A Review on the Production of C4 Platform Chemicals from Biochemical Conversion of Sugar Crop Processing Products and By-Products
Abstract
1. Introduction
2. Microbial Production of 2,3-BDO and Acetoin
2.1. Microbial Production of 2,3-BDO
S. cerevisiae Production of 2,3-BDO
2.2. Microbial Production of Acetoin
S. cerevisiae Production of Acetoin
2.3. Summary of Optimization Strategies for Acetoin and 2,3-Butanediol Production
3. Microbial Production of C4 Dicarboxylic Acids
3.1. Exporter Engineering and Metabolic Engineering
3.2. Malic Acid
3.3. Succinic Acid
3.4. Co-Production Strategies during Fermentation
3.5. Summary of Optimization Strategies for C4 Dicarboxylic Acids
4. Fermentation on Sugar Crop Processing Products and By-Products
4.1. Acetoin and 2,3-Butanediol from Molasses
4.2. C4 Dicarboxylic Acids from Molasses
4.3. C4 Platform Chemicals from Lignocellulosic By-Products
5. Opportunities for Downstream Upgrading
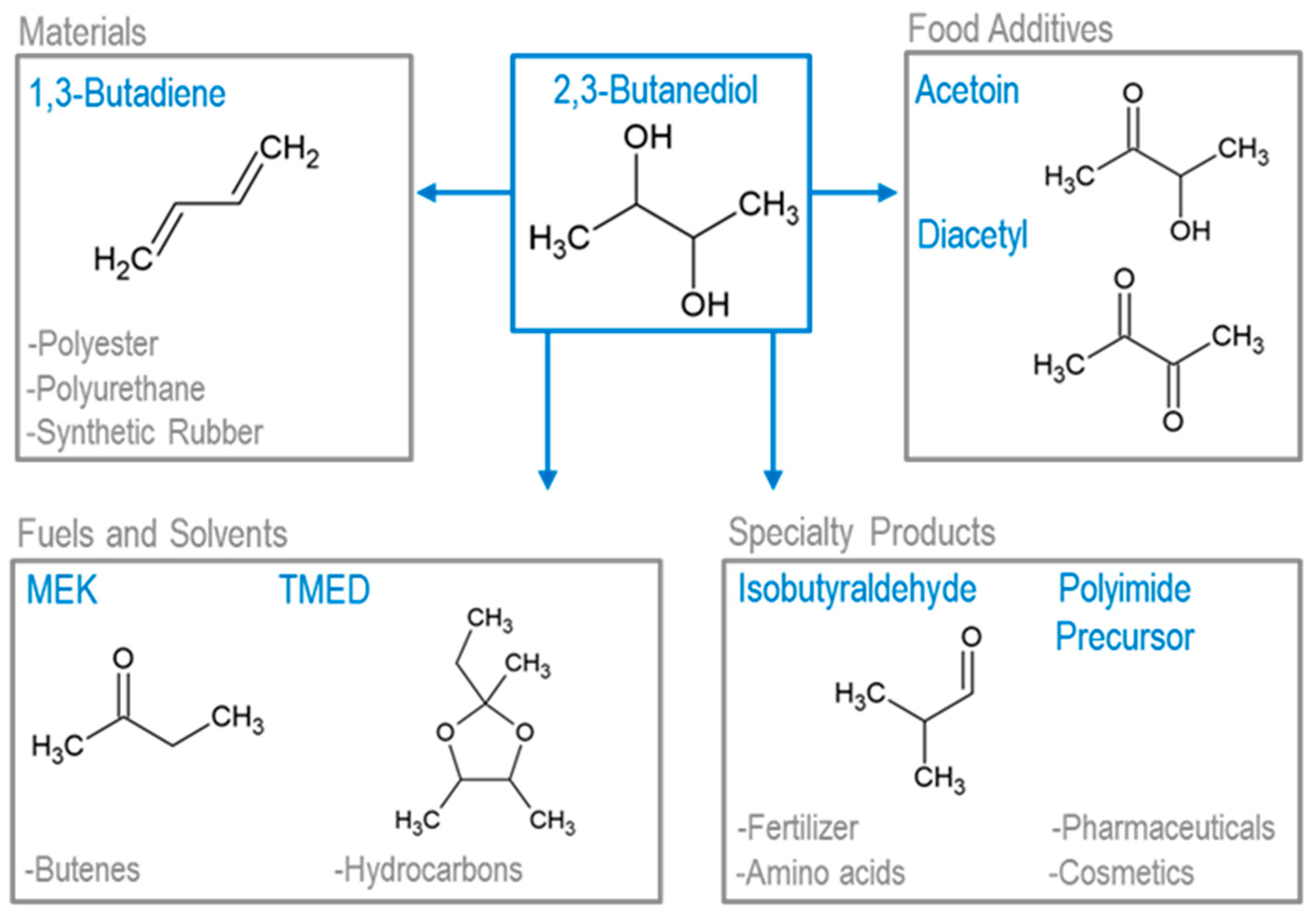
5.1. Upgrading Acetoin and 2,3-Butanediol
5.2. Upgrading C4 Dicarboxylic Acids
6. Summary and Outlook
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefin. 2016, 11, 195–214. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The Road to Biorenewables: Carbohydrates to Commodity Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 4464–4480. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Ou, L.; Kim, H.; Kelley, S.; Park, S. Impacts of feedstock properties on the process economics of fast-pyrolysis biorefineries. Biofuels Bioprod. Biorefin. 2018, 12, 442–452. [Google Scholar] [CrossRef]
- Morales-Vera, R.; Crawford, J.; Dou, C.; Bura, R.; Gustafson, R. Techno-Economic Analysis of Producing Glacial Acetic Acid from Poplar Biomass via Bioconversion. Molecules 2020, 25, 4328. [Google Scholar] [CrossRef]
- Boakye-Boaten, N.A.; Kurkalova, L.; Xiu, S.; Shahbazi, A. Techno-economic analysis for the biochemical conversion of Miscanthus x giganteus into bioethanol. Biomass Bioenergy 2017, 98, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Z.; Li, Y.; Cao, L.; Lu, Z. Techno-economic analysis and environmental impact assessment of citric acid production through different recovery methods. J. Clean. Prod. 2020, 249, 119315. [Google Scholar] [CrossRef]
- Casson Moreno, V.; Iervolino, G.; Tugnoli, A.; Cozzani, V. Techno-economic and environmental sustainability of biomass waste conversion based on thermocatalytic reforming. Waste Manag. 2020, 101, 106–115. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Lima, I. Sustainability Issues and Opportunities in the Sugar and Sugar-Bioproduct Industries. Sustainability 2015, 7, 12209–12235. [Google Scholar] [CrossRef]
- Meghana, M.; Shastri, Y. Sustainable valorization of sugar industry waste: Status, opportunities, and challenges. Bioresour. Technol. 2020, 303, 122929. [Google Scholar] [CrossRef] [PubMed]
- USDA. Sugar: World Markets and Trade. Available online: https://www.fas.usda.gov/data/sugar-world-markets-and-trade (accessed on 3 March 2022).
