Protection against Osteoporosis by Fermented Mulberry Vinegar Supplementation via Inhibiting Osteoclastic Activity in Ovariectomized Rats and Osteoclastic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. MV Production by Fermentation with Microorganisms
2.2. MV Quality
2.3. Ovariectomy (OVX) Operation for Rats
2.4. Experimental Design for Animal Study
2.5. Micro-Computed Tomography (Micro-CT)
2.6. Biochemical Assays for Blood
2.7. Cell Culture and Cytotoxicity Assay
2.8. TRAP Staining
2.9. Real-Time PCR Analysis
2.10. Immunoblot Analysis
2.11. Statistical Analysis
3. Results
3.1. Characteristics of MV and Its Rutin Contents
3.2. Weight Gain and Visceral Fat Mass
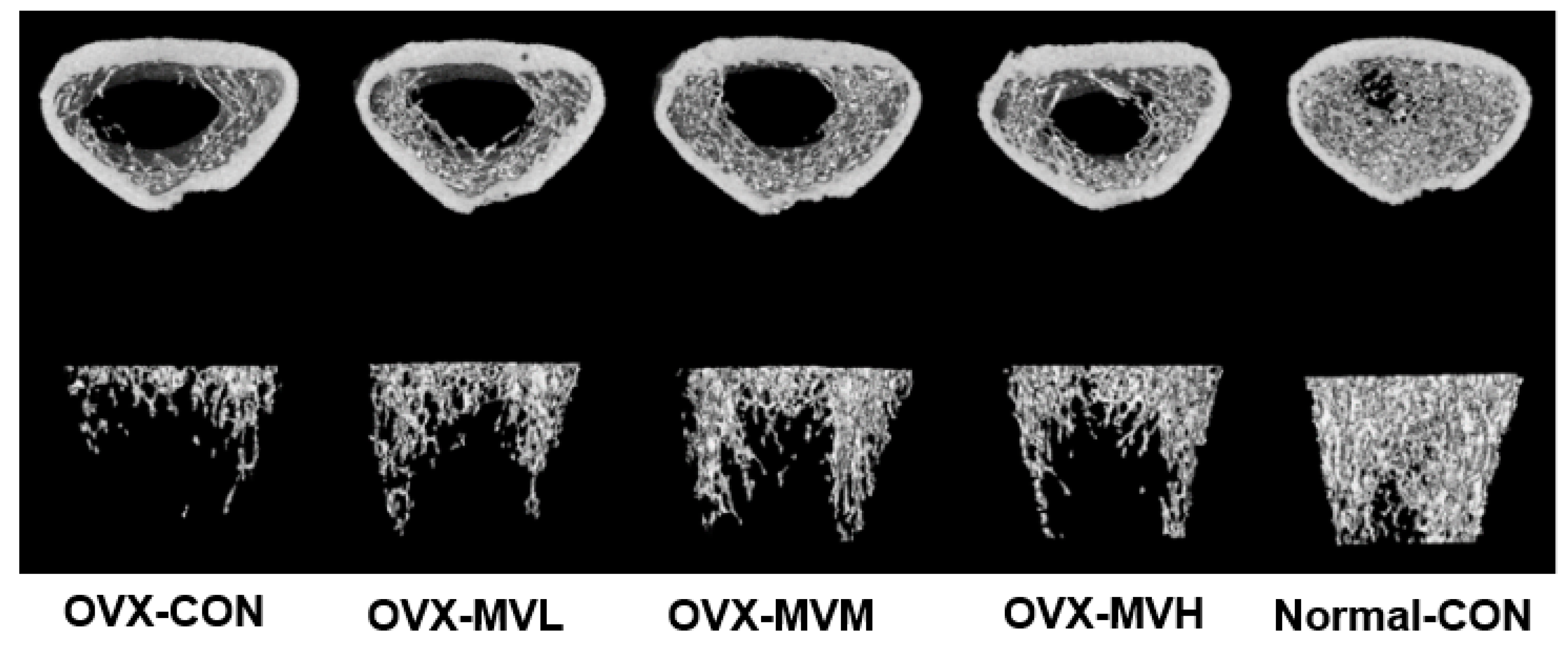
3.3. BMD by Micro-CT
3.4. Cell Viability RANKL-Induced Osteoclasts from RAW 264.7 Cells
3.5. TRAP-Positive Cells and TRAP Activity
3.6. mRNA Expression of Osteoclast-Related Genes
3.7. Osteoclast-Related Protein Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Daily, J.W.; Song, M.Y.; Kwon, H.K. Gene-gene and gene-lifestyle interactions of AKAP11, KCNMA1, PUM1, SPTBN1, and EPDR1 on osteoporosis risk in middle-aged adults. Nutrition 2020, 79–80, 110859. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [Green Version]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, S.; Sun, K.; Wang, J.; Liu, X.; Zhao, Y.; Yang, J.; Zhao, D.; Xue, C.; Tao, Y.; et al. Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet. Disord. 2021, 22, 494. [Google Scholar] [CrossRef]
- Ilesanmi-Oyelere, B.L.; Schollum, L.; Kuhn-Sherlock, B.; McConnell, M.; Mros, S.; Coad, J.; Roy, N.C.; Kruger, M.C. Inflammatory markers and bone health in postmenopausal women: A cross-sectional overview. Immun. Ageing 2019, 16, 15. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Gamsjaeger, S.; Hassler, N.; Fahrleitner-Pammer, A.; Dobnig, H.; Stepan, J.J.; Pavo, I.; Eriksen, E.F.; Klaushofer, K. Vitamin D and calcium supplementation for three years in postmenopausal osteoporosis significantly alters bone mineral and organic matrix quality. Bone 2017, 95, 41–46. [Google Scholar] [CrossRef]
- Park, S.; Park, C.Y.; Ham, J.O.; Lee, B.K. Familial interactions and physical, lifestyle, and dietary factors to affect bone mineral density of children in the KNHANES 2009–2010. J. Bone Miner. Metab. 2014, 32, 455–467. [Google Scholar] [CrossRef]
- Gambacciani, M.; Cagnacci, A.; Lello, S. Hormone replacement therapy and prevention of chronic conditions. Climacteric 2019, 22, 303–306. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed] [Green Version]
- Picherit, C.; Bennetau-Pelissero, C.; Chanteranne, B.; Lebecque, P.; Davicco, M.-J.; Barlet, J.-P.; Coxam, V. Soybean Isoflavones Dose-Dependently Reduce Bone Turnover but Do Not Reverse Established Osteopenia in Adult Ovariectomized Rats. J. Nutr. 2001, 131, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji-Sun, K.; Hyunjung, L.; Farida Sukma, N.; Chang Hwa, J.; Young-Jin, J.; Tae-Youl, H.; Jiyun, A. Dry-Fermented Soybean Food (Cheonggukjang) Ameliorates Senile Osteoporosis in the Senescence-Accelerated Mouse Prone 6 Model. J. Med. Food 2019, 22, 1047–1057. [Google Scholar]
