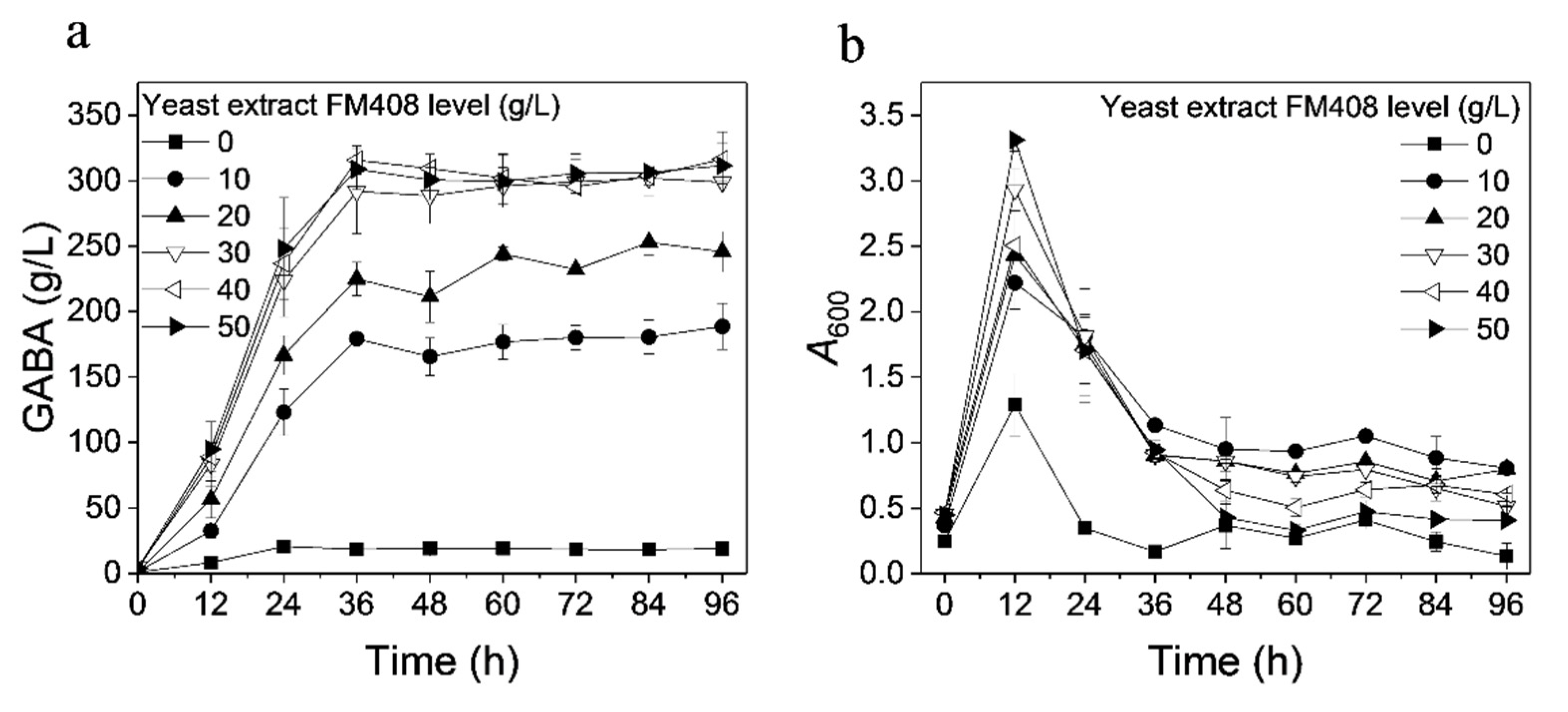
3.2. Effect of Nitrogen Source on GABA Production
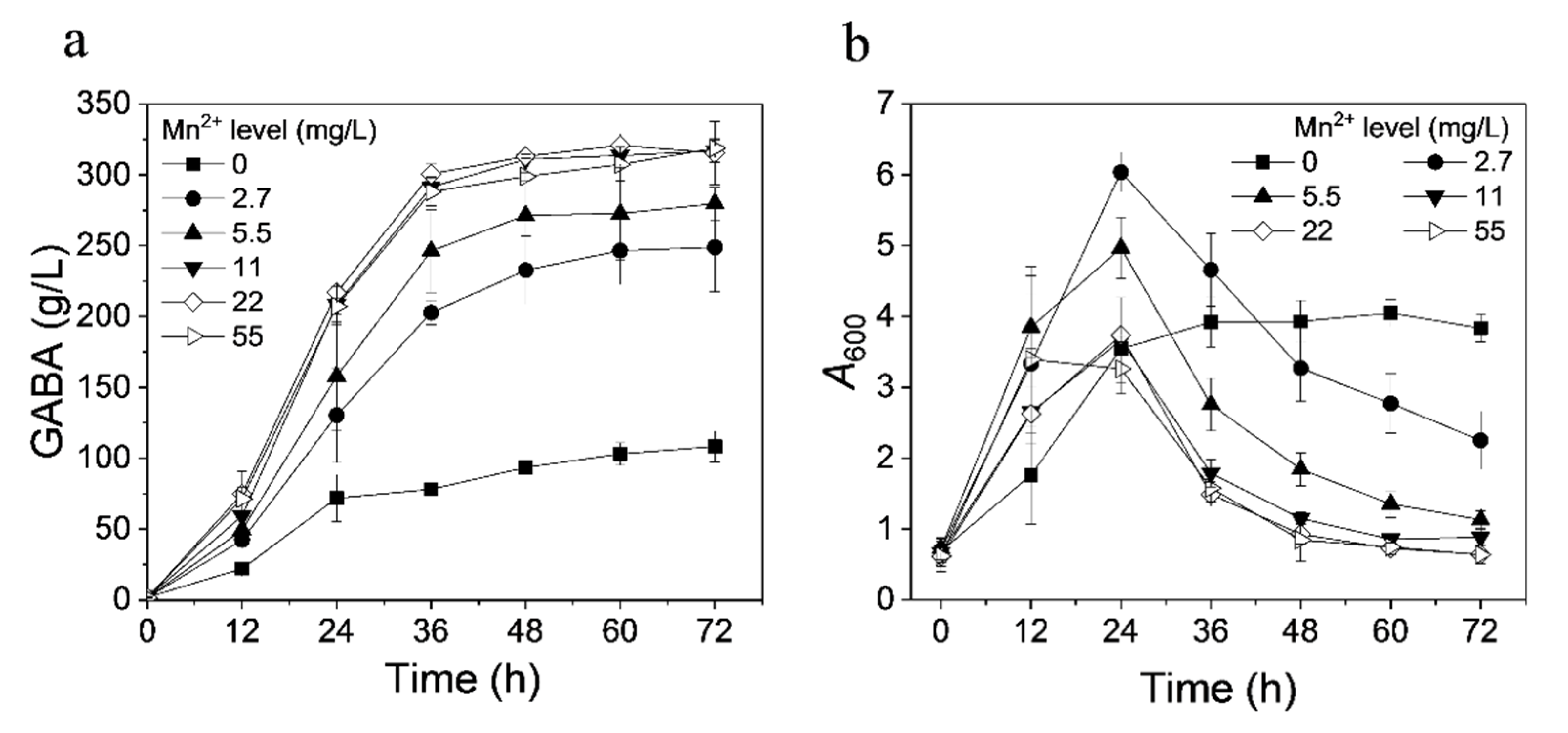
3.3. Effect of Ions on GABA Production
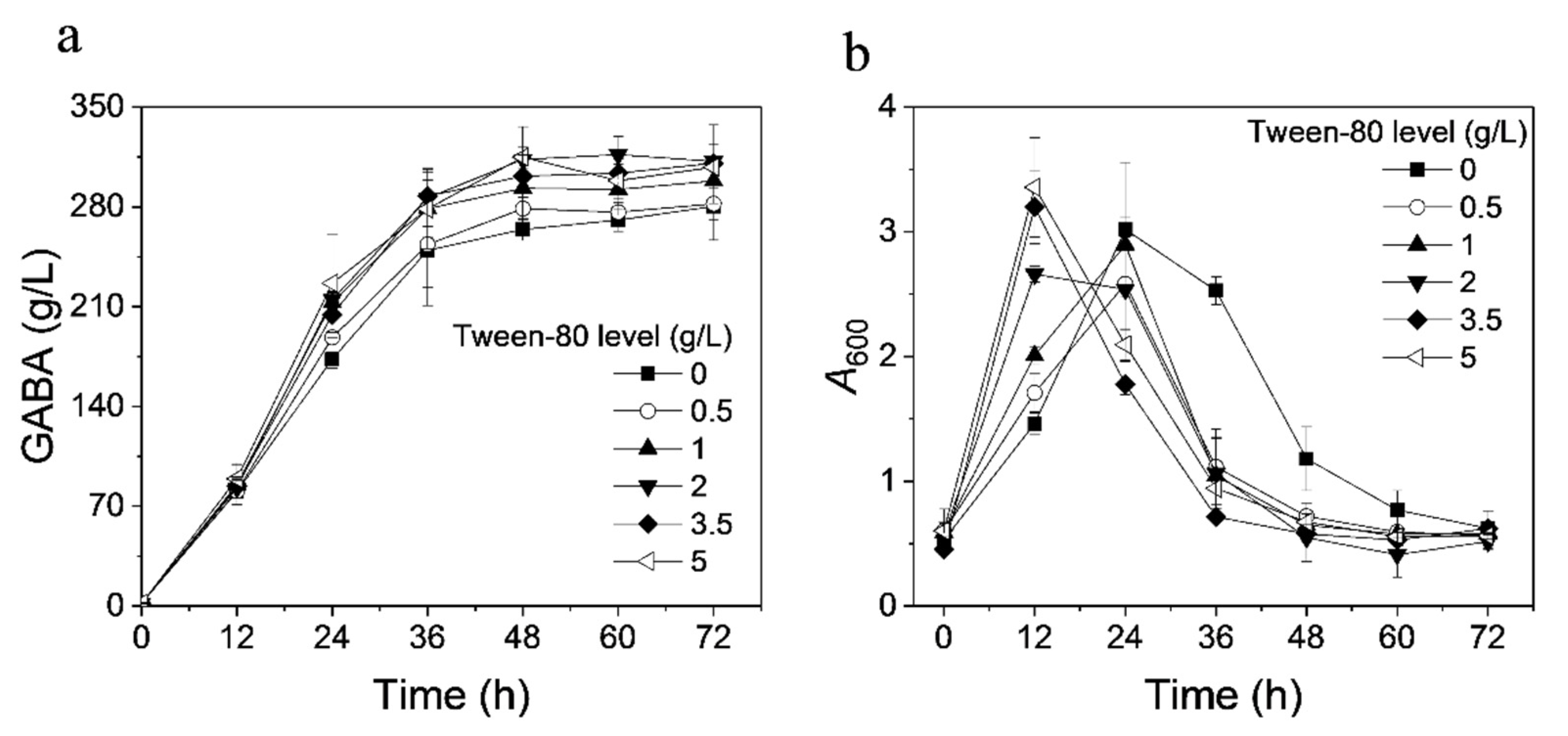
3.4. Effect of Tween-80 on GABA Production
3.5. Effect of Temperature on GABA Production
3.6. Effect of Glucose in Seed Medium on GABA Production
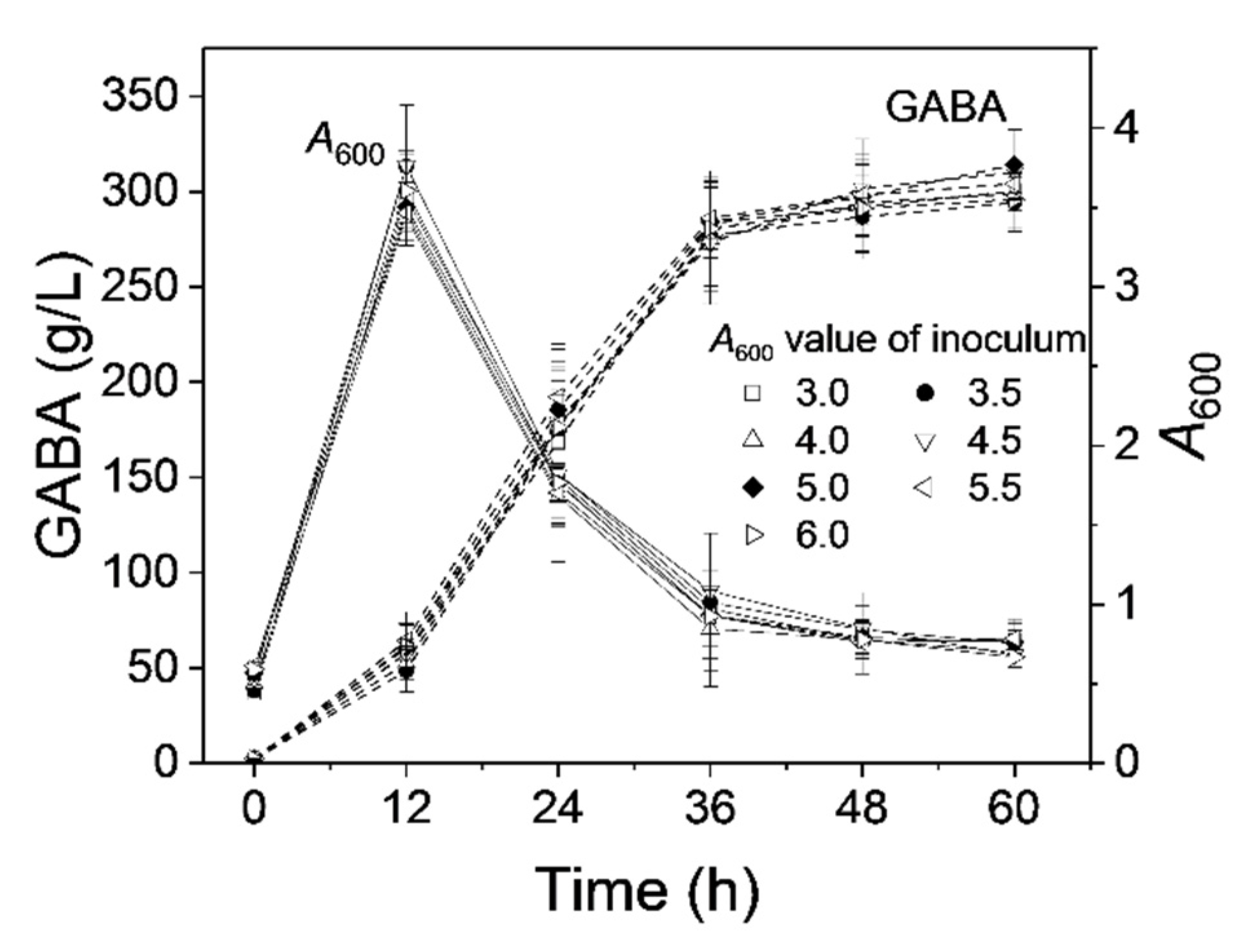
3.7. Effect of Seed Age on GABA Production
3.8. PAS-Based GABA Bioprocess
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ueno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci. Biotechnol. Biochem. 1997, 61, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Skoog, A.; Amend, J.P. Concentration and distribution of dissolved amino acids in a shallow hydrothermal system, Vulcano Island (Italy). Org. Geochem. 2004, 35, 1001–1014. [Google Scholar] [CrossRef]
- Wong, C.G.T.; Bottiglieri, T.; Snead, O.C. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rui, Q.; Han, S.; Wu, X.; Wang, X.; Wu, P.; Shen, Y.; Dai, H.; Xue, Q.; Li, Y. Reduced GABA levels in the medial prefrontal cortex are associated with cognitive impairment in patients with NMOSD. Mult. Scler. Relat. Disord. 2022, 58, 103496. [Google Scholar] [CrossRef]
- Lalwani, P.; Gagnon, H.; Cassady, K.; Simmonite, M.; Peltier, S.; Seidler, R.D.; Taylor, S.F.; Weissman, D.H.; Polk, T.A. Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. NeuroImage 2019, 201, 116033. [Google Scholar] [CrossRef]
- Wenneberg, C.; Glenthoj, B.Y.; Hjorthoj, C.; Zingenberg, F.J.B.; Glenthoj, L.B.; Rostrup, E.; Broberg, B.V.; Nordentoft, M. Cerebral glutamate and GABA levels in high-risk of psychosis states: A focused review and meta-analysis of (1)H-MRS studies. Schizophr. Res. 2020, 215, 38–48. [Google Scholar] [CrossRef]
- Hata, T.; Rehman, F.; Hori, T.; Nguyen, J.H. GABA, gamma-aminobutyric acid, protects against severe liver injury. J. Surg. Res. 2019, 236, 172–183. [Google Scholar] [CrossRef]