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuels Bioprod. Biorefin. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Dotaniya, C.K.; Meena, B.L.; Rajendiran, S.; Regar, K.L.; Lata, M. Use of sugarcane industrial by-products for improving sugarcane productivity and soil health. Int. J. Recycl. Org. Waste Agric. 2016, 5, 185–194. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Crecchio, C.; Pizzigallo, M.D.R.; Ruggiero, P. Synergistic effects of cellulolytic and pectinolytic enzymes in degrading sugar beet pulp. Bioresour. Technol. 1997, 60, 215–222. [Google Scholar] [CrossRef]
- Zieminski, K.; Kowalska-Wentel, M. Effect of Different Sugar Beet Pulp Pretreatments on Biogas Production Efficiency. Appl. Biochem. Biotechnol. 2017, 181, 1211–1227. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. A Review on the Complete Utilization of the Sugarbeet. Sugar Tech 2013, 16, 339–346. [Google Scholar] [CrossRef]
- Klasson, K.T.; Qureshi, N.; Powell, R.; Heckemeyer, M.; Eggleston, G. Fermentation of Sweet Sorghum Syrup to Butanol in the Presence of Natural Nutrients and Inhibitors. Sugar Tech 2018, 20, 224–234. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Klasson, K.T.; Liu, S. Butanol production from sweet sorghum bagasse with high solids content: Part I-comparison of liquid hot water pretreatment with dilute sulfuric acid. Biotechnol. Prog. 2018, 34, 960–966. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Klasson, K.T.; Liu, S. High solid fed-batch butanol fermentation with simultaneous product recovery: Part II-process integration. Biotechnol. Prog. 2018, 34, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Boone, S.A. Bioethanol fermentation of clarified sweet sorghum (Sorghum bicolor (L.) Moench) syrups sealed and stored under vegetable oil. Ind. Crops Prod. 2021, 163, 113330. [Google Scholar] [CrossRef]
- Wright, M.; Klasson, K.T.; Kimura, K. Production of Acetoin from Sweet Sorghum Syrup and Beet Juice via Fermentation. Sugar Tech 2019, 22, 354–359. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Rego, G.C.; Ramos, L.R.; de Menezes, C.A.; Silva, E.L. Improved dark fermentation of cane molasses in mesophilic and thermophilic anaerobic fluidized bed reactors by selecting operational conditions. Int. J. Energy Res. 2020, 44, 10442–10452. [Google Scholar] [CrossRef]
- Xu, C.; Alam, M.A.; Wang, Z.; Peng, Y.; Xie, C.; Gong, W.; Yang, Q.; Huang, S.; Zhuang, W.; Xu, J. Co-fermentation of succinic acid and ethanol from sugarcane bagasse based on full hexose and pentose utilization and carbon dioxide reduction. Bioresour. Technol. 2021, 339, 125578. [Google Scholar] [CrossRef]
- Chacón, S.J.; Matias, G.; Vieira, C.F.d.S.; Ezeji, T.C.; Maciel Filho, R.; Mariano, A.P. Enabling butanol production from crude sugarcane bagasse hemicellulose hydrolysate by batch-feeding it into molasses fermentation. Ind. Crops Prod. 2020, 155, 112837. [Google Scholar] [CrossRef]
- Cavalcante, W.A.; Gehring, T.A.; Santaella, S.T.; Freitas, I.B.F.; Angenent, L.T.; van Haandel, A.C.; Leitão, R.C. Upgrading sugarcane biorefineries: Acetate addition allows for conversion of fermented sugarcane molasses into high-value medium chain carboxylic acids. J. Environ. Chem. Eng. 2020, 8, 103649. [Google Scholar] [CrossRef]
- Wu, R.; Chen, D.; Cao, S.; Lu, Z.; Huang, J.; Lu, Q.; Chen, Y.; Chen, X.; Guan, N.; Wei, Y.; et al. Enhanced ethanol production from sugarcane molasses by industrially engineered Saccharomyces cerevisiae via replacement of the PHO4 gene. RSC Adv. 2020, 10, 2267–2276. [Google Scholar] [CrossRef]
- Unrean, P.; Ketsub, N. Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind. Crops Prod. 2018, 123, 238–246. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Farzad, S.; Görgens, J.F. Furfural production from sugarcane bagasse along with co-production of ethanol from furfural residues. Biomass Convers. Biorefinery 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Valorization of sugar beet pulp through biotechnological approaches: Recent developments. Biotechnol. Lett. 2021, 43, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, J.; Bieliński, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevsky, A.; Witońska, I.A. Products of sugar beet processing as raw materials for chemicals and biodegradable polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, C.; Cheng, Y.-S.; Lee, C.; Simmons, C.W.; Dooley, T.M.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Integrating sugar beet pulp storage, hydrolysis and fermentation for fuel ethanol production. Appl. Energy 2012, 93, 168–175. [Google Scholar] [CrossRef]
- Berlowska, J.; Cieciura, W.; Borowski, S.; Dudkiewicz, M.; Binczarski, M.; Witonska, I.; Otlewska, A.; Kregiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp with Mixed Bacterial Cultures for Lactic Acid and Propylene Glycol Production. Molecules 2016, 21, 1380. [Google Scholar] [CrossRef] [PubMed]
- Bellido, C.; Infante, C.; Coca, M.; Gonzalez-Benito, G.; Lucas, S.; Garcia-Cubero, M.T. Efficient acetone-butanol-ethanol production by Clostridium beijerinckii from sugar beet pulp. Bioresour. Technol. 2015, 190, 332–338. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Sadeghzadeh Hemayati, S.; Tabatabaei, M.; Aghbashlo, M.; Mahmoudi, S.B. A review on beet sugar industry with a focus on implementation of waste-to-energy strategy for power supply. Renew. Sustain. Energy Rev. 2019, 103, 423–442. [Google Scholar] [CrossRef]
- Jang, Y.S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2-C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef]
- Gerardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.M. Continuous Flow Upgrading of Selected C2-C6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Shanks, B.H.; Broadbelt, L.J. A Robust Strategy for Sustainable Organic Chemicals Utilizing Bioprivileged Molecules. ChemSusChem 2019, 12, 2970–2975. [Google Scholar] [CrossRef]
- Shanks, B.H.; Keeling, P.L. Bioprivileged molecules: Creating value from biomass. Green Chem. 2017, 19, 3177–3185. [Google Scholar] [CrossRef]
- Zhou, X.; Brentzel, Z.J.; Kraus, G.A.; Keeling, P.L.; Dumesic, J.A.; Shanks, B.H.; Broadbelt, L.J. Computational Framework for the Identification of Bioprivileged Molecules. ACS Sustain. Chem. Eng. 2018, 7, 2414–2428. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.M.; Shanks, B.H.; Broadbelt, L.J. Identification of bioprivileged molecules: Expansion of a computational approach to broader molecular space. Mol. Syst. Des. Eng. 2021, 6, 445–460. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kasumi, T.; Ogihara, J.; Tamura, M.; Arai, T.; Tomishige, K. Erythritol: Another C4 Platform Chemical in Biomass Refinery. ACS Omega 2020, 5, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Hohn, K.L. Catalytic Conversion of Biomass-derived Compounds to C4 Chemicals. In Catalysis; The Royal Society of Chemistry: London, UK, 2019; pp. 1–36. [Google Scholar]