- Park, S.; Zhang, T.; Qiu, J.Y.; Wu, X. The Combination of Mulberry Extracts and Silk Amino Acids Alleviated High Fat Diet-Induced Nonalcoholic Hepatic Steatosis by Improving Hepatic Insulin Signaling and Normalizing Gut Microbiome Dysbiosis in Rats. Evid. Based Complement. Alternat. Med. 2019, 2019, 8063121. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Wu, X.; Qiu, J.Y. Mulberry and dandelion water extracts prevent alcohol-induced steatosis with alleviating gut microbiome dysbiosis. Exp. Biol. Med. 2018, 243, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Jao, H.Y.; Hsu, J.D.; Lee, Y.R.; Lo, C.S.; Lee, H.J. Mulberry water extract regulates the osteoblast/osteoclast balance in an ovariectomized rat model. Food Funct. 2016, 7, 4753–4763. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Func. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Se-In, B.; Geon Hyeong, G.; Eun Ju, C.; Ah Young, L.; Weon Taek, S. Characteristics of fermented vinegar using mulberry and its antioxidant activity. Korean Soc. Food Preserv. 2020, 27, 651–662. [Google Scholar]
- Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Ryuk, J.A.; Park, S. Supplementing with Opuntia ficus-indica Mill and Dioscorea nipponica Makino extracts synergistically attenuates menopausal symptoms in estrogen-deficient rats. J. Ethnopharmacol. 2014, 155, 267–276. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Method Association of Official Analytical Communities, 19th ed.; AOAC International: Arlington, VA, USA, 2012. [Google Scholar]
- Yang, H.J.; Kim, M.J.; Qiu, J.Y.; Zhang, T.; Wu, X.; Jang, D.J.; Park, S. Rice Porridge Containing Welsh Onion Root Water Extract Alleviates Osteoarthritis-Related Pain Behaviors, Glucose Levels, and Bone Metabolism in Osteoarthritis-Induced Ovariectomized Rats. Nutrients 2019, 11, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, D.S.; Kang, S.; Moon, B.R. Fermented soybeans, Chungkookjang, prevent hippocampal cell death and beta-cell apoptosis by decreasing proinflammatory cytokines in gerbils with transient artery occlusion. Exp. Biol. Med. 2015, 241, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Park, S.Y.; Lee, S.J.; Boo, Y.C.; Choi, J.Y.; Kim, J.E. Ucma, a direct transcriptional target of Runx2 and Osterix, promotes osteoblast differentiation and nodule formation. Osteoarthr. Cartil. 2015, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Uehara, S.; Udagawa, N.; Li, F.; Kadota, S.; Esumi, H.; Kobayashi, Y.; Takahashi, N. Arctigenin Inhibits Osteoclast Differentiation and Function by Suppressing Both Calcineurin-Dependent and Osteoblastic Cell-Dependent NFATc1 Pathways. PLoS ONE 2014, 9, e85878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, D.S.; Kang, E.S.; Kim, D.B.; Kang, S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E99–E109. [Google Scholar] [CrossRef]