- Cohen, B.I. The significance of ammonia/gamma-aminobutyric acid (GABA) ratio for normality and liver disorders. Med. Hypotheses 2002, 59, 757–758. [Google Scholar] [CrossRef]
- Francois, A.; Low, S.A.; Sypek, E.I.; Christensen, A.J.; Sotoudeh, C.; Beier, K.T.; Ramakrishnan, C.; Ritola, K.D.; Sharif-Naeini, R.; Deisseroth, K.; et al. A Brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 2017, 93, 822–839. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Lee, J. Production and its anti-hyperglycemic effects of gamma-aminobutyric acid from the wild yeast strain Pichia silvicola UL6-1 and Sporobolomyces carnicolor 402-JB-1. Mycobiology 2017, 45, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Diana, M.; Quilez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Kawasaki, N.; Nakayama, A.; Yamano, N.; Takeda, S.; Kawata, Y.; Yamamoto, N.; Aiba, S. Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 2005, 46, 9987–9993. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, E.Y.; Noh, W.; Oh, Y.H.; Kim, H.Y.; Song, B.K.; Cho, K.M.; Hong, S.H.; Lee, S.H.; Jegal, J. Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli. Bioprocess Biosyst. Eng. 2013, 36, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Khare, S.K. 2-Pyrrolidone synthesis from gamma-aminobutyric acid produced by Lactobacillus brevis under solid-state fermentation utilizing toxic deoiled cottonseed cake. Bioprocess Biosyst. Eng. 2017, 40, 145–152. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N.P. High gamma-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2016, 57, 3661–3672. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Jung, D.; Cho, E.; Seo, M. Expression, purification, and characterization of glutamate decarboxylase from human gut-originated Lactococcus garvieae MJF010. World J. Microbiol. Biotechnol. 2022, 38, 69. [Google Scholar] [CrossRef]