- Hülsey, M.J.; Yang, H.; Yan, N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 5694–5707. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-Butanediol production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2021, 54, 107783. [Google Scholar] [CrossRef]
- Tinôco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D.M.G. Technological development of the bio-based 2,3-butanediol process. Biofuels Bioprod. Biorefin. 2020, 15, 357–376. [Google Scholar] [CrossRef]
- Mazière, A.; Prinsen, P.; García, A.; Luque, R.; Len, C. A review of progress in (bio)catalytic routes from/to renewable succinic acid. Biofuels Bioprod. Biorefin. 2017, 11, 908–931. [Google Scholar] [CrossRef]
- Klein, B.C.; Silva, J.F.L.; Junqueira, T.L.; Rabelo, S.C.; Arruda, P.V.; Ienczak, J.L.; Mantelatto, P.E.; Pradella, J.G.C.; Junior, S.V.; Bonomi, A. Process development and techno-economic analysis of bio-based succinic acid derived from pentoses integrated to a sugarcane biorefinery. Biofuels Bioprod. Biorefin. 2017, 11, 1051–1064. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable bio-succinic acid production: Superstructure optimization, techno-economic, and lifecycle assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.P.; Wang, G.Y.; Khan, I.; Chi, Z.M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wu, M.; Dai, Z.; Zhang, S.; Zhang, W.; Dong, W.; Zhou, J.; Jiang, M.; Xin, F. Current advances on biological production of fumaric acid. Biochem. Eng. J. 2020, 153, 107397. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Empirical analysis of large-scale bio-succinic acid commercialization from a technoeconomic and innovation value chain perspective: BioAmber biorefinery case study in Canada. Renew. Sustain. Energy Rev. 2021, 137, 110587. [Google Scholar] [CrossRef]
- Drejer, E.B.; Chan, D.T.C.; Haupka, C.; Wendisch, V.F.; Brautaset, T.; Irla, M. Methanol-based acetoin production by genetically engineeredBacillus methanolicus. Green Chem. 2020, 22, 788–802. [Google Scholar] [CrossRef]
- Ji, X.-J.; Huang, H.; Ouyang, P.-K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Jantama, K.; Polyiam, P.; Khunnonkwao, P.; Chan, S.; Sangproo, M.; Khor, K.; Jantama, S.S.; Kanchanatawee, S. Efficient reduction of the formation of by-products and improvement of production yield of 2,3-butanediol by a combined deletion of alcohol dehydrogenase, acetate kinase-phosphotransacetylase, and lactate dehydrogenase genes in metabolically engineered Klebsiella oxytoca in mineral salts medium. Metab. Eng. 2015, 30, 16–26. [Google Scholar] [CrossRef]
- Li, L.; Li, K.; Wang, Y.; Chen, C.; Xu, Y.; Zhang, L.; Han, B.; Gao, C.; Tao, F.; Ma, C.; et al. Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab. Eng. 2015, 28, 19–27. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, J.a.; Hao, Y.; Zhu, J.; Chu, J.; Wei, D.; Shen, Y. Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30. J. Ind. Microbiol. Biotechnol. 2010, 37, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, A.; Qin, J.; Li, L.; Ai, X.; Jiang, T.; Tang, H.; Xu, P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, L.L.; Winkler, A.; Tucker, M.G.; Cosgrove, S.E.; Ross, T.; Lawson, E.; Carroll, K.C.; Perl, T.M. Outbreak of multidrug-resistant Serratia marcescens infection in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2008, 29, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huo, G.; Feng, L.; Mao, Y.; Wang, Z.; Ma, H.; Chen, T.; Zhao, X. Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnol. Biofuels 2016, 9, 90. [Google Scholar] [CrossRef]
- Shrivastav, A.; Lee, J.; Kim, H.Y.; Kim, Y.R. Recent insights in the removal of klebseilla pathogenicity factors for the industrial production of 2,3-butanediol. J. Microbiol. Biotechnol. 2013, 23, 885–896. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.W.; Lee, Y.G.; Park, Y.C.; Seo, J.H. Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.G.; Jin, Y.S.; Rao, C.V. Metabolic engineering of non-pathogenic microorganisms for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2021, 105, 5751–5767. [Google Scholar] [CrossRef]
- Bae, S.-J.; Kim, S.; Hahn, J.-S. Efficient production of acetoin in Saccharomyces cerevisiae by disruption of 2,3-butanediol dehydrogenase and expression of NADH oxidase. Sci. Rep. 2016, 6, 27667. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Bao, T.; Rao, Z.; Yang, T.; Xu, M.; Xu, Z.; Li, H.; Yang, S. The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab. Eng. 2014, 23, 34–41. [Google Scholar] [CrossRef]
- Sun, J.-A.; Zhang, L.-Y.; Rao, B.; Shen, Y.-L.; Wei, D.-Z. Enhanced acetoin production by Serratia marcescens H32 with expression of a water-forming NADH oxidase. Bioresour. Technol. 2012, 119, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Ge, Y.; Li, L.; Gao, C.; Xu, P.; Ma, C. Biotechnological production of acetoin, a bio-based platform chemical, from a lignocellulosic resource by metabolically engineered Enterobacter cloacae. Green Chem. 2016, 18, 1560–1570. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Kandasamy, V.; Lee, S.Y.; Solem, C.; Jensen, P.R. Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis. Metab. Eng. 2017, 44, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Morita, K.; Ida, K.; Kato, H.; Kinoshita, S.; Hataya, S.; Shimizu, H.; Kondo, A.; Matsuda, F. A pyruvate carbon flux tugging strategy for increasing 2,3-butanediol production and reducing ethanol subgeneration in the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2018, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hahn, J.S. Efficient production of 2,3-butanediol in Saccharomyces cerevisiae by eliminating ethanol and glycerol production and redox rebalancing. Metab. Eng. 2015, 31, 94–101. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G.; Kim, S.J.; Jin, Y.S.; Seo, J.H. Deletion of glycerol-3-phosphate dehydrogenase genes improved 2,3-butanediol production by reducing glycerol production in pyruvate decarboxylase-deficient Saccharomyces cerevisiae. J. Biotechnol. 2019, 304, 31–37. [Google Scholar] [CrossRef]
- Ida, Y.; Furusawa, C.; Hirasawa, T.; Shimizu, H. Stable disruption of ethanol production by deletion of the genes encoding alcohol dehydrogenase isozymes in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2012, 113, 192–195. [Google Scholar] [CrossRef]
- de Smidt, O.; du Preez, J.C.; Albertyn, J. Molecular and physiological aspects of alcohol dehydrogenases in the ethanol metabolism of Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 33–47. [Google Scholar] [CrossRef]
- Oud, B.; Flores, C.-L.; Gancedo, C.; Zhang, X.; Trueheart, J.; Daran, J.-M.; Pronk, J.T.; van Maris, A.J.A. An internal deletion in MTH1 enables growth on glucose of pyruvate-decarboxylase negative, non-fermentative Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 131. [Google Scholar] [CrossRef]
- Lian, J.; Chao, R.; Zhao, H. Metabolic engineering of a Saccharomyces cerevisiae strain capable of simultaneously utilizing glucose and galactose to produce enantiopure (2R,3R)-butanediol. Metab. Eng. 2014, 23, 92–99. [Google Scholar] [CrossRef]