- Park, S.; Hong, S.M.; Ahn, I.L.S.; Kim, D.S.; Kim, S.H. Estrogen Replacement Reverses Olanzapine-Induced Weight Gain and Hepatic Insulin Resistance in Ovariectomized Diabetic Rats. Neuropsychobiology 2010, 61, 148–161. [Google Scholar] [CrossRef]
- Yang, H.J.; Ko, B.S.; Kwon, D.Y.; Lee, H.W.; Kim, M.J.; Ryuk, J.; Kang, S.; Kim, D.S.; Park, S. Asian Elm tree inner bark prevents articular cartilage deterioration in ovariectomized obese rats with monoiodoacetate-induced osteoarthritis. Menopause 2016, 23, 197–208. [Google Scholar] [CrossRef]
- Lee, H.W.; Ko, B.S.; Kang, S.; Ryuk, J.A.; Kim, M.J.; Park, S. Dangguijihwang-tang and Dangguijakyak-san prevent menopausal symptoms, and Dangguijihwang-tang prevents articular cartilage deterioration in ovariectomized obese rats with monoiodoacetate-induced osteoarthritis. Evid. Based Complement. Alternat. Med. 2017, 2017, 5658681. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhou, L.; Liu, Q.; Tickner, J.; Tan, Z.; Li, X.; Liu, M.; Lin, X.; Wang, T.; Pavlos, N.J.; et al. Cyanidin Chloride inhibits ovariectomy-induced osteoporosis by suppressing RANKL-mediated osteoclastogenesis and associated signaling pathways. J. Cell Physiol. 2018, 233, 2502–2512. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, H.; Kim, M.; Kim, S.; Song, K.; Jung, H.S.; Sohn, Y. Solanum nigrum Line inhibits osteoclast differentiation and suppresses bone mineral density reduction in the ovariectomy-induced osteoporosis model. Mol. Med. Rep. 2021, 24, 607. [Google Scholar] [CrossRef]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef] [PubMed]
- Novack, D.V. Estrogen and Bone: Osteoclasts Take Center Stage. Cell Metab. 2007, 6, 254–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Q.; Li, Y.; Sun, P.; Kuek, V.; Yuan, J.; Yang, J.; Wen, L.; Wang, H.; Xu, J.; et al. Notopterol Attenuates Estrogen Deficiency-Induced Osteoporosis via Repressing RANKL Signaling and Reactive Oxygen Species. Front. Pharmacol. 2021, 12, 664836. [Google Scholar] [CrossRef]
- Hsiao, Y.-M.; Hu, C.-C.; Chen, M.-F.; Chang, C.-H.; Chiu, Y.-T.; Chang, Y. Serum Insufficiency Induces RANKL-Independent Osteoclast Formation during Developing Ischemic ONFH. Biomedicines 2021, 9, 685. [Google Scholar] [CrossRef]






| Mean ± SD | Mean ± SD | ||
|---|---|---|---|
| pH | 2.26 ± 0.01 | Oxalic acid (mg/mL) | No detection |
| Acidity (%) | 4.07 ± 0.01 | Citric acid (mg/mL) | 106.0 ± 0.06 |
| Live bacteria counts (log CFU/mL) | 7.31 ± 0.23 | Succinic acid (mg/mL) | No detection |
| Rutin (mg/g) | 0.36 ± 0.04 | Lactic acid (mg/mL) | 19.2 ± 0.04 |
| Total phenol (μg gallic acid equivalent/mL) | 570 ± 0.18 | Acetic acid (mg/mL) | 15.0 ± 0.09 |
| Total flavonoids (μg quercetin equivalent/mL) | 80.0 ± 3.03 |
| OVX-CON | OVX-MVL | OVX-MVM | OVX-MVH | Normal-CON | |
|---|---|---|---|---|---|