- Mousavi, R.; Mottawea, W.; Hassan, H.; Gomaa, A.; Audet, M.C.; Hammami, R. Screening, characterization and growth of γ-aminobutyric acid-producing probiotic candidates from food origin under simulated colonic conditions. J. Appl. Microbiol. 2022. [Google Scholar] [CrossRef]
- Yao, L.; Cao, J.; Lyu, C.; Fan, F.; Wang, H.; Cao, H.; Huang, J.; Mei, L. Food-grade γ-aminobutyric acid production by immobilized glutamate decarboxylase from Lactobacillus plantarum in rice vinegar and monosodium glutamate system. Biotechnol. Lett. 2021, 43, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, P.G.; Villegas, J.M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of gamma-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Kozuka, K.; Mimura, K.; Nakano, S.; Ito, S. Design of a full-consensus glutamate decarboxylase and its application to GABA biosynthesis. ChemBioChem 2021, 23, e202100447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, Y.W.; Kim, M.S.; Lee, M.H.; Kim, G.A.; Bae, K.; Yoon, S.S. Gamma-aminobutyric acid fermentation in MRS-based medium by the fructophilic Lactiplantibacillus plantarum Y7. Food Sci. Biotechnol. 2022, 31, 333–341. [Google Scholar] [CrossRef]
- Gong, L.; Ren, C.; Xu, Y. Deciphering the crucial roles of transcriptional regulator GadR on gamma-aminobutyric acid production and acid resistance in Lactobacillus brevis. Microb. Cell Factories 2019, 18, 108. [Google Scholar] [CrossRef]
- Lyu, C.; Yao, L.; Zhu, Q.; Mei, J.; Cao, Y.; Hu, S.; Zhao, W.; Huang, J.; Mei, L.; Yao, S.; et al. Reconstruction of the glutamate decarboxylase system in Lactococcus lactis for biosynthesis of food-grade gamma-aminobutyric acid. Appl. Microbiol. Biotechnol. 2021, 105, 4127–4140. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 2018, 69, 151–158. [Google Scholar] [CrossRef]