- Huang, S.; Geng, A. High-copy genome integration of 2,3-butanediol biosynthesis pathway in Saccharomyces cerevisiae via in vivo DNA assembly and replicative CRISPR-Cas9 mediated delta integration. J. Biotechnol. 2020, 310, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Seo, J.H. Production of 2,3-butanediol from glucose and cassava hydrolysates by metabolically engineered industrial polyploid Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Nadal, I.; Rico, J.; Pérez-Martínez, G.; Yebra, M.J.; Monedero, V. Diacetyl and acetoin production from whey permeate using engineered Lactobacillus casei. J. Ind. Microbiol. Biotechnol. 2009, 36, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, J.; Zhang, X.; Chen, T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnol. Lett. 2012, 34, 1877–1885. [Google Scholar] [CrossRef]
- Fan, X.; Wu, H.; Jia, Z.; Li, G.; Li, Q.; Chen, N.; Xie, X. Metabolic engineering of Bacillus subtilis for the co-production of uridine and acetoin. Appl. Microbiol. Biotechnol. 2018, 102, 8753–8762. [Google Scholar] [CrossRef]
- Cilli, F.; Nazli-Zeka, A.; Arda, B.; Sipahi, O.R.; Aksit-Barik, S.; Kepeli, N.; Ozinel, M.A.; Gulay, Z.; Ulusoy, S. Serratia marcescens sepsis outbreak caused by contaminated propofol. Am. J. Infect. Control 2019, 47, 582–584. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, W.B.; Yoon, J.-K.; Cho, W.-S.; Kim, S.J.; Oh, Y.R.; Jun, K.I.; Kang, C.K.; Choe, P.G.; Kim, J.-I.; et al. Outbreak investigation of Serratia marcescens neurosurgical site infections associated with a contaminated shaving razors. Antimicrob. Resist. Infect. Control 2020, 9, 64. [Google Scholar] [CrossRef]
- Lu, L.; Mao, Y.; Kou, M.; Cui, Z.; Jin, B.; Chang, Z.; Wang, Z.; Ma, H.; Chen, T. Engineering central pathways for industrial-level (3R)-acetoin biosynthesis in Corynebacterium glutamicum. Microb. Cell Factories 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Javidnia, K.; Faghih-Mirzaei, E.; Miri, R.; Attarroshan, M.; Zomorodian, K. Biotransformation of acetoin to 2,3-butanediol: Assessment of plant and microbial biocatalysts. Res. Pharm. Sci. 2016, 11, 349–354. [Google Scholar] [CrossRef]
- González, E.; Rosario Fernández, M.; Marco, D.; Calam, E.; Sumoy, L.; Parés, X.; Dequin, S.; Biosca, J.A. Role of saccharomyces cerevisiae oxidoreductases Bdhlp and Aralp in the metabolism of acetoin and 2,3-butanediol. Appl. Environ. Microbiol. 2010, 76, 670–679. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, S.; Park, H.J.; Kim, J.; Jin, H.; Kim, B.G.; Hahn, J.S. High-yield production of (R)-acetoin in Saccharomyces cerevisiae by deleting genes for NAD(P)H-dependent ketone reductases producing meso-2,3-butanediol and 2,3-dimethylglycerate. Metab. Eng. 2021, 66, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Ruan, H.Z.; Chen, X.L.; Zhang, F.; Zhang, W. Equilibrium of the intracellular redox state for improving cell growth and l-lysine yield of Corynebacterium glutamicum by optimal cofactor swapping. Microb. Cell Factories 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, S.; Zhang, L.; Zhou, Y.J. Advanced Strategies for Production of Natural Products in Yeast. iScience 2020, 23, 100879. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Liu, Y.; Wang, J.; Deng, Y. Recent progress on bio-based production of dicarboxylic acids in yeast. Appl. Microbiol. Biotechnol. 2020, 104, 4259–4272. [Google Scholar] [CrossRef]
- Darbani, B.; Stovicek, V.; van der Hoek, S.A.; Borodina, I. Engineering energetically efficient transport of dicarboxylic acids in yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2019, 116, 19415–19420. [Google Scholar] [CrossRef]
- Yang, L.; Christakou, E.; Vang, J.; Lubeck, M.; Lubeck, P.S. Overexpression of a C4-dicarboxylate transporter is the key for rerouting citric acid to C4-dicarboxylic acid production in Aspergillus carbonarius. Microb. Cell Factories 2017, 16, 43. [Google Scholar] [CrossRef]
- Yang, L.; Henriksen, M.M.; Hansen, R.S.; Lubeck, M.; Vang, J.; Andersen, J.E.; Bille, S.; Lubeck, P.S. Metabolic engineering of Aspergillus niger via ribonucleoprotein-based CRISPR-Cas9 system for succinic acid production from renewable biomass. Biotechnol. Biofuels 2020, 13, 206. [Google Scholar] [CrossRef]
- Cao, W.; Yan, L.; Li, M.; Liu, X.; Xu, Y.; Xie, Z.; Liu, H. Identification and engineering a C4-dicarboxylate transporter for improvement of malic acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 2020, 104, 9773–9783. [Google Scholar] [CrossRef]
- Knuf, C. Malic Acid Production by Aspergillus oryzae; Chalmers University Of Technology: Gothenburg, Sweden, 2014. [Google Scholar]
- Liu, J.; Xie, Z.; Shin, H.D.; Li, J.; Du, G.; Chen, J.; Liu, L. Rewiring the reductive tricarboxylic acid pathway and L-malate transport pathway of Aspergillus oryzae for overproduction of L-malate. J. Biotechnol. 2017, 253, 1–9. [Google Scholar] [CrossRef]
- Kovilein, A.; Umpfenbach, J.; Ochsenreither, K. Acetate as substrate for L-malic acid production with Aspergillus oryzae DSM 1863. Biotechnol. Biofuels 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shan, L.; Zhou, Y.; Xie, Z.; Ball, A.S.; Cao, W.; Liu, H. Development of a Cre-loxP-based genetic system in Aspergillus niger ATCC1015 and its application to construction of efficient organic acid-producing cell factories. Appl. Microbiol. Biotechnol. 2019, 103, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Guettler, M.; Jain, M.; Rumler, D. Method for Making Succinic Acid, Bacterial Variants for Use in The Process, and Methods for Obtaining Variants. U.S. Patent No. 5573931, 12 November 1996. [Google Scholar]
- Ercole, A.; Raganati, F.; Salatino, P.; Marzocchella, A. Continuous succinic acid production by immobilized cells of Actinobacillus succinogenes in a fluidized bed reactor: Entrapment in alginate beads. Biochem. Eng. J. 2021, 169, 107968. [Google Scholar] [CrossRef]
- Litsanov, B.; Brocker, M.; Bott, M. Toward homosuccinate fermentation: Metabolic engineering of Corynebacterium glutamicum for anaerobic production of succinate from glucose and formate. Appl. Environ. Microbiol. 2012, 78, 3325–3337. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Guo, S.; Lemma, W.D.; Zhang, W.; Ma, J.; Jia, H.; Wu, H.; Jiang, M.; Ouyang, P. Enhanced succinic acid production under acidic conditions by introduction of glutamate decarboxylase system in E. coli AFP111. Bioprocess Biosyst. Eng. 2017, 40, 549–557. [Google Scholar] [CrossRef]
- Grabar, T.; Gong, W.; Yocum, R.R. Metabolic Evolution of Escherichia coli Strains that Produce Organic Acids; Myriant Corporation: Quincy, MA, USA, 2018. [Google Scholar]
- Zhang, X.; Jantama, K.; Moore, J.C.; Jarboe, L.R.; Shanmugam, K.T.; Ingram, L.O. Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106, 20180–20185. [Google Scholar] [CrossRef]
- Schroder, H.; Haefner, S.; Abendroth, V.; Hollmann, R.; Raddatz, A.; Ernst, H.; Gurski, H. Microbial Succinic Acid Producers and Purification of Succinic Acid. U.S. Patent No. 8673598 B2, 18 March 2014. [Google Scholar]