| Final weight (g) | 411.2 ± 4.92 *** | 413.7 ± 9.38 *** | 402.5 ± 3.50 *** | 408.8 ± 5.52 *** | 320.5 ± 3.95 |
| Weight gain (g) | 138.6 ± 5.56 *** | 142.9 ± 6.77 *** | 129.4 ± 5.27 *** | 136.5 ± 5.48 *** | 54.04 ± 2.10 |
| Uterus weight (g) | 0.105 ± 0.003 *** | 0.119 ± 0.005 *** | 0.126 ± 0.009 *** | 0.121 ± 0.010 *** | 0.695 ± 0.098 |
| Serum 17β-estradiol(pg/mL) | 35.28 ± 3.96 ** | 37.29 ± 5.32 ** | 42.20 ± 5.32 # | 38.64 ± 3.93 * | 45.27 ± 4.81 |
| Serum CTX-1 (ng/mL) | 3.79 ± 0.62 ** | 3.60 ± 0.48 ** | 3.21 ± 0.66 * | 2.78 ± 0.55 # | 2.46 ± 0.36 |
| Serum OPG (ng/mL) | 1.17 ± 0.09 * | 1.42 ± 0.11 # | 1.46 ± 0.07 *,# | 1.50 ± 0.11 *,#,+ | 1.31 ± 0.15 |
| Serum RANKL (pg/mL) | 30.31 ± 6.17 ** | 23.86 ± 4.97 # | 25.33 ± 4.20 *,# | 24.27 ± 3.06 # | 20.01 ± 2.79 |
| Serum TRAP (U/L) | 4.39 ± 0.22 ** | 3.95 ± 0.29 **,# | 4.01 ± 0.30 ***,# | 4.05 ± 0.26 ***,# | 3.10 ± 0.20 |
| Parameter | OVX-CON | OVX-MVL | OVX-MVM | OVX-MVH | Normal-CON |
|---|---|---|---|---|---|
| BV/TV (%) | 54.50 ± 1.22 | 54.38 ± 0.902 | 53.12 ± 0.691 | 53.22 ± 0.434 | 57.75 ± 1.07 |
| BS/BV (1/mm) | 4.751 ± 0.227 | 5.356 ± 0.301 | 5.697 ± 0.504 | 5.250 ± 0.208 | 7.581 ± 0.182 |
| TB_Th (mm) | 0.082 ± 0.003 | 0.096 ± 0.006 | 0.100 ± 0.002 | 0.106 ± 0.004 | 0.096 ± 0.006 |
| Tb.N (1/mm) | 1.312 ± 0.049 | 1.325 ± 0.067 | 1.382 ± 0.072 | 1.318 ± 0.047 | 1.878 ± 0.049 |
| Tb.Sp (mm) | 0.777 ± 0.034 | 0.776 ± 0.023 | 0.734 ± 0.022 | 0.749 ± 0.018 | 0.520 ± 0.030 |
| BMD (mg/cm3) | 125.9 ± 4.42 *** | 152.1 ± 12.52 *** | 168.8 ± 21.59 ***,# | 146.5 ± 6.150 *** | 321.6 ± 8.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, E.J.; Jo, S.W.; Kang, H.J.; Park, S.K.; Yu, K.Y.; Jeong, D.-Y.; Park, S. Protection against Osteoporosis by Fermented Mulberry Vinegar Supplementation via Inhibiting Osteoclastic Activity in Ovariectomized Rats and Osteoclastic Cells. Fermentation 2022, 8, 211. https://doi.org/10.3390/fermentation8050211
Yim EJ, Jo SW, Kang HJ, Park SK, Yu KY, Jeong D-Y, Park S. Protection against Osteoporosis by Fermented Mulberry Vinegar Supplementation via Inhibiting Osteoclastic Activity in Ovariectomized Rats and Osteoclastic Cells. Fermentation. 2022; 8(5):211. https://doi.org/10.3390/fermentation8050211
Chicago/Turabian StyleYim, Eun Jung, Seung Wha Jo, Hyeon Jin Kang, Seul Ki Park, Kang Yeol Yu, Do-Youn Jeong, and Sunmin Park. 2022. "Protection against Osteoporosis by Fermented Mulberry Vinegar Supplementation via Inhibiting Osteoclastic Activity in Ovariectomized Rats and Osteoclastic Cells" Fermentation 8, no. 5: 211. https://doi.org/10.3390/fermentation8050211
APA StyleYim, E. J., Jo, S. W., Kang, H. J., Park, S. K., Yu, K. Y., Jeong, D.-Y., & Park, S. (2022). Protection against Osteoporosis by Fermented Mulberry Vinegar Supplementation via Inhibiting Osteoclastic Activity in Ovariectomized Rats and Osteoclastic Cells. Fermentation, 8(5), 211. https://doi.org/10.3390/fermentation8050211