- Gong, L.; Ren, C.; Xu, Y. GlnR negatively regulates glutamate-dependent acid resistance in Lactobacillus brevis. Appl. Environ. Microbiol. 2020, 86, e02615–e02619. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Gao, Q.; Yu, S.; Li, L.; Gao, N. The two-step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Appl. Microbiol. Biotechnol. 2012, 94, 1619–1627. [Google Scholar] [CrossRef]
- Binh, T.T.T.; Ju, W.; Jung, W.; Park, R. Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef]
- Zhao, A.; Hu, X.; Pan, L.; Wang, X. Isolation and characterization of a gamma-aminobutyric acid producing strain Lactobacillus buchneri WPZ001 that could efficiently utilize xylose and corncob hydrolysate. Appl. Microbiol. Biotechnol. 2015, 99, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Hayashi, H.; Abe, K. Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997, 179, 3362–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Factories 2010, 9, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liu, X.; Fu, J.; Wang, S.; Chen, Y.; Chang, K.; Li, H. Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microb. Cell Factories 2018, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Chang, K.; Ding, G.; Wu, H.; Chen, Y.; Jia, M.; Liu, X.; Wang, S.; Jin, Y.; Pan, H.; et al. Genomic insights into a robust gamma-aminobutyric acid-producer Lactobacillus brevis CD0817. AMB Express 2019, 9, 72. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Cao, Y.; Xu, H. A high gamma-aminobutyric acid-producing Lactobacillus brevis isolated from Chinese traditional paocai. Ann. Microbiol. 2008, 58, 649–653. [Google Scholar] [CrossRef]
- Wu, C.; Hsueh, Y.; Kuo, J.; Liu, S. Characterization of a potential probiotic Lactobacillus brevis RK03 and efficient production of γ-aminobutyric acid in batch fermentation. Int. J. Mol. Sci. 2018, 19, 143. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Zhang, L.; Xin, Y.; Xu, Z.; He, H.; Kong, J. Oxygen-inducible conversion of lactate to acetate in heterofermentative Lactobacillus brevis ATCC 367. Appl. Environ. Microbiol. 2017, 83, e01659-17. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P. Lactobacillus: Lactobacillus brevis. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 418–424. [Google Scholar] [CrossRef]