- Becker, J.; Reinefeld, J.; Stellmacher, R.; Schafer, R.; Lange, A.; Meyer, H.; Lalk, M.; Zelder, O.; von Abendroth, G.; Schroder, H.; et al. Systems-wide analysis and engineering of metabolic pathway fluxes in bio-succinate producing Basfia succiniciproducens. Biotechnol. Bioeng. 2013, 110, 3013–3023. [Google Scholar] [CrossRef]
- Lee, P.C.; Lee, S.Y.; Hong, S.H.; Chang, H.N. Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBEL55E, from bovine rumen. Appl. Microbiol. Biotechnol. 2002, 58, 663–668. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.M.; Song, H.; Lee, J.W.; Kim, T.Y.; Jang, Y.S. From genome sequence to integrated bioprocess for succinic acid production by Mannheimia succiniciproducens. Appl. Microbiol. Biotechnol. 2008, 79, 11–22. [Google Scholar] [CrossRef]
- Hong, K.K.; Kim, J.H.; Yoon, J.H.; Park, H.M.; Choi, S.J.; Song, G.H.; Lee, J.C.; Yang, Y.L.; Shin, H.K.; Kim, J.N.; et al. O-Succinyl-L-homoserine-based C4-chemical production: Succinic acid, homoserine lactone, gamma-butyrolactone, gamma-butyrolactone derivatives, and 1,4-butanediol. J. Ind. Microbiol. Biotechnol. 2014, 41, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.W.; Choi, S.; Yi, J. Mutant Microorganism Producing Succinic Acid Simultaneously Using Sucrose and Glycerol, and Method for Preparing Succinic Acid Using Same. U.S. Patent No. 8691516 B2, 8 April 2014. [Google Scholar]
- Lee, J.W.; Yi, J.; Kim, T.Y.; Choi, S.; Ahn, J.H.; Song, H.; Lee, M.H.; Lee, S.Y. Homo-succinic acid production by metabolically engineered Mannheimia succiniciproducens. Metab. Eng. 2016, 38, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, H.U.; Park, J.M.; Song, H.; Kim, J.S.; Lee, S.Y. Genome-scale analysis of Mannheimia succiniciproducens metabolism. Biotechnol. Bioeng. 2007, 97, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Yun, H.; Park, S.; Lee, S.Y. MetaFluxNet: The management of metabolic reaction information and quantitative metabolic flux analysis. Bioinformatics 2003, 19, 2144–2146. [Google Scholar] [CrossRef]
- Choi, S.; Song, H.; Lim, S.W.; Kim, T.Y.; Ahn, J.H.; Lee, J.W.; Lee, M.H.; Lee, S.Y. Highly selective production of succinic acid by metabolically engineered Mannheimia succiniciproducens and its efficient purification. Biotechnol. Bioeng. 2016, 113, 2168–2177. [Google Scholar] [CrossRef]
- Kim, W.J.; Ahn, J.H.; Kim, H.U.; Kim, T.Y.; Lee, S.Y. Metabolic engineering of Mannheimia succiniciproducens for succinic acid production based on elementary mode analysis with clustering. Biotechnol. J. 2017, 12, 1600701. [Google Scholar] [CrossRef]
- Molenaar, D.; van der Rest, M.E.; Drysch, A.; Yucel, R. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J. Bacteriol. 2000, 182, 6884–6891. [Google Scholar] [CrossRef]
- Ahn, J.H.; Seo, H.; Park, W.; Seok, J.; Lee, J.A.; Kim, W.J.; Kim, G.B.; Kim, K.J.; Lee, S.Y. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase. Nat. Commun. 2020, 11, 1970. [Google Scholar] [CrossRef]
- Meyer, V.; Wu, B.; Ram, A.F. Aspergillus as a multi-purpose cell factory: Current status and perspectives. Biotechnol. Lett. 2011, 33, 469–476. [Google Scholar] [CrossRef]
- Yang, L.; Lubeck, M.; Lubeck, P.S. Effects of heterologous expression of phosphoenolpyruvate carboxykinase and phosphoenolpyruvate carboxylase on organic acid production in Aspergillus carbonarius. J. Ind. Microbiol. Biotechnol. 2015, 42, 1533–1545. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Lubeck, M.; Ahring, B.K.; Lubeck, P.S. Enhanced succinic acid production in Aspergillus saccharolyticus by heterologous expression of fumarate reductase from Trypanosoma brucei. Appl. Microbiol. Biotechnol. 2016, 100, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Li, H.Y.; Xie, C.Y.; Fei, Q.; Cheng, K.K. Co-production of acetoin and succinic acid by metabolically engineered Enterobacter cloacae. Biotechnol. Biofuels 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.-J.; Yan, X.; Zhou, Y.-J.; Lin, Z.-N.; Cheng, K.-K.; Zhang, J.-A. Co-production of 2,3-BDO and succinic acid using xylose by Enterobacter cloacae. J. Chem. Technol. Biotechnol. 2018, 93, 1462–1467. [Google Scholar] [CrossRef]
- Li, X.; Wei, L.; Wang, Z.; Wang, Y.; Su, Z. Efficient co-production of propionic acid and succinic acid by Propionibacterium acidipropionici using membrane separation coupled technology. Eng. Life Sci. 2021, 21, 429–437. [Google Scholar] [CrossRef]
- Bu, J.; Yan, X.; Wang, Y.-T.; Zhu, S.-M.; Zhu, M.-J. Co-production of high-gravity bioethanol and succinic acid from potassium peroxymonosulfate and deacetylation sequentially pretreated sugarcane bagasse by simultaneous saccharification and co-fermentation. Energy Convers. Manag. 2019, 186, 131–139. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [Google Scholar] [CrossRef]
- Cheng, K.K.; Wu, J.; Wang, G.Y.; Li, W.Y.; Feng, J.; Zhang, J.A. Effects of pH and dissolved CO2 level on simultaneous production of 2,3-butanediol and succinic acid using Klebsiella pneumoniae. Bioresour. Technol. 2013, 135, 500–503. [Google Scholar] [CrossRef]
- Xi, Y.L.; Chen, K.Q.; Li, J.; Fang, X.J.; Zheng, X.Y.; Sui, S.S.; Jiang, M.; Wei, P. Optimization of culture conditions in CO2 fixation for succinic acid production using Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1605–1612. [Google Scholar] [CrossRef]
- Mitrea, L.; Vodnar, D.C. Klebsiella pneumoniae—A useful pathogenic strain for biotechnological purposes: Diols biosynthesis under controlled and uncontrolled pH levels. Pathogens 2019, 8, 293. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mishra, C.; Behera, S.S.; Thatoi, H. Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass—A review. Renew. Sustain. Energy Rev. 2017, 78, 1007–1032. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and fermentation of lignocellulosic biomass: Reaction mechanisms and process engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Alokika, A.; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Seo, S.O.; Oh, E.J.; Seo, J.H.; Cate, J.H.D.; Jin, Y.S. 2,3-Butanediol production from cellobiose by engineered Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2014, 98, 5757–5764. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Nair Salini, C.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Deshmukh, A.N.; Nipanikar-Gokhale, P.; Jain, R. Engineering of Bacillus subtilis for the Production of 2,3-Butanediol from Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 179, 321–331. [Google Scholar] [CrossRef]
- Tinôco, D.; Pateraki, C.; Koutinas, A.A.; Freire, D.M.G. Bioprocess Development for 2,3-Butanediol Production by Paenibacillus Strains. ChemBioEng Rev. 2021, 8, 44–62. [Google Scholar] [CrossRef]
- Tinôco, D.; de Castro, A.M.; Seldin, L.; Freire, D.M.G. Production of (2R,3R)-butanediol by Paenibacillus polymyxa PM 3605 from crude glycerol supplemented with sugarcane molasses. Process Biochem. 2021, 106, 88–95. [Google Scholar] [CrossRef]
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Fermentation of biodiesel-derived glycerol by Bacillus amyloliquefaciens: Effects of co-substrates on 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2013, 97, 7651–7658. [Google Scholar] [CrossRef]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.T. Enhanced 2,3-butanediol production from biodiesel-derived glycerol by engineering of cofactor regeneration and manipulating carbon flux in Bacillus amyloliquefaciens. Microb. Cell Factories 2015, 14, 122. [Google Scholar] [CrossRef]
- Maina, S.; Mallouchos, A.; Nychas, G.J.E.; Freire, D.M.G.; Castro, A.M.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess development for (2R,3R)-butanediol and acetoin production using very high polarity cane sugar and sugarcane molasses by a Bacillus amyloliquefaciens strain. J. Chem. Technol. Biotechnol. 2019, 94, 2167–2177. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Liu, P.H.; Qin, J.Y.; Xu, P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007, 74, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Y.; Cheng, L.; He, Q.-F.; Xiu, Z.-L. High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Process Biochem. 2015, 50, 1730–1734. [Google Scholar] [CrossRef]
- Ripoll, V.; Ladero, M.; Santos, V.E. Kinetic modelling of 2,3-butanediol production by Raoultella terrigena CECT 4519 resting cells: Effect of fluid dynamics conditions and initial glycerol concentration. Biochem. Eng. J. 2021, 176, 108185. [Google Scholar] [CrossRef]
- Ripoll, V.; Rodríguez, A.; Ladero, M.; Santos, V.E. High 2,3-butanediol production from glycerol by Raoultella terrigena CECT 4519. Bioprocess Biosyst. Eng. 2020, 43, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Ripoll, V.; Santos, V.E.; Gomez, E.; Garcia-Ochoa, F. Effect of fluid dynamic conditions on 2,3-butanediol production by Raoultella terrigena in SBTR: Oxygen transfer and uptake rates. J. Chem. Technol. Biotechnol. 2017, 92, 1266–1275. [Google Scholar] [CrossRef]
- Mancini, E.; Mansouri, S.S.; Gernaey, K.V.; Luo, J.; Pinelo, M. From second generation feed-stocks to innovative fermentation and downstream techniques for succinic acid production. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1829–1873. [Google Scholar] [CrossRef]
- Jiang, M.; Dai, W.; Xi, Y.; Wu, M.; Kong, X.; Ma, J.; Zhang, M.; Chen, K.; Wei, P. Succinic acid production from sucrose by Actinobacillus succinogenes NJ113. Bioresour. Technol. 2014, 153, 327–332. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, R.; Xi, Y.L.; Zhang, J.H.; Dai, W.Y.; Wan, Y.J.; Chen, K.Q.; Wei, P. Succinic acid production from cellobiose by Actinobacillus succinogenes. Bioresour. Technol. 2013, 135, 469–474. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, M.; Dai, Z.; Xin, F.; Zhou, J.; Dong, W.; Ma, J.; Jiang, M.; Zhang, W. Comprehensive investigation of succinic acid production by Actinobacillus succinogenes: A promising native succinic acid producer. Biofuels Bioprod. Biorefin. 2019, 14, 950–964. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Succinic acid biosynthesis from cane molasses under low pH by Actinobacillus succinogenes immobilized in luffa sponge matrices. Bioresour. Technol. 2018, 268, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Feng, J.; Zhang, A.; Ying, H.; He, X.; Jiang, M.; Chen, K.; Ouyang, P. Enhanced succinic acid production from polyacrylamide-pretreated cane molasses in microbial electrolysis cells. J. Chem. Technol. Biotechnol. 2017, 93, 855–860. [Google Scholar] [CrossRef]
- Shen, N.; Qin, Y.; Wang, Q.; Liao, S.; Zhu, J.; Zhu, Q.; Mi, H.; Adhikari, B.; Wei, Y.; Huang, R. Production of succinic acid from sugarcane molasses supplemented with a mixture of corn steep liquor powder and peanut meal as nitrogen sources by Actinobacillus succinogenes. Lett. Appl. Microbiol. 2015, 60, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Sturm, M.P.; Cole, M.R. Acid hydrolysis of sucrose in sweet sorghum syrup followed by succinic acid production using a genetically engineered Escherichia coli. Biocatal. Agric. Biotechnol. 2022, 39, 102231. [Google Scholar] [CrossRef]
- West, T. Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass. Fermentation 2017, 3, 14. [Google Scholar] [CrossRef]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2019, 95, 513–526. [Google Scholar] [CrossRef]
- Feng, J.; Yang, J.; Yang, W.; Chen, J.; Jiang, M.; Zou, X. Metabolome- and genome-scale model analyses for engineering of Aureobasidium pullulans to enhance polymalic acid and malic acid production from sugarcane molasses. Biotechnol. Biofuels 2018, 11, 94. [Google Scholar] [CrossRef]
- Wei, P.; Cheng, C.; Lin, M.; Zhou, Y.; Yang, S.T. Production of poly(malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: Kinetics and process economics. Bioresour. Technol. 2017, 224, 581–589. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.; Santos, V.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef]
- Papadaki, A.; Papapostolou, H.; Alexandri, M.; Kopsahelis, N.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Koutinas, A.A. Fumaric acid production using renewable resources from biodiesel and cane sugar production processes. Environ. Sci. Pollut. Res. Int. 2018, 25, 35960–35970. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, A.; Ostrowski, K.A.; Aslam, F.; Joyklad, P.; Zajdel, P. Sustainable approach of using sugarcane bagasse ash in cement-based composites: A systematic review. Case Stud. Constr. Mater. 2021, 15, e00698. [Google Scholar] [CrossRef]
- Kumar, A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of sugarcane bagasse using pretreatment strategies for bioethanol production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Papapostolou, H.; Ladakis, D.; Koutinas, A.; Venus, J. Restructuring the Conventional Sugar Beet Industry into a Novel Biorefinery: Fractionation and Bioconversion of Sugar Beet Pulp into Succinic Acid and Value-Added Coproducts. ACS Sustain. Chem. Eng. 2019, 7, 6569–6579. [Google Scholar] [CrossRef]
- Zetty-Arenas, A.M.; Tovar, L.P.; Alves, R.F.; Mariano, A.P.; van Gulik, W.; Maciel Filho, R.; Freitas, S. Co-fermentation of sugarcane bagasse hydrolysate and molasses by Clostridium saccharoperbutylacetonicum: Effect on sugar consumption and butanol production. Ind. Crops Prod. 2021, 167, 113512. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Narisetty, V.; Amraoui, Y.; Abdullah, A.; Ahmad, E.; Agrawal, D.; Parameswaran, B.; Pandey, A.; Goel, S.; Kumar, V. High yield recovery of 2,3-butanediol from fermented broth accumulated on xylose rich sugarcane bagasse hydrolysate using aqueous two-phase extraction system. Bioresour. Technol. 2021, 337, 125463. [Google Scholar] [CrossRef]
- Um, J.; Kim, D.G.; Jung, M.Y.; Saratale, G.D.; Oh, M.K. Metabolic engineering of Enterobacter aerogenes for 2,3-butanediol production from sugarcane bagasse hydrolysate. Bioresour. Technol. 2017, 245, 1567–1574. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Alam, M.A.; Wang, Z.; Zhang, J.; Huang, S.; Zhuang, W.; Xu, C.; Xu, J. Novel biorefining method for succinic acid processed from sugarcane bagasse. Bioresour. Technol. 2021, 324, 124615. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Li, C.; Li, X.; Zhang, Y.; Xu, J.; Lin, C.S.K. Co-fermentation of glucose and xylose from sugarcane bagasse into succinic acid by Yarrowia lipolytica. Biochem. Eng. J. 2019, 148, 108–115. [Google Scholar] [CrossRef]