- Sa, H.D.; Park, J.Y.; Jeong, S.J.; Lee, K.W.; Kim, J.H. Characterization of glutamate decarboxylase (GAD) from Lactobacillus sakei A156 isolated from Jeot-gal. J. Microbiol. Biotechnol. 2015, 25, 696–703. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate decarboxylase from lactic acid bacteria-a key enzyme in GABA synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Seo, M.; Nam, Y.; Lee, S.; Park, S.; Yi, S.; Lim, S. Expression and characterization of a glutamate decarboxylase from Lactobacillus brevis 877G producing γ-aminobutyric acid. Biosci. Biotechnol. Biochem. 2013, 77, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tun, H.M.; Law, Y.S.; Khafipour, E.; Shan, N.P. Common distribution of gad operon in Lactobacillus brevis and its GadA contributes to efficient GABA synthesis toward cytosolic near-neutral pH. Front. Microbiol. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, C.; Zhao, W.; Peng, C.; Hu, S.; Fang, H.; Hua, Y.; Yao, S.; Huang, J.; Mei, L. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Factories 2018, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Cha, I.; Roh, S.; Shin, H.; Seo, M. Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J. Microbiol. Biotechnol. 2017, 27, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.; Yang, J.; Jung, Y.H. Enhanced production of gamma-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT-Food Sci. Technol. 2021, 137, 110443. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Jooyandeh, H.; Falah, F.; Vasiee, A. Gamma-aminobutyric acid production by Lactobacillus brevis A3: Optimization of production, antioxidant potential, cell toxicity, and antimicrobial activity. Food Sci. Nutr. 2020, 8, 5330–5339. [Google Scholar] [CrossRef]