- Lo, E.; Brabo-Catala, L.; Dogaris, I.; Ammar, E.M.; Philippidis, G.P. Biochemical conversion of sweet sorghum bagasse to succinic acid. J. Biosci. Bioeng. 2020, 129, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Cao, W.; Shen, F.; Luo, J.; Yin, J.; Qiao, C.; Wan, Y. Membrane-assisted beta-poly(L-malic acid) production from bagasse hydrolysates by Aureobasidium pullulans ipe-1. Bioresour. Technol. 2020, 295, 122260. [Google Scholar] [CrossRef]
- Deng, F.; Aita, G.M. Fumaric Acid Production by Rhizopus oryzae ATCC® 20344™ from Lignocellulosic Syrup. BioEnergy Research 2018, 11, 330–340. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Zhang, S.; Zhang, W.; Yang, Q.; Dong, W.; Jiang, M.; Ma, J.; Xin, F. Bio-based succinic acid: An overview of strain development, substrate utilization, and downstream purification. Biofuels Bioprod. Biorefin. 2019, 14, 965–985. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Shibamoto, T. Diacetyl: Occurrence, analysis, and toxicity. J. Agric. Food Chem. 2014, 62, 4048–4053. [Google Scholar] [CrossRef]
- Huchede, M.; Gu, Q.; Gauthier, G.; Bellière-Baca, V.; Michel, C.; Millet, J.M.M. New process for producing butane-2,3-dione by oxidative dehydrogenation of 3-hydroxybutanone. React. Chem. Eng. 2019, 4, 932–938. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, T.; Liu, D.; Wu, J.; Chen, Y.; Wang, L.; Guo, K.; Ying, H.; Ouyang, P. Production of liquid hydrocarbon fuels with acetoin and platform molecules derived from lignocellulose. Green Chem. 2016, 18, 2165–2174. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Vapor-phase hydrogenation of acetoin and diacetyl into 2,3-butanediol over supported metal catalysts. Catal. Commun. 2017, 99, 53–56. [Google Scholar] [CrossRef]
- Maina, S.; Dheskali, E.; Papapostolou, H.; Castro, A.M.d.; Guimaraes Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess Development for 2,3-Butanediol Production from Crude Glycerol and Conceptual Process Design for Aqueous Conversion into Methyl Ethyl Ketone. ACS Sustain. Chem. Eng. 2021, 9, 8692–8705. [Google Scholar] [CrossRef]
- Bai, Y.; Page, S.J.; Zhang, J.; Liu, D.; Zhao, X. Kinetic modelling of acid-catalyzed liquid-phase dehydration of bio-based 2, 3-butanediol considering a newly identified by-product and an updated reaction network. Chem. Eng. J. 2020, 389, 124451. [Google Scholar] [CrossRef]
- Harvey, B.G.; Merriman, W.W.; Quintana, R.L. Renewable Gasoline, Solvents, and Fuel Additives from 2,3-Butanediol. ChemSusChem 2016, 9, 1814–1819. [Google Scholar] [CrossRef]
- Hoppe, F.; Burke, U.; Thewes, M.; Heufer, A.; Kremer, F.; Pischinger, S. Tailor-Made Fuels from Biomass: Potentials of 2-butanone and 2-methylfuran in direct injection spark ignition engines. Fuel 2016, 167, 106–117. [Google Scholar] [CrossRef]
- Wildenberg, A.; Fenard, Y.; Carbonnier, M.; Kéromnès, A.; Lefort, B.; Serinyel, Z.; Dayma, G.; Le Moyne, L.; Dagaut, P.; Heufer, K.A. An experimental and kinetic modeling study on the oxidation of 1,3-dioxolane. Proc. Combust. Inst. 2021, 38, 543–553. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Zhang, J.; Guo, Q.; Unocic, K.A.; Tao, L.; Li, Z. A hybrid pathway to biojet fuel via 2,3-butanediol. Sustain. Energy Fuels 2020, 4, 3904–3914. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Yang, C.; Su, Y.; Yamada, Y.; Sato, S. Production of 1,3-butadiene from biomass-derived C4 alcohols. Fuel Process. Technol. 2020, 197, 106193. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Matei-Rutkovska, F.; Huchede, M.; Jaillardon, K.; Qingyi, G.; Michel, C.; Millet, J.M.M. Production of 1,3-butadiene in one step catalytic dehydration of 2,3-butanediol. Catal. Today 2019, 323, 62–68. [Google Scholar] [CrossRef]
- Lima, M.T.; Finelli, F.G.; de Oliveira, A.V.B.; Kartnaller, V.; Cajaiba, J.F.; Leão, R.A.C.; de Souza, R.O.M.A. Continuous-flow synthesis of dimethyl fumarate: A powerful small molecule for the treatment of psoriasis and multiple sclerosis. RSC Adv. 2020, 10, 2490–2494. [Google Scholar] [CrossRef]
- Gérardy, R.; Winter, M.; Horn, C.R.; Vizza, A.; Van Hecke, K.; Monbaliu, J.-C.M. Continuous-Flow Preparation of γ-Butyrolactone Scaffolds from Renewable Fumaric and Itaconic Acids under Photosensitized Conditions. Org. Process Res. Dev. 2017, 21, 2012–2017. [Google Scholar] [CrossRef]
- Stojkovič, G.; Plazl, I.; Žnidaršič-Plazl, P. l-Malic acid production within a microreactor with surface immobilised fumarase. Microfluid. Nanofluidics 2010, 10, 627–635. [Google Scholar] [CrossRef]
- Nghiem, N.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Verma, M.; Mandyal, P.; Singh, D.; Gupta, N. Recent Developments in Heterogeneous Catalytic Routes for the Sustainable Production of Succinic Acid from Biomass Resources. ChemSusChem 2020, 13, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Bogel-Łukasik, E. Valuable new platform chemicals obtained by valorisation of a model succinic acid and bio-succinic acid with an ionic liquid and high-pressure carbon dioxide. Green Chem. 2017, 19, 4048–4060. [Google Scholar] [CrossRef]
- Aguzín, F.L.; Martínez, M.L.; Beltramone, A.R.; Padró, C.L.; Okulik, N.B. Esterification of Succinic Acid Using Sulfated Zirconia Supported on SBA-15. Chem. Eng. Technol. 2021, 44, 1185–1194. [Google Scholar] [CrossRef]
- Vardon, D.R.; Settle, A.E.; Vorotnikov, V.; Menart, M.J.; Eaton, T.R.; Unocic, K.A.; Steirer, K.X.; Wood, K.N.; Cleveland, N.S.; Moyer, K.E.; et al. Ru-Sn/AC for the Aqueous-Phase Reduction of Succinic Acid to 1,4-Butanediol under Continuous Process Conditions. ACS Catal. 2017, 7, 6207–6219. [Google Scholar] [CrossRef]
- Kang, K.H.; Hong, U.G.; Bang, Y.; Choi, J.H.; Kim, J.K.; Lee, J.K.; Han, S.J.; Song, I.K. Hydrogenation of succinic acid to 1,4-butanediol over Re–Ru bimetallic catalysts supported on mesoporous carbon. Appl. Catal. Gen. 2015, 490, 153–162. [Google Scholar] [CrossRef]
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. A critical view on the environmental sustainability of biorefinery systems. Curr. Opin. Green Sustain. Chem. 2021, 27, 100392. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J. Exnovating for a renewable energy transition. Nat. Energy 2019, 4, 254–256. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. A framework for the design and analysis of integrated multi-product biorefineries from agricultural and forestry wastes. Renew. Sustain. Energy Rev. 2021, 139, 110687. [Google Scholar] [CrossRef]
- Martinez-Valencia, L.; Garcia-Perez, M.; Wolcott, M.P. Supply chain configuration of sustainable aviation fuel: Review, challenges, and pathways for including environmental and social benefits. Renew. Sustain. Energy Rev. 2021, 152, 111680. [Google Scholar] [CrossRef]
- Krishna, S.H.; Huang, K.; Barnett, K.J.; He, J.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W.; De bruyn, M.; Weckhuysen, B.M. Oxygenated commodity chemicals from chemo-catalytic conversion of biomass derived heterocycles. AIChE J. 2018, 64, 1910–1922. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Wahl, S.A.; Benjamin, K.R.; Takors, R.; Wierckx, N.; Noorman, H.J. Grand Research Challenges for Sustainable Industrial Biotechnology. Trends Biotechnol. 2019, 37, 1042–1050. [Google Scholar] [CrossRef]
- Stalidzans, E.; Dace, E. Sustainable metabolic engineering for sustainability optimisation of industrial biotechnology. Comput. Str. Biotechnol. J. 2021, 19, 4770–4776. [Google Scholar] [CrossRef]
- Kampers, L.F.C.; Asin-Garcia, E.; Schaap, P.J.; Wagemakers, A.; Martins Dos Santos, V.A.P. From Innovation to Application: Bridging the Valley of Death in Industrial Biotechnology. Trends Biotechnol. 2021, 39, 1240–1242. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Martinez-Valencia, L.; Tanzil, A.H.; Garcia-Perez, M.; García-Ojeda, J.C.; Bertok, B.; Heckl, I.; Argoti, A.; Friedler, F. Synthesis and Techno-Economic Analysis of Pyrolysis-Oil-Based Biorefineries Using P-Graph. Energy Fuels 2021, 35, 13159–13169. [Google Scholar] [CrossRef]
- Benjamin, M.F.D.; Ventura, J.-R.S.; Sangalang, K.P.H.; Adorna, J.A.; Belmonte, B.A.; Andiappan, V. Optimal synthesis of Philippine agricultural residue-based integrated biorefinery via the P-graph method under supply and demand constraints. J. Clean. Prod. 2021, 308, 127348. [Google Scholar] [CrossRef]





| Product | Applications | Market Price | Reference |
|---|---|---|---|
| Acetoin | Food additive, flavor/fragrance additive in tobacco products, cosmetics, soaps and detergents, chemical precursor, plant growth promoter and postharvest decay control | USD 30–50/kg | [48,58] |
| 2,3-BDO | Chemical precursor for fuels and solvents, flavor and fragrance additives, pharmaceuticals, polymers and materials | USD 2–3/kg | [49,50,51] |
| Succinic Acid | Food additive, chemical precursor for fuels and solvents, pharmaceuticals, polymers and materials, precursor for 1,4-butanediol | USD 2–3/kg | [52,53,54] |
| Malic Acid | Primarily a food and flavor additive, further applications in pharmaceuticals, textiles and polymers/materials | USD 2/kg | [39,55] |
| Fumaric Acid | Primarily a food and flavor additive, further applications in polymers/resins and paper | USD 1.5/kg | [39,56] |
| Product | Feedstock | Brief Summary | Reference |
|---|---|---|---|
| 2,3-BDO | Sugarcane bagasse | 2,3-BDO is produced from mutant E. ludwigii with xylose-rich hydrolysate from sugarcane bagasse as a feedstock. Fed-batch fermentation resulted in accumulation of 68 g/L with yield of 38% and productivity of 0.9 g/L/h, with acetic acid by-product. Separation with an optimized aqueous two-phase system resulted in recovery of 97%. Techno-economic analysis using ASPEN Plus software is also presented for estimation of capital and operating costs. | [176] |
| 2,3-BDO | Sugarcane bagasse | Metabolic/pathway engineering of E. aerogenes with gene deletions is explored for the improvement of xylose consumption and 2,3-BDO yield. Sugarcane bagasse hydrolysate was utilized as a feedstock for 2,3-BDO production. A carbon yield of 70% is reported after 36 h, decreasing to 40% at 72 h. By-products include succinate, acetate, ethanol, and acetoin. | [177] |
| Succinic Acid | Sugarcane bagasse | Sugarcane bagasse is pretreated with three different methods (hot water, ethanol, sodium hydroxide) and subsequently utilized in fermentations with A. succinogenes for succinic acid production. Sodium hydroxide pretreatment is reported as the most successful. The authors report maximum yield of 41 g/L with productivity of 0.3 g/L/h and present an assessment of energy and water consumption of the developed process. | [178] |
| Succinic Acid | Sugarcane bagasse | The co-utilization of glucose and xylose from sugarcane bagasse hydrolysates is explored for the production of succinic acid from Y. lipolytica. Mixed glucose-xylose carbon source resulted in a titer of 28 g/L with 55% yield and 0.36 g/L/h productivity; bagasse hydrolysates resulted in a higher titer of 33 g/L and higher yield of 58% with 0.33 g/L/h productivity. | [179] |
| Succinic Acid | Sugar beet pulp | Sugar beet pulp is used in an integrated bio-refinery study for the extraction/production of antioxidants and pectins along with fermentation of hydrolysates for succinic acid from A. succinogenes. Fed-batch pilot-scale (50 L) fermentation resulted in production of 30 g/L succinic acid, with 90% yield and 0.75 g/L/h productivity (similar to 5 L lab-scale fermentation). Estimated succinic acid production cost (at 40 kton capacity) is reported to be USD 2.4/kg. | [173] |
| Succinic Acid | Sweet sorghum bagasse | Phosphoric acid-pretreated sweet sorghum bagasse hydrolysate is used in the production of succinic acid from A. succinogenes. The authors report final concentration of 17.8 g/L with 61% yield, comparable to yield from pure glucose as sole carbon source. The authors utilize a 3.5 L, CO2-sparged bioreactor and suggest the feasibility of this process as a sustainable route for carbon sequestration. | [180] |
| Polymalic Acid | Sugarcane bagasse | Bagasse hydrolysates are utilized in the production of β-poly(L-malic acid) from A. pullulans. The authors conclude that the mixture of acid and enzyme hydrolysates is ultimately not recommended as a fermentation substrate, due to effects from sugar ratios and concentration. Further processing is suggested to optimize suitable quantities/concentrations of sugars and acid for effective bagasse hydrolysate utilization. | [181] |
| Fumaric Acid | Energy cane bagasse | Hydrolysates from pre-treated energy cane bagasse were utilized in the production of fumaric acid with R. oryzae. Powdered activated carbon was applied to hydrolysates to remove potential fermentation inhibitors. Optimized conditions resulted in the production of 34 g/L fumaric acid with 43% yield and 0.2 g/L/h productivity, comparable to fermentation using pure glucose and xylose media. | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, G.O.; Terrell, E. A Review on the Production of C4 Platform Chemicals from Biochemical Conversion of Sugar Crop Processing Products and By-Products. Fermentation 2022, 8, 216. https://doi.org/10.3390/fermentation8050216
Bruni GO, Terrell E. A Review on the Production of C4 Platform Chemicals from Biochemical Conversion of Sugar Crop Processing Products and By-Products. Fermentation. 2022; 8(5):216. https://doi.org/10.3390/fermentation8050216
Chicago/Turabian StyleBruni, Gillian O., and Evan Terrell. 2022. "A Review on the Production of C4 Platform Chemicals from Biochemical Conversion of Sugar Crop Processing Products and By-Products" Fermentation 8, no. 5: 216. https://doi.org/10.3390/fermentation8050216
APA StyleBruni, G. O., & Terrell, E. (2022). A Review on the Production of C4 Platform Chemicals from Biochemical Conversion of Sugar Crop Processing Products and By-Products. Fermentation, 8(5), 216. https://doi.org/10.3390/fermentation8050216