- Laroute, V.; Mazzoli, R.; Loubiere, P.; Pessione, E.; Cocaign-Bousquet, M. Environmental conditions affecting GABA production in Lactococcus lactis NCDO 2118. Microorganisms 2021, 9, 122. [Google Scholar] [CrossRef]
- Yang, S.; Lin, Q.; Lu, Z.; Lu, F.; Bie, X.; Zou, X.; Sun, L. Characterization of a novel glutamate decarboxylase from Streptococcus salivarius ssp thermophilus Y2. J. Chem. Technol. Biotechnol. 2008, 83, 855–861. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, S.J.; Kim, J.H. Characterization of a glutamate decarboxylase (GAD) gene from Lactobacillus zymae. Biotechnol. Lett. 2014, 36, 1791. [Google Scholar] [CrossRef]
- Ueno, H. Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Bashokouh, F.; Ramakrishnan, N.R.; Mustafa, S.; Ariff, A.B. Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: A review. RSC Adv. 2017, 7, 29395–29420. [Google Scholar] [CrossRef]
- Del Valle, M.J.; Laiño, J.E.; de Giori, G.S.; Leblanc, J.G. Factors stimulating riboflavin produced by Lactobacillus plantarum CRL 725 grown in a semi-defined medium. J. Basic Microbiol. 2017, 57, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tanamool, V.; Hongsachart, P.; Soemphol, W. Screening and characterisation of gamma-aminobutyric acid (GABA) producing lactic acid bacteria isolated from Thai fermented fish (Plaa-som) in Nong Khai and its application in Thai fermented vegetables (Som-pak). Food Sci. Technol. 2020, 40, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Villegas, J.M.; Brown, L.; de Giori, G.S.; Hebert, E.M. Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT-Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Bremer, E.; Krämer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Small, P.L.C.; Waterman, S.R. Acid stress, anaerobiosis and gadCB lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 1998, 6, 214–216. [Google Scholar] [CrossRef]
- Xiao, T.; Shah, N.P. Lactic acid produced by Streptococcus thermophilus activated glutamate decarboxylase (GadA) in Lactobacillus brevis NPS-QW 145 to improve gamma-amino butyric acid production during soymilk fermentation. LWT-Food Sci. Technol. 2021, 137, 110474. [Google Scholar] [CrossRef]


| Strain | Method | Substrate | Titer (g/L) | Conversion Rate (%) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|
| Lactobacillus brevis CD0817 | pH auto-sustain | L-glutamic acid | 321.9 | 99.6 | 6.7 | This study |
| Lactobacillusbrevis ATCC 367ΔglnR | pH-controlled | MSG | 284.7 | NS | NS | [28] |
| Lactobacillus brevis D17 | pH-controlled | MSG | 177.7 | NS | 4.9 | [25] |
| Lactobacillus brevis 9530: pNZ8148-gadBC | pH-controlled | MSG | 104.4 | NS | NS | [44] |
| Lactobacillus buchneri WPZ001 | NS | MSG | 75.5 | NS | NS | [31] |
| Lactobacillus brevis RK03 | NS | MSG | 62.5 | 93.3 | NS | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Zhu, Y.; Wang, L.; Sun, T.; Pan, H.; Li, H. pH Auto-Sustain-Based Fermentation Supports Efficient Gamma-Aminobutyric Acid Production by Lactobacillus brevis CD0817. Fermentation 2022, 8, 208. https://doi.org/10.3390/fermentation8050208
Jia M, Zhu Y, Wang L, Sun T, Pan H, Li H. pH Auto-Sustain-Based Fermentation Supports Efficient Gamma-Aminobutyric Acid Production by Lactobacillus brevis CD0817. Fermentation. 2022; 8(5):208. https://doi.org/10.3390/fermentation8050208
Chicago/Turabian StyleJia, Mengya, Yisong Zhu, Lingqin Wang, Tianyi Sun, Hao Pan, and Haixing Li. 2022. "pH Auto-Sustain-Based Fermentation Supports Efficient Gamma-Aminobutyric Acid Production by Lactobacillus brevis CD0817" Fermentation 8, no. 5: 208. https://doi.org/10.3390/fermentation8050208
APA StyleJia, M., Zhu, Y., Wang, L., Sun, T., Pan, H., & Li, H. (2022). pH Auto-Sustain-Based Fermentation Supports Efficient Gamma-Aminobutyric Acid Production by Lactobacillus brevis CD0817. Fermentation, 8(5), 208. https://doi.org/10.3390/fermentation8050208








